Abstract
The adhesive manufacturing industry needs more eco-sustainable processes. In this regard, the main road is to replace raw fossil materials with renewable resources or waste biomass, and simultaneously improve synthetic steps by using clean and greener reagents under mild conditions. In this paper, a synthetic pathway for producing biobased succinyl peroxide (SP) from waste biomass is reported, and then the application range of this polymerization agent to methacrylates and styrene-free resins is extended. At the same time, new formulations of pastes based on benzoyl or succinyl peroxide, displaying an almost complete biobased carbon content, are investigated and tested as cross-linking agents for mastic marble and unsaturated polyester resins. Physicochemical characterization of the final products and polymers is carried out with thermogravimetric analysis (TGA), differential scanning calorimetry (DSC), Fourier transform infrared (FT-IR), Gel Permeation Chromatography (GPC), Nuclear Magnetic Resonance (NMR) and peak exothermic curve analyses.
1. Introduction
Industrial adhesives are used to bond various substrates by means of adhesion and cohesion [1].
The various kinds of adhesive can be classified by chemical composition, including a wide range of compounds that perform the bonding work, such as polyurethanes, polyesters, cyanoacrylates, phenols, and epoxides [2]. Another means of classification is based on the application procedure, e.g., hot melt adhesives, instant adhesives, pressure-sensitive adhesives, thread lockers, structural adhesives, and others [3].
Further categorizations concern the physical form, such as liquid adhesives or highly viscous pastes [2], and finally reactive or non-reactive adhesives [4].
The industrial adhesives sector is still anchored to fossil sources and solvent-based chemistry but, as with all the other industries, needs eco-sustainable processes. Therefore, the development of new solventless and biobased adhesives, especially those of vegetable origin, is gaining increasing interest to reduce the environmental impact of these materials [4]. Studies have been carried out on the adhesive properties of Jatropha proteins [5,6]; moreover, as regards pressure-sensitive adhesives (PSA), new adhesives have been developed based on methyl esters of vegetable fatty acids [7,8,9,10] or epoxidized and hydroxylated soybean oil [10,11].
In the adhesives sector, there is also the field of mastics for marble and other surfaces; in particular, mastics are two-component systems consisting of a resin and a cross-linking agent that acts as hardener [12]. Due to their high reactivity, peroxides are commonly used as cross-linking agents, and resins have been specifically studied for different types of them. Among these, the study of marble adhesives is of industrial interest, especially because it is essential that curing occurs at room temperature and in a short time [12].
A well-known polymerizing and cross-linking agent is represented by succinyl peroxide (SP), an initiator already present on the market, and a useful hardener for some types of resins that find a large range of applications. For materials such as marble, a mastic based on unsaturated polyester resin dissolved in styrene is generally used, for which benzoyl peroxide is employed as a cross-linking agent. Generally, hardeners are used in the form of a paste or liquid to facilitate their dispersion into the resin [4].
Both the above-mentioned commercially available polymerizing agents are synthesized from (or combined with) chemical components arising from fossil sources. As an example, benzoyl peroxide is used as a paste containing chemical components such as dimethyl phthalate and ethylene glycol, which are of fossil origin.
Following our ongoing interest in green oxidation [13], and in developing green protocols obeying the Circular Economy principles [14,15], we decided to adopt the following three strategies: (i) planning a synthetic pathway for producing a succinyl peroxide of biobased origin by using a waste biomass such as soy husks, (ii) extending the application range of this peroxide to the polymerization of methacrylates [16], and (iii) formulating novel peroxide reactive pastes composed of biobased products (component B) for the preparation of a mastic for marble using unsaturated polyesters (component A). This paper is in line with the new requirements of the market, which are now based on renewable raw materials [4]. Using adhesives that contain a high quantity of renewable raw materials, it is possible to contribute to sustainability objectives, such as reducing the carbon footprint. Obviously, research must be oriented towards obtaining products that are at least equivalent to those of petrochemical origin.
2. Materials and Methods
2.1. Materials
Anhydrous benzoyl peroxide, Urea–H2O2 complex, dibasic esters (DBE, mixture of dimethyl glutarate, dimethyl succinate, and dimethyl adipate), (hydroxylpropyl)methylcellulose, acids, and bases were purchased from Aldrich, Milan, Italy and used without further purification. Reactions were conducted using dry glassware and anhydrous organic solvents. Commercial benzoyl peroxide paste (Luperox® ANS55 benzoyl peroxide paste, Arkema Milan, Italy) and vinyl versatate polymer (Versatate Craymul 2322, Arkema, pH 4.5, solid content 50%; viscosity 5000 mPa-s; minimum film forming temperature 5 °C) were supplied by ILPA Adesivi S.r.L. (Bari, Italy). The synthesis reaction of levulinic acid was carried out in a Nuova Fima EN837-1 autoclave. GC-MS analyses were run on a Shimadzu GLC 17-A instrument (Shimadzu, Milan, Italy) using a SLB-5MS Column (30 m × 0.25 mm id, 0.25 µm film thickness). Mass spectra were conducted in EI mode (70 eV). Gel permeation chromatography (GPC) analyses for the polymer synthesized were performed on a KNAUER S 2520 HPLC system, equipped with an ultraviolet detector (wavelength: 254 nm), using a column TOSOH Corporation (Tokyo, Japan) TSKgel G3000HHR, column size 7.8 mm I.D. × 30 cm, particle size 5 µm with exclusion limit (Polystyrene) equal to 6 × 104. FTIR analyses were conducted on a Perkin-Elmer UATR Two spectrophotometer (Achelit, Milan, Italy) equipped with a single-reflection diamond Attenuated total reflection (ATR) crystal (refractive index 2.4). Spectra were acquired by performing 32 scans in the range 4000–650 cm−1. The NMR spectra were acquired on a 500 MHz Bruker spectrometer (Bruker, Milan, Italy): 1H NMR spectra (500 MHz) refer to the residual isotope impurities of CDCl3 (7.25 ppm) and 13C-NMR spectra (125 MHz) refer to 77.00 ppm. Thermogravimetric and differential scanning calorimetry measurements were conducted on a TGA Q600 SDT (Perkin Elmer, Milan, Italy) instrument from TA Instruments. Finally, an ALC Centrifuge PK110 (Thermosùfosher, Milan, Italy) was used. During the activity regarding the preparation of the formulation, the laboratory was conditioned at 27 °C and a relative humidity of 40–60%.
2.2. Levulinic Acid Synthesis
The reaction was conducted following the literature procedure [17]. A 30 mL autoclave test tube, equipped with a magnetic stirrer, is charged with 1 g of soy husks, 20 mL of distilled water, and the cation exchange resin Amberlyst 15 as a heterogeneous catalyst (2.44 g). The system is conditioned at a temperature of 200 °C and kept under stirring for around 2 h. Then, the reaction is stopped by placing the autoclave in an ice bath for around 20 min. All the contents of the test tube are collected with distilled water and subjected to sedimentation by centrifugation at 4000 rpm for 20 min. Subsequently, supernatant is extracted with ethyl acetate (2 × 20 mL). The combined organic phases are dried over sodium sulfate and the solvent removed in vacuo to give levulinic acid as crude oil. The yield 0. 620 g (62% w/w of soy husks) was obtained with a calibration curve and levulinic acid was identified by comparison of the MS spectrum with that reported in the literature [18]. GC/MS (70 eV) m/z (relative intensity): 116 (M+, 3.06), 56 (29.58), 43 (100) (see Supplementary Materials for NMR characterization).
2.3. Succinic Acid Synthesis
A 50 mL 3-necked flask, equipped with a magnetic stirrer, is charged with 2 g of levulinic acid (17 mmol), 40 mL of trifluoroacetic acid, and with 8 mL of hydrogen peroxide 50% (added dropwise). Mixture is kept under stirring at room temperature and then in a sand bath at 90 °C for 3.5 h. Subsequently, two aliquots of 50% hydrogen peroxide (2 × 2 mL) are added at a distance of 20 min from each other. After 20 min, the solution is cooled to room temperature. Reaction product is extracted with ethyl acetate (3 × 20 mL) and the combined organic phases are washed with a 5% NaHCO3 aqueous solution (2 × 20 mL). Then, organic phase is dried, filtered, and the solvent is removed in vacuo. After the work-up of the reaction, the crude mixture showed only the presence of succinic acid as a white solid with an isolated yield of 57% (9.59 mmol, 1.14 g). Succinic acid was characterized by GC-MS and FTIR-ATR analyses, and 1H-NMR and 13C-NMR (see Supplementary Materials for NMR spectra).
All the spectra agreed with those reported in the literature [19]. FTIR-ATR (32 scans 4000–650 cm−1): a broad and intense band between 3550 and 2550 cm−1 due to the carboxylic O–H stretching, an intense peak at 1690 cm−1 due to the C=O carbonyl stretching. GC/MS (70 eV) m/z (relative intensity): 119.00 ([M + H+], 0.93), 100 (54.94), 74 (73.34), 55 (100), 45 (95.15).
The succinic acid was used as well as to prepare succinic anhydride using the literature procedure [20]. A 50 mL round-bottomed flask equipped with a magnetic stirrer was charged with succinic acid (2.0 g, 16.94 mmol). Acetic anhydride (25.0 mL) was added, the flask was fitted with a condenser, and the reaction mixture was heated at 80 °C for 2 h. The excess acetic anhydride was removed in vacuo to obtain 9 as a white solid (1.69 g, 16.9 mmol 100%). Both spectra and m.p. 117–121 were in agreement with the literature [20].
2.4. Synthesis of Succinyl Peroxide with Urea–H2O2 Complex (UHP)
A 50 mL flask, equipped with a magnetic stirrer and placed in a water bath, is charged with 14.11 g of Urea–Hydrogen Peroxide complex and 42.7 mL of methanesulfonic acid. [21] Mixture is stirred at room temperature for 5 min; then, 5 g (50 mmol) of succinic anhydride is added, and the system is left under stirring at room temperature for 24 h. Reaction is stopped by adding 150 mL of ethyl acetate together with 120 g of ice. The product is extracted with ethyl acetate (3 × 150 mL), and the combined organic phases are washed with a 5% NaHCO3 aqueous solution (2 × 80 mL) and 100 mL of brine. The resulting organic phase is dried, filtered, and the solvent is removed in vacuo, giving an isolated yield of 3.29 g 28% (1 mmol) of succinyl peroxide, which was characterized by in accordance with the literature [22]. FTIR-ATR analysis (32 scans 4000–650 cm−1): a broad band in the 3500–3000 cm−1 region attributable to carboxylic OH stretches, peaks of CH stretching in the range 3000–2850 cm−1, absorptions close to 1700 cm−1 characteristic of the acid carbonyl groups, and pairs of peaks at 1765 and 1740 cm−1 assigned to the carbonyl peroxides. The stability of peroxide was determined using iodometric methods [23,24]. In a typical procedure, in a 50 mL flask containing 3 mL of a solution of glacial acetic acid (Aldrich), acetone (3:2, V/V), 3 mL of saturated aqueous solution of KI (RPE-ACS, Aldrich), and 50 mg of peroxide paste are added quickly. The iodine developed by immediate and quantitative reaction between peroxide species and iodide ion in the acidic environment is titrated with a standard sodium thiosulphate solution (Na2S2O3), adding starch balance near the equivalence point.
2.5. Polymerization of Ethyl Methacrylate with Succinyl Peroxide
In a 200 mL beaker, 100 g of ethyl methacrylate and 0.5 g of succinyl peroxide are placed. The system is maintained at a temperature of 80 °C for 18 h. Polyethyl methacrylate obtained was characterized by FTIR-ATR, thermogravimetric analysis, differential scanning calorimetry (30 °C 2 min, to 600 °C, rate 5.0 °C/min, 600.0 °C hold for 2 min), and gel permeation chromatography (GPC). Tetrahydrofuran was used as the mobile phase at a flow rate of 1.0 mL min−1. Polystyrene standards were used for establishing the calibration curve. FTIR-ATR (32 scans 4000–650 cm−1): CH stretching peaks in the range 2850–3000 cm−1, intense peak at 1720 cm−1 typical of ester carbonyl (see Supplementary Materials for FTIR-ATR spectrum).
2.6. Formulation of Biobased Peroxide Paste
Formulation experiments were conducted on the scale of 10 g. In a typical procedure, a 100 mL beaker was charged with reagents according to the sequence and the percentage ranges listed in Section 3.3. Mixture was vigorously stirred with a spatula until reaching the appearance of a paste. The percentage of biobased was calculated using equations previously reported [25].
2.7. Preparation of Mastic for Marble
The mastic was prepared by mixing component A, composed of an unsaturated polyester resin solubilized in styrene 45% and silica 5% (w/w) provided by IPLA S.r.L., and component B, composed of 2% (w/w) of biobased benzoyl reactive paste. Mixture was stirred by means of a mechanic stirrer for 2 min (pot life) Next, it was analyzed by using a thermocouple to determine the curing time [26]. Same procedure was used for the succinyl peroxide paste, using as component A an unsaturated polyester resin solubilized in methacrylate and silica 45/5 w/w provided by ILPA S.r.L.
3. Results and Discussion
3.1. Improving Eco-Sustainability in the Synthesis of Biobased Succinyl Peroxide (SP)
To make the protocol of the preparation of succinyl peroxide more eco-friendly, both the first and the fourth steps of the synthetic strategy were revised and improved according to green chemistry standpoints as listed in Figure 1.
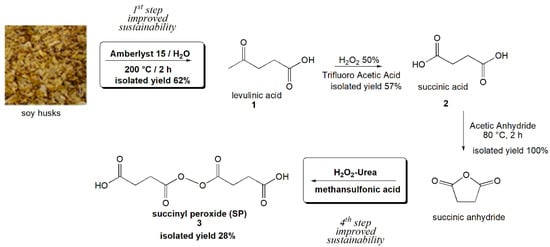
Figure 1.
The improved synthetic strategy for producing biobased succinyl peroxide.
In the first step, in line with Circular Economy principles, a waste ligno-cellulosic biomass such as soy husks was used as a source of monosaccharides for preparing the first synthetic intermediate, which is levulinic acid. To improve the eco-sustainability of this reaction, thermal hydrolysis was conducted with Amberlyst 15 [17], a solid cation exchange resin bearing sulfonic groups that can act as a heterogeneous acid catalyst in place of the toxic and aggressive strong mineral acids H2SO4, HCl [18]. Besides limiting the corrosion of the plants, the use of the Amberlyst 15 would allow the easy recycling of the acid catalyst. This step of the reaction proceeds through a series of acid–base equilibria that lead to a series of intermediates, among which 5-Hydroxymethylfurfural (5-HMF) is the most stable. The presence of free H+ ions pushes HMF to open and rearrange itself into levulinic acid (1) [18]. Levulinic acid (1) has been oxidized in succinic acid (2), using trifluoroacetic acid, to avoid the by-products due to the Baeyer–Villiger reaction; this procedure also allows the recovery of the acid catalyst [19]. After the preparation of succinic anhydride (3), in the fourth step of the peroxidation reaction, a commercially available H2O2–Urea complex (UHP) was preferred to the concentrated H2O2 alone as a peroxidizing agent [21]. This complex appears as a white solid and results as a less hazardous and safe-to-handle reagent, representing an alternative to hydrogen peroxide as it is [22], or at oxidation obtained in organocatalysis as, for example, of dioxirans [24]. Thanks to this approach, we obtained the linear succinyl peroxide, with high yields and selectivity [22,25].
The formation of the succinyl peroxide has been verified by the comparison of FT-IR-ATR spectra reported in Figure 2, which showed a signal at 1700 cm−1 characteristic of the acid carbonyl groups and pairs of peaks at 1765 and 1740 cm−1 assigned to the carbonyl peroxides, while the disappearance of the signal close to 1750 cm−1 was assigned to the carbonyl of anhydride.
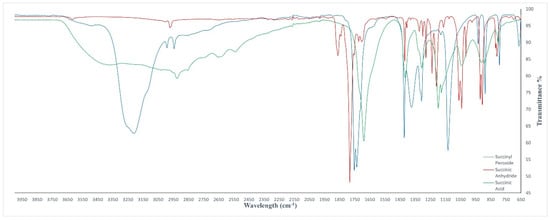
Figure 2.
FT-IR ATR spectra of succinic acid (green line), succinic anhydride (red line) and succinyl anhydride (blue line).
Finally, the synthesized biobased succinyl peroxide was tested as a polymerizing agent of ethyl methacrylate.
3.2. Succinyl Peroxide as Polymerizing Agent of Ethyl Methacrylate
As a further goal of the present work, investigations were conducted to extend the field of applications of succinyl peroxide to the polymerization of methacrylates, the well-known class of reactive vinyl monomers, for which this peroxide has been little used until now [16]. Ethyl methacrylate was chosen as a model monomer, due to the interesting properties of poly(ethyl methacrylate) (PEMA) as a component of bone cement and dentures, and the advanced properties shown with respect to those of the most famous poly(methyl methacrylate) (PMMA), from both a physiochemical and biological point of view [27].
The polymerization reaction was accomplished on 100 g of monomer with 0.5 g of SP as an initiator and with the heating of the reaction mixture at 80 °C, to allow the breakdown of the peroxidic bond and the formation of radicals that would initiate the reaction (see Section 2.5). The synthesized PEMA was subjected to some physicochemical investigations to ascertain the polymerization degree and thermal behavior and compare such parameters with those reported in the literature for analogous initiators.
The weight average molecular weight (Mw), number average molecular weight (Mn), and the polydispersity index (Ð) of PEMA were determined by gel permeation chromatography analyses, with values that proved to be very similar to those obtained with other polymerizing agents [28]. The molecular weights obtained were 20,818 (g mol−1) Mw, 17,781 (g mol−1) Mn, and D corresponding to 1.17.
Thermal behavior, evaluated by TGA-DSC profiles, showed that PEMA is an amorphous thermoplastic polymer, (Figure 3). Two glass transition temperatures (Tg) were identified from the DSC profile corresponding to the atactic (a) and syndiotactic (s) polymer (Figure 3B). Tg (a) and Tg (s) were 43 and 137 °C, respectively, in agreement with literature values of 51 and 120 °C [29]. Variation in the glass transition temperature can be ascribed to different polymerization degrees (PD). Furthermore, a melting peak is evident in both DSC and DTG profiles; the peak is centered at the melting temperature (Tm) of ~288 °C and the fusion enthalpy ΔHf was found to be 19 J/g. Finally, a decomposition temperature (Td) was detected at around 371 °C, corresponding to the onset of the final decay in the TGA profile.
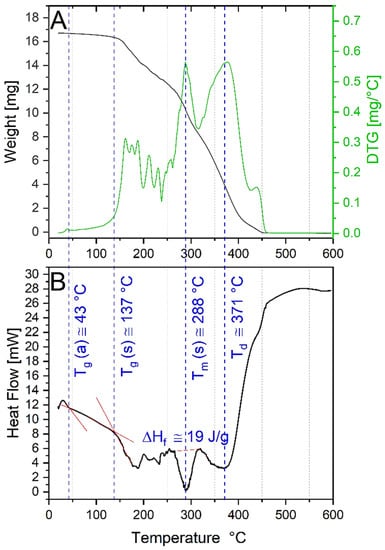
Figure 3.
(A) Thermogravimetric analysis (TGA) curves together with derivative thermogravimetric (DTG) profiles, and (B) differential scanning calorimetry (DSC) of the PEMA synthetized by using the biobased succinyl peroxide as a polymeric agent. Blue dotted lines represent the glass transition temperatures (Tg), melting temperature (Tm), and decomposition temperature (Td).
3.3. Formulation of a Biobased Benzoyl Peroxide Paste
Benzoyl peroxide is commonly used as a cross-linking agent for unsaturated polyester resins. Mastic for marble is realized by mixing such polyester resins with a commercially available paste containing benzoyl peroxide, dimethyl phthalate, and ethylene glycol as the main components. The composition is carefully studied to ensure that the paste holds the needed viscosity and is suitable to be modeled with a spatula. Recently, biobased formulations were studied for reactive plasticizers and sealants derived from plant oils, functionalized with alkoxy silane groups [4]. Following this idea, a novel formulation was studied to obtain a paste possessing the same physicochemical properties of the commercially analogous product but with a carbon content suitable for certification as a “biobased” product.
Preliminary tests were carried out on epoxidized soybean oil and methyl oleate as components of a biobased formulation for the peroxide paste.
The best compositions obtained are reported in Table 1 and Table 2. However, even though the two formulations displayed sufficient viscosity to hold up on a spatula, they proved to be not homogeneously dispersed, and within 0.5 h, the organic phase began to separate from the aqueous one.

Table 1.
Formulation of epoxidized soybean oil paste as cross-linking agent for unsaturated polyester resins.

Table 2.
Formulation of methyl oleate paste as cross-linking agent for unsaturated polyester resins.
Due to the instability of these formulations, other organic dispersing phases were investigated.
To this end, a naturally derived commercial mixture of methyl esters of succinic acid (20%), glutaric acid (60%), and adipic acid (20%), namely dibasic esters (DBE, Aldrich), was chosen as the organic phase in which benzoyl peroxide was to be dissolved. Usually, DBE are used as a co-solvent for ternary system solvents [30], and as stabilizers for coating resins [31].
This mixture of non-flammable, readily biodegradable, and non-corrosive esters provided the suitable solvent properties to the paste. As a thickener for distilled water, (hydroxypropyl)methylcellulose was used, of obvious natural origin.
Vinyl versatate was used as the coupling agent, which is the same component of fossil origin used in commercial pulp, as a viable biobased alternative could not be found. Fortunately, it was used in a very low percentage within the formulation.
At first, the original commercial paste based on benzoyl peroxide was re-formulated, replacing dimethyl phthalate and ethylene glycol with the above-mentioned additives by investigating the concentration range shown in Table 3 (second column) according to suggestions by ILPA Adesivi.

Table 3.
Formulation of biobased paste as cross-linking agent for unsaturated polyester resins a.
The optimized formulation furnished a paste displaying good cross-linking and hardening properties towards the unsaturated styrene-based polyester resins, with performance, as regards surface drying times, comparable to that of commercial products.
Despite having a viscosity of 109.39 Pa∙s, calculated at a shear rate of 0.631 s−1, which was lower than that of a commercial paste (208.86 Pa∙s), the new formulation proved to be easy to handle, being viscous enough to hold up on a spatula.
Furthermore, the difference in the benzoyl peroxide dispersion technique, compared to the commercial one, justifies both the difference in viscosity and the difference in the appearance of the paste.
The new paste based on benzoyl peroxide contains 28% of biobased carbon, which exceeds the threshold of 20% that is necessary for certification as a biobased product (DIN CERTCO) [32]. To further increase the biobased carbon content of this material, a new formulation was studied by replacing benzoyl peroxide with succinyl peroxide, the latter being obtained from a waste biomass in a very easy way, as demonstrated (see Section 3.1).
Due to the very similar molecular weights of the two peroxides, the same mass percentages were maintained in the novel formulation (last column of Table 3). The new hardener paste, which possesses 96.3% of biobased carbon content, despite displaying a high value of viscosity of 922.86 Pa∙s (calculated at a shear rate of 0.631 s−1), proved suitable for the requested application of being modeled by a spatula.
In addition, both the new biobased formulations listed in Table 3 showed the same hardener properties as the commercial paste; hence, they have potential applications as a new biobased paste for mastics of marble.
For each formulation paste, we conducted the performance evaluation of the final cured product obtained using a peak exothermic curve (Figure 4). The highest temperature was measured as the “peak exothermic time”, as the elapsed time from the start of mixing and corresponding to cure time [26].
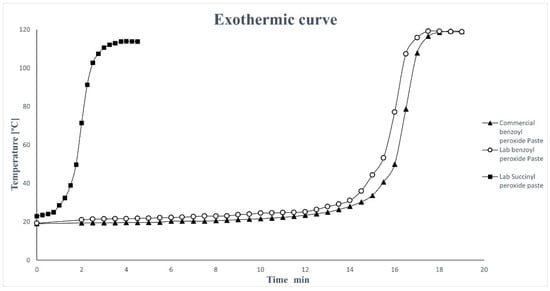
Figure 4.
Peak exothermic curve for the biobased formulation compared with commercial benzoyl peroxide paste.
Comparing the graphics of the biobased paste with benzoyl peroxide, it is evident that the curing time is very similar to that obtained from the commercial product, while it is evident that the paste with the succinyl peroxide is much more reactive and therefore the curing time is considerably reduced.
This drawback is largely balanced by the prolonged stability shown by SP in the paste compared to when it is used in pure form. In fact, a water solution of pure succinyl peroxide having a water content similar to that of the biobased formulation (88/11 w/w) undergoes, in 3 h at 25 °C, a reduction in peroxide titer of 60% [24]. In contrast, succinyl peroxide in paste shows high stability at room temperature for several days, enabling its potential industrial applications.
4. Conclusions
In this work, a commercial radical initiator such as succinyl peroxide has been synthesized starting from an agricultural waste such as soy husks. The synthetic pathway has been modified to improve its sustainability and the biobased peroxide has been tested as a polymerizing agent of ethyl methacrylate to extend its range of applications.
In addition, an innovative and biobased formulation for benzoyl peroxide paste was realized, which is used as a hardener for unsaturated polyester resins dissolved in styrene. Moreover, an almost completely biobased formulation was achieved by substituting the fossil-based initiator with the biobased succinyl peroxide.
Comparison of the cross-linking agent properties of biobased pastes with those of commercial analogs showed similar behavior for benzoyl peroxide, while succinyl peroxide displayed a reduced curing time, a drawback that is counterbalanced by the increased stability of this peroxide when used in the paste.
In conclusion, we have shown that an innovative and more sustainable approach in the field of formulations represents a possibility also from an industrial point of view, thus paving the way towards achieving the objectives of economic and environmental sustainability as envisaged by the international community.
The possibility of using agricultural waste such as soy husks to produce a biobased initiator is one type of evidence that Circular Economy principles are always applicable, even with very reactive fine chemicals such as peroxides.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/coatings12030361/s1, Figure S1: 1H-NMR (500 MHz, in CDCl3) of levulinic acid, Figure S2: 13C-NMR (125 MHz, in CDCl3) of levulinic acid, Figure S3: 1H-NMR spectrum of succinic acid, Figure S4: 13C-NMR spectrum of succinic acid, Figure S5: FTIR-ATR spectrum of polymer of ethyl methacrylate.
Author Contributions
L.V., M.C. and P.C., investigation; A.M. and C.F., methodology; M.A., validation; L.G., conceptualization, data curation; L.D., conceptualization, writing—original draft preparation; L.G. and L.D., review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We acknowledge the owner of Ilpa Industry, Amedeo Borricelli, for making it possible to carry out this work and for allowing us to use its blue resin, which was donated to us.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ebnesajjad, S. Adhesives Technology Handbook, 2rd ed.; Publisher William Andrew Inc.: Norwich, NY, USA, 2008; pp. 1–387. [Google Scholar]
- Gierenz, G.; Karmann, W. Adhesives and Adhesive Tapes; John Wiley & Sons: Hoboken, NJ, USA, 2001. [Google Scholar] [CrossRef]
- Sanghvi, M.R.; Tambare, O.H.; More, A.P. Performance of various fillers in adhesives applications: A review. Polym. Bull. 2022. [Google Scholar] [CrossRef]
- Brandt, A.L.; Beck, H.; Klein, J.; Damke, J.-E.; Schimdt, S.; Kuz, A.; De vries, J.G.; Hinze, S.; Van Heck, R. Bio-Based Reactive Plasticizer and Adhesives and Sealants Containing Them. Patent WO 2019166224 A1, 6 September 2019. [Google Scholar]
- Lestari, D.; Mulder, W.J.; Sanders, J.P.M. Jatropha seed protein functional properties for technical applications. Biochem. Eng. J. 2011, 53, 297–304. [Google Scholar] [CrossRef]
- Hamarneh, A.I.; Heeres, H.J.; Broekhuis, A.A.; Picchioni, F. Extraction of Jatropha curcas proteins and application in polyketone-based wood adhesives. Int. J. Adhes. Adhes. 2010, 30, 615–625. [Google Scholar] [CrossRef]
- Bunker, S.P.; Wool, R.P. Synthesis and Characterization of Monomers and Polymers for Adhesives from Methyl Oleate. J. Polym. Sci. A Polym. Chem. 2002, 40, 451–458. [Google Scholar] [CrossRef]
- Maassen, W.; Meier, M.A.R.; Willenbacher, N. Unique adhesive properties of pressure sensitive adhesives from plant oils. Int. J. Adhes. Adhes. 2016, 64, 65–71. [Google Scholar] [CrossRef]
- Lia, A.; Li, K. Pressure-Sensitive Adhesives Based on Tung Oil. RSC Adv. 2015, 5, 85264–85271. [Google Scholar] [CrossRef]
- Ahn, B.K.; Kraft, S.; Wang, D.; Sun, X.S. Thermally Stable, Transparent, Pressure-Sensitive Adhesives from Epoxidized and Dihydroxyl Soybean Oil. Biomacromolecules 2011, 12, 1839–1843. [Google Scholar] [CrossRef]
- Wool, R.P.; Bunker, S.P. Pressure Sensitive Adhesives from Plant Oils. U.S. Patent 20020188056A1, 12 December 2002. [Google Scholar]
- Guangyi, S.; Xia, J. Marble Glue Composition and Preparation Method Thereof. Patent CN111040705 (A), 21 April 2020. [Google Scholar]
- Annese, C.; D’Accolti, L.; Fusco, C.; Licini, G.; Zonta, C. Heterolytic (2 e) vs Homolytic (1 e) Oxidation Reactivity: N−H versus C−H Switch in the Oxidation of Lactams by Dioxirans. Chem. Eur. J. 2017, 23, 259–262. [Google Scholar] [CrossRef]
- Tarzia, A.; Montanaro, J.; Casiello, M.; Annese, C.; Nacci, A.; Maffezzoli, A. Synthesis, curing and properties of an epoxy resin derived from gallic acid. BioResource 2018, 13, 632–645. [Google Scholar] [CrossRef] [Green Version]
- Rizzi, V.; Fiorini, F.; Lamanna, G.; Gubitosa, J.; Prasetyanto, E.A.; Fini, P.; Fanelli, F.; Nacci, A.; De Cola, L.; Cosma, P. Polyamidoamine-Based Hydrogel for Removal of Blue and Red Dyes from Wastewater. Adv. Sustain. Syst. 2018, 2, 1700146. [Google Scholar] [CrossRef]
- Crawford, J.W.C. Production of Polymerized Unsaturated Compounds. U.S. Patent 2097586, 25 February 1937. [Google Scholar]
- Pileidis, F.D.; Titirici, M.M. Levulinic Acid Biorefineries: New Challenges for Efficient Utilization of Biomass. ChemSusChem 2016, 9, 562–582. [Google Scholar] [CrossRef] [PubMed]
- Laurenza, G.A.; Losito, O.; Casiello, M.; Fusco, C.; Nacci, A.; Pantone, V.; D’Accolti, L. Valorization of cigarette butts for top value-added chemicals: Levulinic Acid. Sci. Rep. 2021, 11, 15775. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Wu, L.; Mascal, M. Efficient, Metal-Free Production of Succinic Acid by Oxidation of biomass-derived Levulinic Acid with Hydrogen Peroxide. Green Chem. 2015, 17, 2335–2338. [Google Scholar] [CrossRef]
- Manoni, F.; Cornaggia, C.; Murray, J.; Tallon, S.; Connon, S.J. Catalytic, enantio- and diastereoselective synthesis of c-butyrolactones incorporating quaternary stereocentres. ChemComm 2012, 48, 6502–6504. [Google Scholar] [CrossRef]
- Pilevar, A.; Hosseini, A.; Becker, J.; Schreiner, P.R. Syn-Dihidroxylation of Alkenes Using a Sterically Demanding Cyclic Diacyl Peroxide. J. Org. Chem. 2019, 84, 12377–12386. [Google Scholar] [CrossRef]
- Peng, H.; Alemany, L.B.; Margrave, J.L.; Khabashesku, V.N. Sidewall Carboxylic Acid Functionalization of Single-Walled Carbon Nanotubes. J. Am. Chem. Soc. 2003, 125, 15174–15182. [Google Scholar] [CrossRef]
- Annese, C.; D’Accolti, L.; Filardi, R.; Tommasi, I.; Fusco, C. Oxidative cleavage of lactams in water using dioxiranes: An expedient and environmentally safe route to ω-nitro acids. TeLe 2013, 54, 515–517. [Google Scholar] [CrossRef]
- Brocklehurst, P.; Sheffield, C.N.; Ferguson, A.S. Stable Peroxide Composition and Method of preparation Thereof. U.S. Patent 4402853, 6 September 1983. [Google Scholar]
- Pantone, V.; Laurenza, A.G.; Annese, C.; Comparelli, R.; Fracassi, F.; Fini, P.; Nacci, A.; Russo, A.; Fusco, C.; D’Accolti, L. Preparation and Characterization of Soybean Oil-Based Polyurethanes for Digital Doming Applications. Materials 2017, 10, 848. [Google Scholar] [CrossRef]
- ASTM D2471-99 Standard Test Method for Gel Time and Peak Exothermic Temperature of Reacting Thermosetting Resins. Available online: https://www.mystandards.biz/standard/astm-d2471-99-10.11.1999.html (accessed on 25 January 2022).
- Harper, E.J.; Behiri, J.C.; Bonfield, W. Flexural and fatigue properties of a bone cement based upon polyethylmethacrylate and hydroxyapatite. J. Mater. Sci. Mater. Med. 1995, 6, 799–803. [Google Scholar] [CrossRef]
- Meena, M.; Nanjundan, S.; Umapathy, M.J. Free Radical Polymerization of Methyl and Ethyl Methacrylates by Green Methodology. Int. J. Appl. Eng. Res. 2016, 11, 2177–2184. [Google Scholar] [CrossRef]
- Karasz, F.E.; MacKnight, W.J. The Influence of Stereoregularity on the Glass Transition Temperatures of Vinyl Polymers. Macromolecules 1968, 1, 537–540. [Google Scholar] [CrossRef]
- Uusi-Penttilä, M.; Richards, R.J.; Blowers, P.; Torgerson, B.A.; Berglund, K.A. Liquid−Liquid Equilibria of Selected Dibasic Ester + Water + Solvent Ternary Systems. J. Chem. Eng. Data 1996, 41, 235–238. [Google Scholar] [CrossRef]
- Kamath, V.R.; Sargent, J.D. Polymer Bound Light Stabilizer Coating Resins. U.S. Patent US4927891, 22 May 1990. [Google Scholar]
- Certif TÜV Rheinland®- DIN CERTCO, Certification Scheme Biobased Products. Available online: https://www.dincertco.de/din-certco/en/main-navigation/products-and-services/certification-of-products/packaging/biobased-products/ (accessed on 25 January 2022).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).