Abstract
As the hydroxyl-terminated polybutadiene (HTPB)-based binder system is widely applied in many industries, the curing process plays an important role in the final properties of the resulting product containing such a binder system. This study used a viscometer to measure viscosity buildup in the curing process of the binder system with various curing agents under isothermal conditions. Key parameters such as rheological reaction rate constant (kƞ) and pot life of different were measured and calculated. The rheological reaction rate constants of the HTPB-based binder systems included 0.0423 min−1 (MDI), 0.0049 min−1 (HDI-trimer) and 0.0014 min−1 (HMDI). The pot lives of the HTPB-MDI, HTPB-TDI, and HTPB-HDI-trimer were 0.6 h, 3.6 h and 8.1 h, respectively. One interesting finding is that HTPB-HDI-trimer binder, which had the long pot life, exhibited an accelerated trend in the viscosity buildup in the late phase. This feature is of great significance to improving the final properties of the products generated in propellant manufacturing and other fields. The cause of this phenomenon and the curing process of HTPB-HDI-trimer binder system were analyzed and discussed in the present study.
1. Introduction
The hydroxyl-terminated polybutadiene (HTPB)-based binder system is broadly applied in many industries, such as coatings, adhesives, epoxy resin, polyurethane elastomers, composite propellants and explosives [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15]. With the addition of the binder, the slurry fluidity and mechanical properties of the final products can be adjusted so that the requirements for the physical forms and specific shapes of products can be fulfilled in various situations. The binder system matters a great deal to the mechanical properties, process properties, stability and explosive properties [1].
A polyisocyanate participates in the curing reaction and produces hard segments of polyurethane elastomer (PU elastomer) whose structure directly affects the mechanical properties of the PU elastomer. Polyisocyanates have a significant effect on the curing reaction rate and mechanical properties of the products. Both the reactivity and the curing reaction rate of their isocyanate groups differ, thereby having different effects on the length of the pot life, the initial/late phase curing reaction rate, and the length of the final curing time [6,7,8,9]. Therefore, studying the effect of single and composite curing agents on the curing reaction rate of the binder system is of great importance to adjusting the system’s pot life, curing reaction rate and final curing time. Although there are numerous methods to investigate the curing process of the binder system, the viscosity approach is simpler and more practicable than others as it measures the viscosity change in the curing reaction [16,17]. In addition, the viscosity approach reflects the pot life of the binder system and slurry. However, there are very few reports on the systematic rheological research of the curing of the HTPB–isocyanate binder system.
This paper studied the effect of single curing agents such as methylene diphenyl diisocyanate (MDI), toluene diisocyanate (TDI), trimer of hexamethylene-1,6-diisocyanate (HDI-trimer), isophorone diisocyanate (IPDI) and hexamethylene diisocyanate (HMDI) on the curing reactions of the HTPB-based binder system. The study involved the use of a viscometer to observe the curing reactions of HTPB with multiple single curing agents in given temperature conditions. The curve of viscosity buildup versus time served as a basis for computing the curing reaction rates and pot life, anatomizing the curve changes and the curing reaction mechanism. Additionally, the findings here are of great significance for improving the performance of HTPB-based binder-cured products.
2. Experimental Instruments and Materials
2.1. Instruments and Materials
The experimental equipment includes the following: a digital thermostatic oil bath (manufactured by Changzhou Saipu; model: HH-SA, Changzhou, China); a digital thermostatic water bath (manufactured by Changzhou Zhibo; model: HH-1, Changzhou, China); a digital viscometer (manufactured by Shanghai Fangrui; model: SNB-1A, Shanghai, China); a vacuum drier (manufactured by BÜICH; model: V-700, Shanghai, China); an electronic balance (manufactured by Mettler Toledo; model: AL 201-IC, Shanghai, China); a digital electric mixer (manufactured by Shanghai Angni; model: AM 90L-H, Shanghai, China).
The experimental chemicals include HTPB (produced by Luoyang Liming Chemicals, Luoyang, China), technical pure, vacuum-dehydrated for 2 h before use under 105 °C/0.05 MPa and sealed and kept in a dark place; HMDI (produced by BASF, Chongqing, China), technical pure; and TDI, MDI, IPDI and HDI-trimer (produced by Bayer, Leverkusen, Germany), technical pure.
2.2. Binder System Formulas
Supposing the isocyanate index (R) of the binder system is 1, the amounts of HTPB and the curing agent can be computed based on Formula (1) through Formula (3):
where R is the isocyanate index, nNCO is the molecular concentration of the hydroxyl group in the isocyanate, and nOH is the molecular concentration of the hydroxyl group in HTPB.
2.3. Sample Preparation and Testing
Approximately 200.00 g of HTPB and each kind of curing agent was measured with electronic balance and transferred into a dry beaker; the mixture was churned for 5 min at a rate of 1000 rpm, transferred into the vacuum dryer and deaerated for 5 min under a vacuum pressure of 100 Pa; the binder mixture was transferred into a small measuring cup which was in turn transferred into the thermostatic water bath at 45 °C for the viscosity buildup test.
2.4. Rheology
Normally, there is an exponential relationship between the binder system viscosity change and the curing reaction time, which conforms to the Arrhenius equation:
where ƞ(t)T is the system viscosity at time t at temp T, ƞ0 is the initial system viscosity at temp T, kƞ is the rheological reaction rate constant of the system, and t is the system curing time.
The logarithm of Formula (2) is adopted on both sides to obtain the viscosity versus time linear equation, i.e., the Arrhenius chemorheological model under isothermal condition as shown in Formula (3). A linear fit of time t is plotted based on the logarithm of the system viscosity (ln(ƞ(t)T)). The slope of the straight line is taken as the rheological reaction rate constant (kƞ), and the logarithm of the initial viscosity (ln(ƞ0)) as the intercept. Then, we obtain the following:
where ƞ(t)T is the viscosity of the system at temperature T at any given time t, ƞ0(T) is the initial viscosity of the system at temperature T, kƞ is the rheological reaction rate constant of the system, and t is the curing time.
3. Results and Discussion
The viscosity buildup of the binder system with time in the reaction of HTPB with each single curing agent, i.e., MDI, HMDI, TDI, IPDI and HDI-trimer, was recorded and is shown in Figure 1. The curing temperature of all the reactions is set as 45 °C so the measurements of these agents could have comparability.
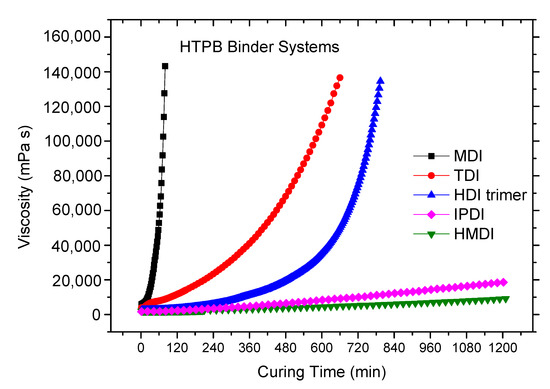
Figure 1.
The viscosity increases with the curing time at 45 °C (binder systems with a single curing agent).
As can be seen in Figure 1, there are obvious differences in the reactivity of different curing agents. One can find that MDI has the highest reactivity and the fastest curing rate. TDI and HDI-trimer have the moderate reactivity and curing reaction rate. The reactivity and curing reaction rate of IPDI and HMDI are relatively low. An interesting finding is that the curing reaction of the HTPB-HDI-trimer binder system accelerates in the late phase of the reaction.
In order to obtain the quantitative data of the curing reaction process, such as rheological reaction rate constant and the pot life, we take the logarithm of the viscosity value in Figure 1 and plot the curing time to obtain Figure 2. In this way, one can visually compare the reactivity of the various systems. As shown in Formula (3), the univariate linear regression is implemented to fit lnƞ to time t, and the slope of the straight line is the rheological reaction rate constant (kƞ) at a given temperature. Table 1 provides the rheological reaction rate constants, the related coefficients and pot lives of the various HTPB–isocyanate binder systems, respectively.

Figure 2.
The fit of lnƞ to the curing time (t) at 45 °C (binder systems with a single curing agent).

Table 1.
The rheological reaction rate constant and pot life of binder systems with a single curing agent at 45 °C.
The pot life of a binder system is a very important process parameter of the cast-cure product as the pot life matters a great deal to the fluidity, processibility and initial/late phase properties of the products. The pot life of a binder system is usually defined as the time that it takes to achieve a viscosity of 20,000 mPa·s [16]. The pot life of HTPB-MDI binder is 0.6 h, which may be too short for propellant and PBX casting [18]. The pot life of HTPB-TDI is 3.6 h, which is suitable for propellant and PBX casting. As for HTPB HDI-trimer/ IPDI binder systems, the pot lives are 8.1 h and 20.0 h.
Table 1 shows that the rheological reaction rate constant of MDI is 0.0423 min−1, which is approximately 20 times more than that of IPDI (0.0020 min−1) and 30 times more than that of HMDI (0.0014 min−1). The rheological reaction rate constants of TDI and HDI-trimer are 0.0051 min−1 and 0.0049 min−1, respectively. In Figure 1 and Table 1, the various HTPB–isocyanate binder systems rank by reactivity (MDI > TDI > HDI-trimer > IPDI > HMDI). Bina [19] conducted a test of viscosity buildup in HTPB, TDI, IPDI and HMDI at a curing temperature of 45 °C, and the findings were proved to be in line with the conclusion of these experiments.
It is worth noting that as the curing reaction progresses, the different curing agent-binder systems show different viscosity growth trends, which can be basically divided into three types. As can be seen in Figure 2, the first type is that the viscosity builds up linearly with time, such as MDI and HMDI, the second type is that with the progress of the curing reaction, the curing reaction rate and viscosity buildup gradually decreases, such as TDI and IPDI, and the third type is that the viscosity of the binder system built up slowly in the initial phase and accelerated in the late phase, such as HDI-trimer. In the following paragraphs, we try to analyze the causes for these differences.
According to Aranguren [20], HTPB has a relatively high molecular mass, the hydroxyl groups differing in bonds vary very insignificantly in the reactivity, with an unremarkable effect on the curing reaction rate. Considering the identical curing temperature, R-value and preparation method, only the curing agents differ. As a consequence, the reaction rate varies primarily with the curing agents.
Generally, the aromatic isocyanates are more reactive than the aliphatic isocyanates. MDI, an aromatic isocyanate with two benzene rings, has a higher rheological reaction rate than TDI with one benzene ring. While the curing agent HDI-trimer has no benzene ring, its tri-functionality makes the three-dimensional network structure generate faster in the late phase of the curing reaction. Therefore, its reaction rate is slightly lower than that of TDI. As for IPDI and HMDI, they are less reactive cycloaliphatic isocyanates.
As indicated in Figure 2, MDI and HMDI have viscosity buildup curves which approximate to the fitted curve. The TDI and IPDI curing systems deviate downward to the straight lines, while HDI-trimer curing system deviates upward from the straight line. The MDI and HMDI systems have a good linear relationship in regard of curve fitting and have coefficients of correlation which approximate to 1. It is obvious that the MDI and HMDI systems undergo almost no change in the reaction rate in either the initial or late phase of the curing reactions. As shown by the structural formulas of MDI and HMDI in Figure 3, the isocyanate groups are symmetrically structured, with the same reactivity and steric hindrance and no remarkable change in the reaction rate between the initial and late phases [2,16].
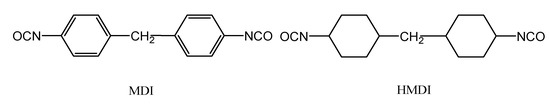
Figure 3.
The structural formulas of MDI and HMDI.
The curves of both TDI and IPDI tend to deviate to the lower right at a certain time point of the reaction. Two of the curing agents have different isomers and are asymmetrically structured. The industrial product of IPDI is a mixture of the cis-isomer and trans-isomer, with very complex actual structures. IPDI exists in the form of four isomers, as shown in Figure 4 [20]. The two isocyanate groups in IPDI which differ in the reactivity are the secondary isocyanate group connected to cyclohexane, and the primary isocyanate group connected to cyclohexane via the methylene group. In IPDI, the primary isocyanate group (NCOprim) is much less reactive than the secondary isocyanate group (NCOsec) as the primary isocyanate group is subject to the steric hindrance effect of cyclohexane and the α-substituent group. In this experiment, we observe a two-stage curing reaction mainly because the curing agent (IPDI) has two isocyanate groups which differ in position and reactivity. That explains why the curing reaction rate constant is faster in the initial phase than that in the late phase. This experimental phenomenon is consistent with the research of Lucio [7].

Figure 4.
The four isomers of IPDI.
Commercial TDI is a mixture of two isomers, 2,4-TDI and 2,6-TDI, at a proportion of 80% to 20% (refer to Figure 5). As -CH3 is an electron-donating group and there is a steric hindrance effect, the 2,4-isomer is more reactive than 2,6-isomer. Now that there is much more 2,4-TDI than 2,6-TDI and 2,4-TDI is more reactive, we give consideration mainly to the reaction of 2,4-TDI with HTPB for the purpose of explaining why the initial phase is faster than its later counterpart. As for 2,4-TDI, the 4-isocyanate group is more reactive than the 2-isocyanate group and participates earlier in the curing reaction. Due to the electron-withdrawing feature of the isocyanate group, upon the reaction of the 4-isocyanate group being completed, it no longer contributes to the reaction with the 2-isocyanate groups, whose reactivity therefore drops significantly, hence a fall in viscosity buildup rate. This mainly explains why the viscosity buildup of HTPB-TDI binder slowed down in the late phase of the curing reaction.
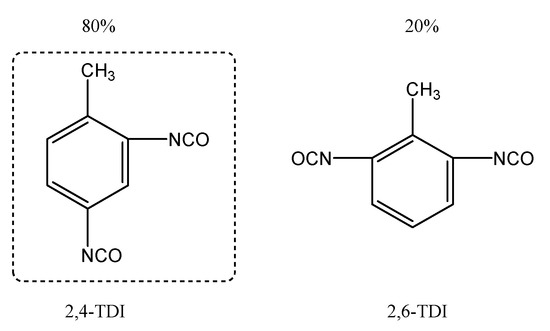
Figure 5.
The structural formula of TDI.
What attracts our attention most is the viscosity buildup during the curing process of HTPB-HDI-trimer binder. From Figure 1 and Figure 2, one can find that within the initial 480 to 600 min of the curing reaction, the curing reaction rate and the viscosity buildup of the HTPB-HDI-trimer binder are relatively slow. After that, the viscosity buildup of the binder shows an accelerated trend. This characteristic of mild reaction in the initial phase and accelerated viscosity growth in the late phase has important engineering practical significance in propellant and other fields. This phenomenon may be mainly attributable to the structure of HDI-trimer.
As a common homopolymer, HDI-trimer exists in the form of a medium-viscosity yellowish transparent liquid which can be prepared in a solvent-free trimer binder system (see Figure 6 for the structure) [21,22]. Compared with HDI-biuret, HDI-trimer has a lower viscosity, causing a low viscosity in the initial reaction and a long pot life. Structurally, HDI-trimer has three equivalent isocyanate groups which theoretically do not differ in reactivity. In view of this aspect, the viscosity buildup of HTPB-HDI-trimer binder should be similar to that of HTPB-MDI and HTPB-HMDI binders, which showed a linear growth relationship between the viscosity and the curing time. In reality however, when the curing reaction remains for 480~600 min, the viscosity buildup of the HTPB-HDI-trimer binder shows an accelerated trend. The cause may be that as the reaction goes on, the molecular chain of the binder system lengthens until the participation of only a small amount of HDI-trimer in the late-phase reaction quickens the growth of the three-dimensional PU network while increasing the viscosity, as shown in Figure 6 (the curing reaction process of the HTPB-HDI-trimer binder system).
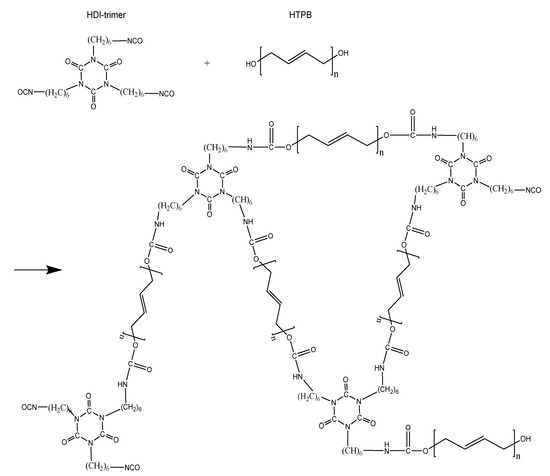
Figure 6.
The HTPB-HDI-trimer curing reaction.
4. Conclusions
In this study, the curing reaction of the HTPB-based binder system with different curing agents has been investigated via viscosity rheological testing. The rheological reaction rate constants and pot lives of different binder systems have been obtained.
The rheological reaction rate constant of HTPB-MDI is 0.0423 min−1, which is the highest of the five binders. On the contrary, the rheological reaction rate constant of HTPB-HMDI is the slowest, merely 0.0014 min−1. The rheological reaction rate constants of HTPB-TDI and HTPB-HDI-trimer are relatively moderate, which are 0.0051 min−1 and 0.0049 min−1, respectively. With regard to the curing reaction rate of the binder systems, MDI ranks highest, followed by TDI, HDI-trimer, IPDI and HMDI. The pot life of a binder system is a vital parameter in industrial production. The pot life of HTPB-MDI binder is 0.6 h, which is too short. In that case, its application may be limited in some cases. Nonetheless, the pot lives of HTPB-IPDI and HTPB-HMDI are too long, which necessitates the addition of a curing catalyst before being put into actual utilization.
One interesting finding is that the HTPB-HDI-trimer binder shows a trend of mild viscosity buildup in the initial phase and an accelerated one in the late phase. This may be mainly attributed to the three equally active isocyanate groups that form a three-dimensional network structure in the late phase of the curing reaction. Such a structure results in a shorter final curing time, conducive to preventing solid phase settlement. As a result, this feature is no doubt significant in improving the performance of the resulting products in propellant manufacturing and other fields.
Author Contributions
Y.L. and T.C. conceived and designed the experiments; H.M., J.G. and L.Z. performed the experiments; Y.Y. and H.M. analyzed the data; Q.Z. contributed reagents/materials/analysis tools; H.M. and J.G. wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by NSAF (Grant No. U1330131). The authors wish to express their gratitude to Chengdu Technological University Start-up Project of Introducing Talents (Grant No. 2019RC010), Education Reform Project of Sichuan Province (Grant No. JG2018-1243), and Shenzhen Free Exploration Project for Basic Research of Science and Technology Program (Grant No. JCYJ20180306174135470).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations and Symbols
| HTPB | Hydroxyl-terminated polybutadiene |
| HDI-trimer | Trimer of hexamethylene-1,6-diisocyanate |
| HMDI | Hexamethylene diisocyanate |
| PU elastomer | Polyurethane elastomer |
| MDI | Methylene diphenyl diisocyanate |
| TDI | toluene diisocyanate |
| IPDI | Isophorone diisocyanate |
| R | Isocyanate index |
| nNCO | Molecular concentration of the hydroxyl group in the isocyanate |
| nOH | Molecular concentration of the hydroxyl group in HTPB |
| ƞ(t)T | System viscosity at time t at temp T |
| ƞ0 | Initial system viscosity at temp T |
| kƞ | Rheological reaction rate constant of the system |
| (t)T | Viscosity of the system at temperature T and at any given time t |
| 0(T) | Initial viscosity of the system at temperature T |
| t | Curing time |
| T | Curing temperature |
References
- Toosi, F.S.; Shahidzadeh, M.; Ramezanzadeh, B. An investigation of the effects of pre-polymer functionality on the curing behavior and mechanical properties of HTPB-based polyurethane. J. Ind. Eng. Chem. 2015, 24, 166–173. [Google Scholar] [CrossRef]
- Guo, J.H.; Chai, T.; Liu, Y.C.; Cui, J.L.; Ma, H.; Jing, S.M.; Zhong, L.C.; Qin, S.D.; Wang, G.D.; Ren, X. Kinetic research on the curing reaction of hydroxyl-terminated polybutadiene based polyurethane binder system via FT-IR measurements. Coatings 2018, 8, 175. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.H.; Liu, Y.C.; Chai, T. Synthesis and properties of a nano-silica modified environmentally friendly polyurethane adhesive. RSC. Adv. 2015, 5, 44990–44997. [Google Scholar]
- Kou, Y.J.; Zhou, W.Y.; Li, B.; Dong, L.N.; Duan, Y.E.; Hou, Q.W.; Liu, X.R.; Cai, H.W.; Chen, Q.G.; Dang, Z.M. Enhanced mechanical and dielectric properties of an epoxy resin modified with hydroxyl-terminated polybutadiene. Compos. Part A: Appl. Sci. Manuf. 2018, 114, 97–106. [Google Scholar] [CrossRef]
- Zhang, P.A.; Tan, W.J.Y.; Zhang, X.L.; Chen, J.; Yuan, J.M.; Deng, J.R. Chemical modification of hydroxyl-terminated polybutadiene and its application in composite propellants. Ind. Eng. Chem. Res. 2021, 60, 3819–3829. [Google Scholar] [CrossRef]
- Ganivada, M.N.; Dhara, M.; Jana, S.; Jana, T. Synthetic routes to modify hydroxyl terminated polybutadiene for various potential applications. J. Macromol. Sci. Part A 2022, 59, 167–179. [Google Scholar] [CrossRef]
- Lucio, B.; de la Fuente, J.L. Rheokinetic analysis on the formation of metallo-polyurethanes based on hydroxyl-terminated polybutadiene. Eur. Polym. J. 2014, 50, 117–126. [Google Scholar] [CrossRef]
- Gong, Y.H.; Yang, P.F.; Tao, F.R.; Zhang, D.T.; Guo, X.F.; Li, T.D. Kinetic and thermodynamic studies of the urethane reaction based on 1, 3-diazetidine-2, 4-dione and 4-(Tetrahydro-Pyran-2-yloxy)-butan-1-ol. J. Int. J. Polymanal. Ch. 2014, 19, 107–114. [Google Scholar] [CrossRef]
- Rao, B.N.; Malkappa, K.; Kumar, N.; Jana, T. Ferrocene grafted hydroxyl terminated polybutadiene: A binder for propellant with improved burn rate. Polymer 2019, 163, 162–170. [Google Scholar] [CrossRef]
- Chen, S.H.; Tang, Y.; Yu, H.S.; Guan, X.Y.; DeLuca, L.T.; Zhang, W.; Shen, R.Q.; Ye, Y.H. Combustion enhancement of hydroxyl-terminated polybutadiene by doping multiwall carbon nanotubes. Carbon 2019, 144, 472–480. [Google Scholar] [CrossRef]
- Sikder, B.K.; Jana, T. Effect of solvent and functionality on the physical properties of hydroxyl-terminated polybutadiene (HTPB)-based polyurethane. ACS Omega 2018, 3, 3004–3013. [Google Scholar] [CrossRef] [PubMed]
- Sekkar, V.; Raunija, T.S.K. Issues related with pot life extension for hydroxyl-terminated polybutadiene-based solid propellant binder system. Prop. Expl. Pyro. 2015, 40, 267–274. [Google Scholar] [CrossRef]
- Zhang, Q.; Shu, Y.J.; Liu, N.; Lu, X.M.; Shu, Y.; Wang, X.C.; Mo, H.C.; Xu, M.H. Hydroxyl terminated polybutadiene: Chemical modification and application of these modifiers in propellants and explosives. Cent. Eur. J. Energetic Mater. 2019, 16, 153–193. [Google Scholar] [CrossRef]
- Yadav, N.; Srivastava, P.K.; Varma, M. Recent advances in catalytic combustion of AP-based composite solid propellants. Def. Technol. 2021, 17, 1013–1031. [Google Scholar] [CrossRef]
- Akram, N.; Zia, K.M.; Sattar, R.; Tabassum, S.; Saeed, M. Thermomechanical investigation of hydroxyl-terminated polybutadiene-based linear polyurethane elastomers. J. Appl. Polym. Sci. 2019, 136, 47289. [Google Scholar] [CrossRef]
- Ma, H.; Liu, Y.C.; Guo, J.H.; Chai, T.; Yu, Y.W.; Yuan, J.M.; Jing, S.M.; Feng, F.W.; Zhong, L.C.; Zhou, Y.M.; et al. Catalyzed HTPB/HDI-trimer curing reactions and influence on pot life. Coatings 2020, 10, 1073. [Google Scholar] [CrossRef]
- Kazachenko, A.S.; Akman, F.; Abdelmoulahi, H.; Issaoui, N.; Malyar, Y.N.; Al-Dossary, O.; Wojcik, M.J. Intermolecular hydrogen bonds interactions in water clusters of ammonium sulfamate: FTIR, X-ray diffraction, AIM, DFT, RDG, ELF, NBO analysis. J. Mol. Liq. 2021, 342, 117475. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.C.; Chai, T.; Ma, Z.L.; Jia, K.H. Curing reaction kinetics of the EHTPB-based PBX binder system and its mechanical properties. Coatings 2020, 10, 1266. [Google Scholar] [CrossRef]
- Bina, C.K.; Kannan, K.G.; Ninan, K.N. DSC study on the effect of isocyanates and catalysts on the HTPB cure reaction. J. Therm. Anal. Calorim. 2004, 78, 753–760. [Google Scholar] [CrossRef]
- Hailu, K.; Guthausen, G.; Becker, W.; Konig, A.; Bendfeld, A.; Geissler, E. In-situ characterization of the cure reaction of HTPB and IPDI by simultaneous NMR and IR measurements. Polym. Test. 2010, 29, 513–519. [Google Scholar] [CrossRef]
- Bae, J.H.; Won, J.C.; Lim, W.B.; Kim, B.J.; Lee, J.H.; Min, J.G.; Seo, M.J.; Mo, Y.H.; Huh, P. Tacky-free polyurethanes pressure-sensitive adhesives by molecular-weight and HDI trimer design. Materials 2021, 14, 2164. [Google Scholar] [CrossRef] [PubMed]
- Çakmakçi, E. HDI trimer based fluorine containing urethane methacrylates for hydrophobic photocured coatings. Polym. -Plast. Technol. Mater. 2019, 58, 854–865. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).