YSZ/LSM Composite Cathode Deposited by Solution Precursor Plasma Spraying
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Characterization of YSZ/LSM Composite Cathode
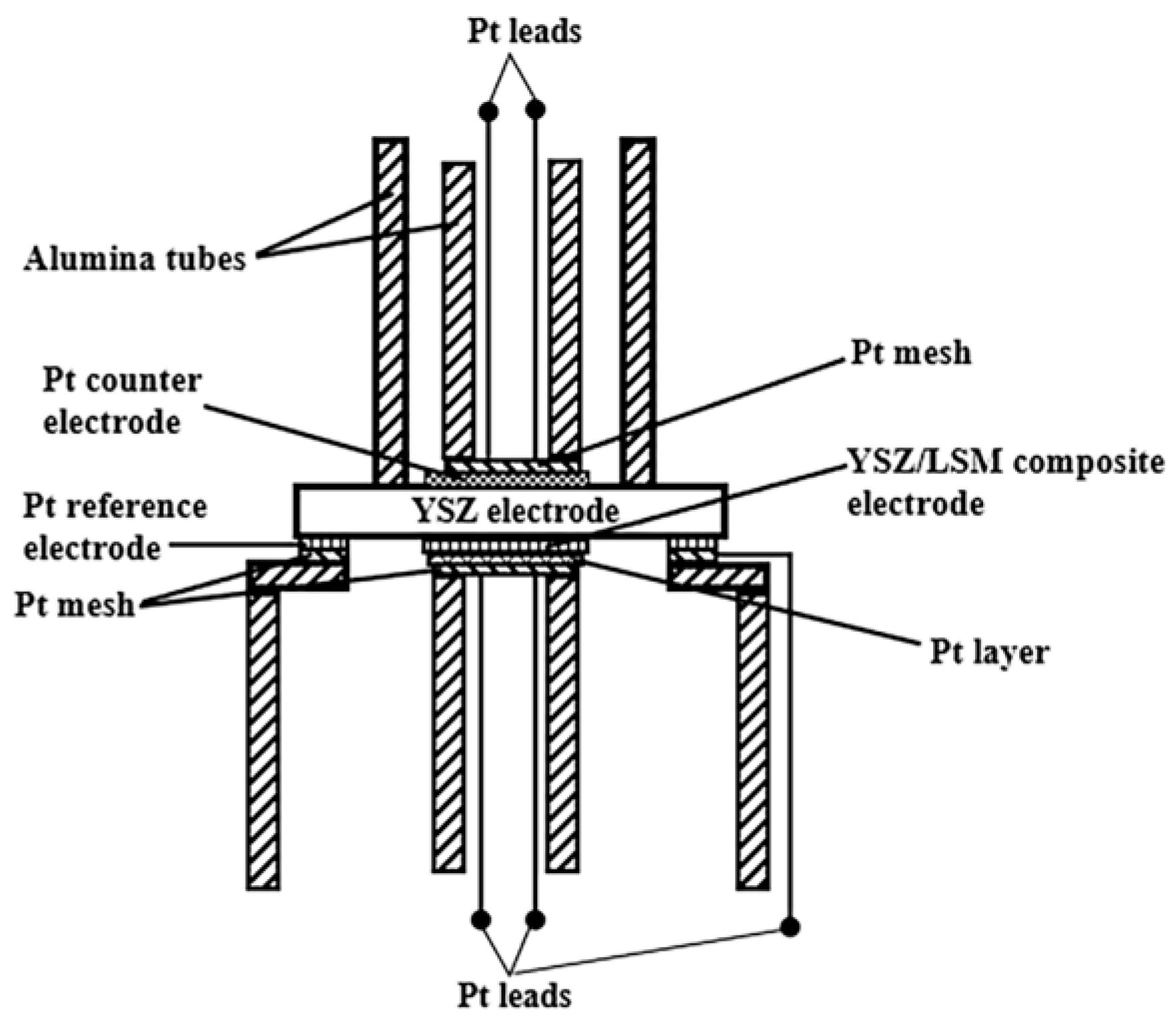
2.3. Characterization of Polarization Properties of Composite Cathode
3. Results and Discussion
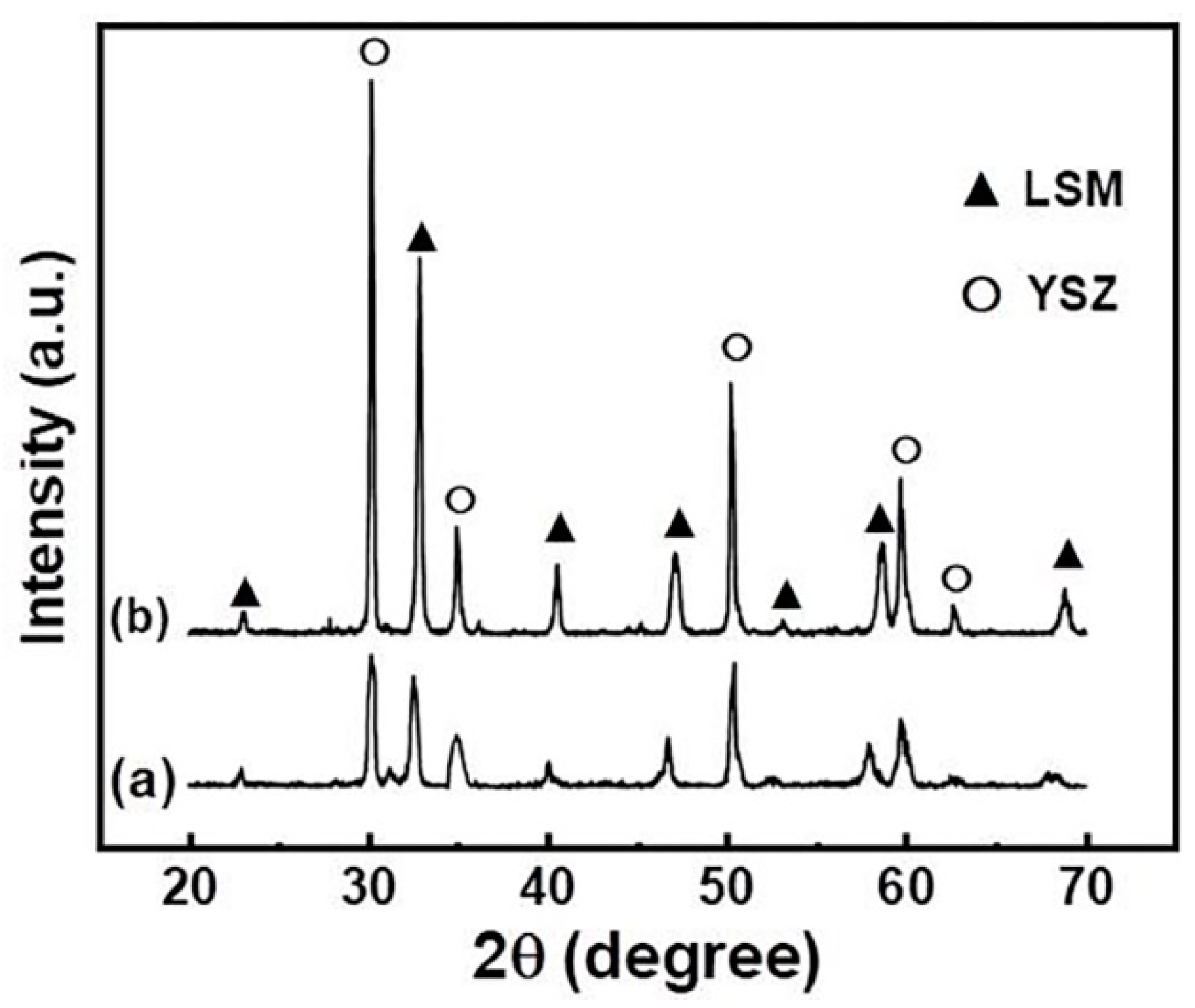
3.1. Phase Structure of YSZ/LSM Deposits
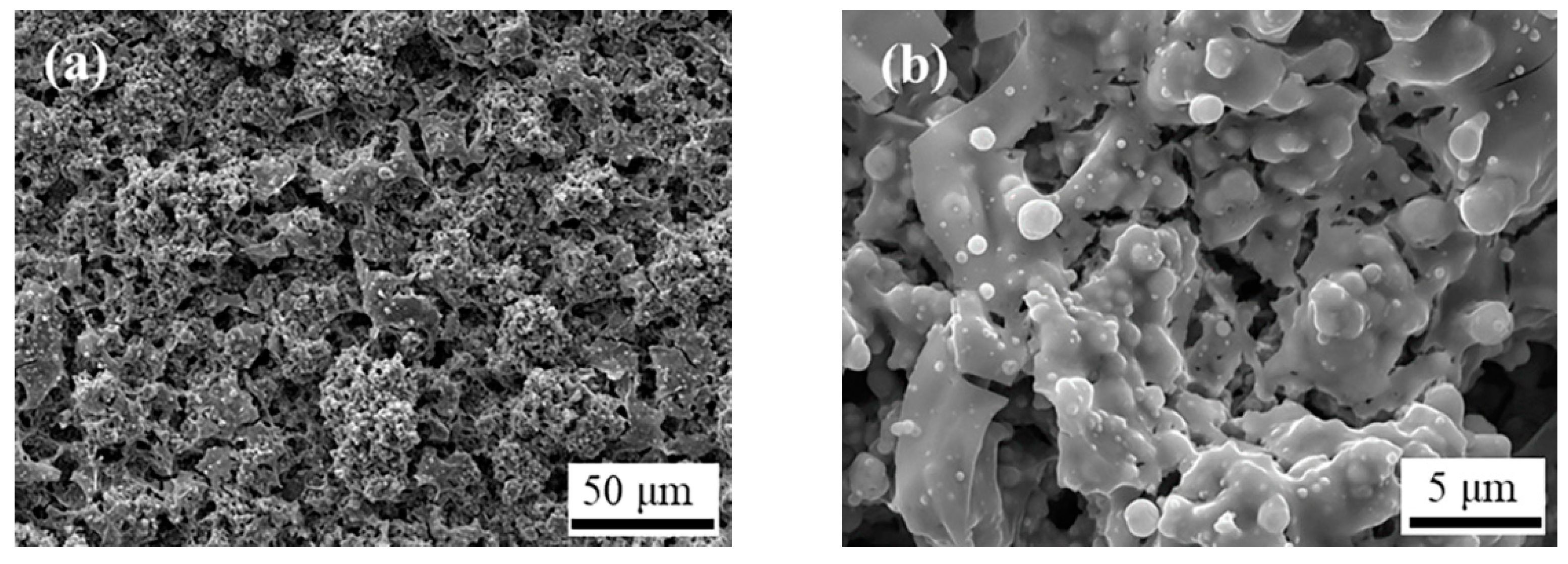
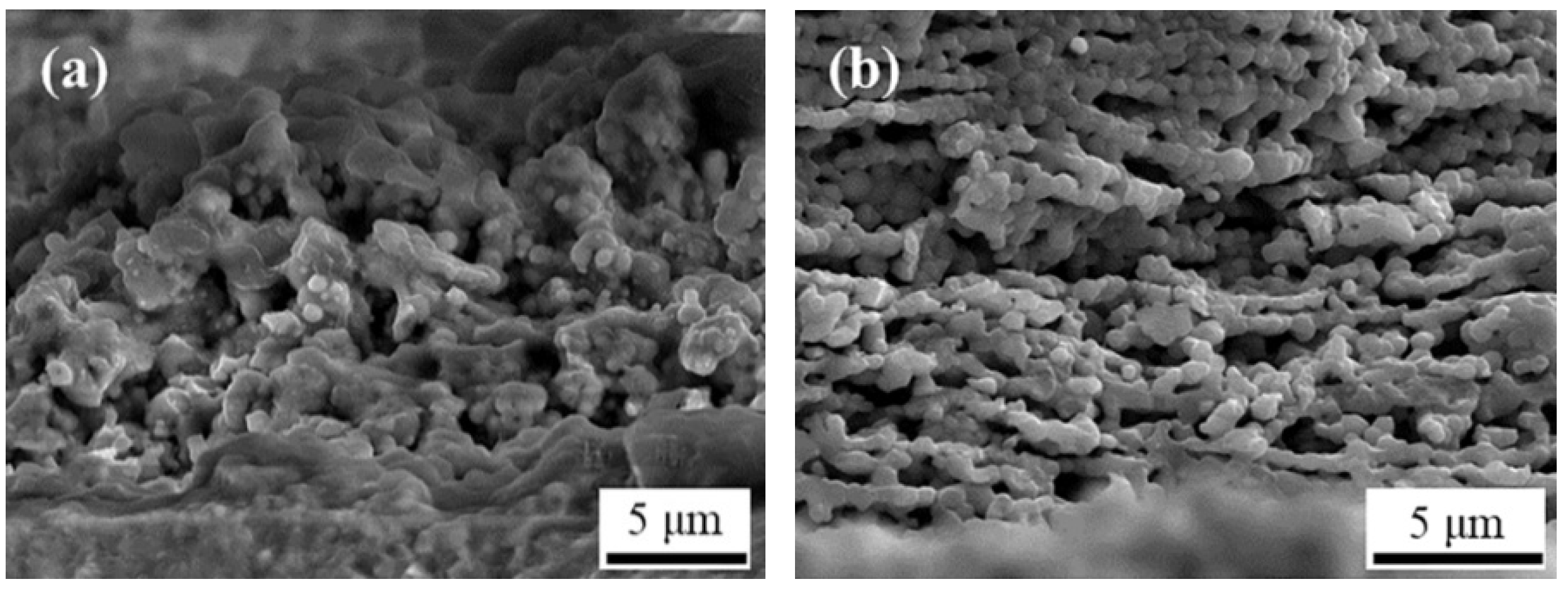
3.2. Microstructure of YSZ/LSM Prepared by SPPS
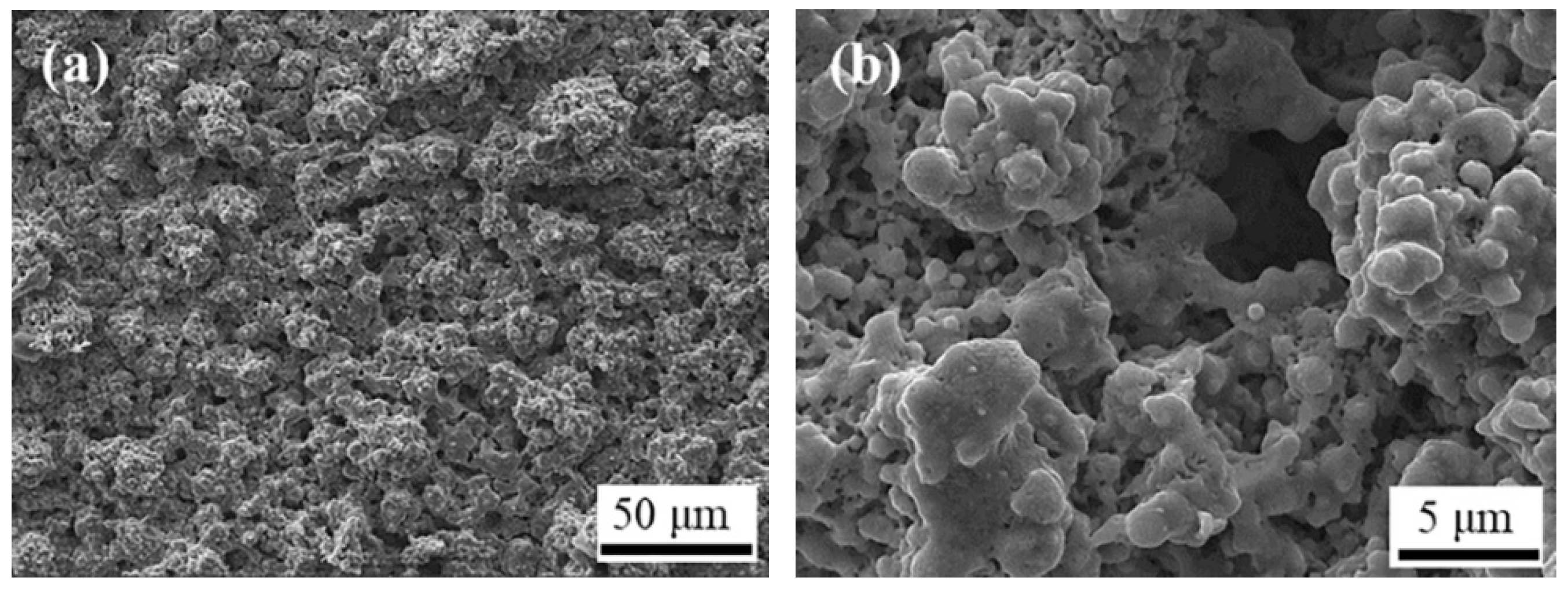
3.3. Effect of Annealing Treatment on the Microstructure of YSZ/LSM Composite Cathode
3.4. The Electrochemical Performance of YSZ/LSM Composite Cathode
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xie, J.; Hao, W.; Wang, F. The analysis of interfacial thermal stresses of solid oxide fuel cell applied for submarine power. Int. J. Energy Res. 2018, 42, 2010–2020. [Google Scholar] [CrossRef]
- Boldrin, P.; Brandon, N.P. Progress and outlook for solid oxide fuel cells for transportation applications. Nat. Catal. 2019, 2, 571–577. [Google Scholar] [CrossRef] [Green Version]
- Waters, D.F.; Pratt, L.M.; Cadou, C.P. Gas Turbine/Solid Oxide Fuel Cell Hybrids for Aircraft Propulsion and Power. J. Propuls. Power 2021, 37, 349–361. [Google Scholar] [CrossRef]
- Mozdzierz, M.; Chalusiak, M.; Kimijima, S.; Szmyd, J.S.; Brus, G. An afterburner-powered methane/steam reformer for a solid oxide fuel cells application. Heat Mass Transf. 2018, 54, 2331–2341. [Google Scholar] [CrossRef] [Green Version]
- Singh, M.; Zappa, D.; Comini, E. Solid oxide fuel cell: Decade of progress, future perspectives and challenges. Int. J. Hydrogen Energy 2021, 46, 27643–27674. [Google Scholar] [CrossRef]
- Du, Y.; Finnerty, C.; Jiang, J. Thermal stability of portable microtubular SOFCs and stacks. J. Electrochem. Soc. 2008, 155, B972–B977. [Google Scholar] [CrossRef]
- Alenazey, F.; Alyousef, Y.; AlOtaibi, B.; Almutairi, G.; Minakshi, M.; Cheng, C.K.; Vo, D.V.N. Degradation behaviors of solid oxide fuel cell stacks in steady-state and cycling conditions. Energy Fuels 2020, 34, 14864–14873. [Google Scholar] [CrossRef]
- Lyu, Y.; Xie, J.; Wang, D.; Wang, J. Review of cell performance in solid oxide fuel cells. J. Mater. Sci. 2020, 55, 7184–7207. [Google Scholar] [CrossRef]
- Istomin, S.Y.; Lyskov, N.V.; Mazo, G.N.; Antipov, E.V. Electrode materials based on complex d-metal oxides for symmetrical solid oxide fuel cells. Russ. Chem. Rev. 2021, 90, 644–676. [Google Scholar] [CrossRef]
- Dwivedi, S. Solid oxide fuel cell: Materials for anode, cathode and electrolyte. Int. J. Hydrogen Energy 2020, 45, 23988–24013. [Google Scholar] [CrossRef]
- Sažinas, R.; Andersen, K.B.; Simonsen, S.B.; Holtappels, P.; Hansen, K.K. Silver modified cathodes for solid oxide fuel cells. J. Electrochem. Soc. 2019, 166, F79–F88. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.L.; Zhang, A.P.; Li, C.X.; Yang, G.J.; Li, C.J. Suspension Plasma Sprayed Sr2Fe1.4Mo0.6O6−δ Electrodes for Solid Oxide Fuel Cells. J. Therm. Spray Technol. 2017, 26, 432–440. [Google Scholar] [CrossRef]
- Meepho, M.; Wattanasiriwech, S.; Angkavatana, P.; Wattanasiriwech, D. Application of 8YSZ nanopowder synthesized by the modified solvothermal process for anode supported solid oxide fuel cells. J. Nanosci. Nanotechnol. 2015, 15, 2570–2574. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.L.; Huang, J.Y.; Li, C.X.; Yang, G.J.; Li, C.J. Relationship Between Designed Three-Dimensional YSZ Electrolyte Surface Area and Performance of Solution-Precursor Plasma-Sprayed La0.8Sr0.2MnO3−δ Cathodes. J. Therm. Spray Technol. 2016, 25, 1692–1699. [Google Scholar] [CrossRef]
- Jeon, O.S.; Park, M.G.; Song, R.H.; Ryu, K.H.; Na, C.W.; Shul, Y.G.; Lee, J.G. Effects of Fe2O3 doping on structural and electrical properties of 8 mol% yttria-stabilized zirconia electrolyte for solid oxide fuel cells. J. Mater. Sci. Mater. Electron. 2022, 33, 3208–3214. [Google Scholar] [CrossRef]
- Khan, M.; Zeng, Y.; Hu, N.; Lan, Z.; Wang, Y. Optimizing the structure and properties of Y2O3 stabilized zirconia: An atmospheric plasma spray (APS) and solution precursor plasma spray (SPPS) based comparative study. Ceram. Int. 2018, 44, 18135–18142. [Google Scholar] [CrossRef]
- Tahir, N.N.M.; Baharuddin, N.A.; Samat, A.A.; Osman, N.; Somalu, M.R. A review on cathode materials for conventional and proton-conducting solid oxide fuel cells. J. Alloys Compd. 2022, 894, 162458. [Google Scholar] [CrossRef]
- Shi, H.; Su, C.; Ran, R.; Cao, J.; Shao, Z. Electrolyte materials for intermediate-temperature solid oxide fuel cells. Prog. Nat. Sci. Mater. 2020, 30, 764–774. [Google Scholar] [CrossRef]
- Hussain, S.; Li, Y.P. Review of solid oxide fuel cell materials: Cathode, anode, and electrolyte. Energy Trans. 2020, 4, 113–126. [Google Scholar] [CrossRef]
- Rafique, M.; Nawaz, H.; Shahid Rafique, M.; Bilal Tahir, M.; Nabi, G.; Khalid, N.R. Material and method selection for efficient solid oxide fuel cell anode: Recent advancements and reviews. Int. J. Energy Res. 2019, 43, 2423–2446. [Google Scholar] [CrossRef]
- Zhang, S.L.; Li, C.J.; Li, C.X.; Yang, G.J.; Huang, K.; Liu, M. Liquid plasma sprayed nano-network La0.4Sr0.6Co0.2Fe0.8O3/Ce0.8Gd0.2O2 composite as a high-performance cathode for intermediate-temperature solid oxide fuel cells. J. Power Sources 2016, 327, 622–628. [Google Scholar] [CrossRef]
- Timurkutluk, B.; Timurkutluk, C.; Mat, M.D.; Kaplan, Y. A review on cell/stack designs for high performance solid oxide fuel cells. Renew. Sustain. Energy Rev. 2016, 56, 1101–1121. [Google Scholar] [CrossRef]
- Agbede, O.O.; Hellgardt, K.; Kelsall, G.H. Electrical conductivities and microstructures of LSM, LSM-YSZ and LSM-YSZ/LSM cathodes fabricated on YSZ electrolyte hollow fibres by dip-coating. Mater. Today Chem. 2020, 16, 100252. [Google Scholar] [CrossRef]
- Joulia, A.; Bolelli, G.; Gualtieri, E.; Lusvarghi, L.; Valeri, S.; Vardelle, M.; Rossignol, S.; Vardelle, A. Comparing the deposition mechanisms in suspension plasma spray (SPS) and solution precursor plasma spray (SPPS) deposition of yttria-stabilised zirconia (YSZ). J. Eur. Ceram. Soc. 2014, 34, 3925–3940. [Google Scholar] [CrossRef] [Green Version]
- Pawlowski, L. Finely grained nanometric and submicrometric coatings by thermal spraying: A review. Surf. Coat. Technol. 2008, 202, 4318–4328. [Google Scholar] [CrossRef]
- Wang, X.M.; Li, C.X.; Li, C.J.; Tian, L.H.; Yang, G.J. Microstructure and electrochemical behavior of La0.8Sr0.2MnO3 deposited by solution precursor plasma spraying. Rare Met. Mater. Eng. 2011, 40, 1881–1886. [Google Scholar]
- Wang, X.M.; Li, C.X.; Li, C.J.; Yang, G.J. Microstructure and polarization of La0.8Sr0.2MnO3 cathode deposited by alcohol solution precursor plasma spraying. Int. J. Hydrogen Energy 2012, 37, 12879–12885. [Google Scholar] [CrossRef]
- Prakash, B.S.; Kumar, S.S.; Aruna, S.T. Microstructure and performance of LSM/YSZ based solid oxide fuel cell cathodes fabricated from solution combustion co-synthesized powders and by solution precursor plasma spraying. Surf. Coat. Technol. 2017, 310, 25–32. [Google Scholar] [CrossRef]
- Wang, X.M.; Li, C.X.; Li, C.J.; Yang, G.J. Effect of microstructures on electrochemical behavior of La0.8Sr0.2MnO3 deposited by suspension plasma spraying. Int. J. Hydrogen Energy 2010, 35, 3152–3158. [Google Scholar] [CrossRef]
- Chen, D.; Jordan, E.; Gell, M. Thermal and crystallization behavior of zirconia precursor used in the solution precursor plasma spray process. J. Mater. Sci. 2007, 42, 5576–5580. [Google Scholar] [CrossRef]
- Deng, X.; Petric, A. Geometrical modeling of the triple-phase-boundary in solid oxide fuel cells. J. Power Sources 2005, 140, 297–303. [Google Scholar] [CrossRef]


| Parameter | Value |
|---|---|
| Spraying power | 36 kW |
| Arc current | 600 A |
| Arc voltage | 60 V |
| Main gas (Ar) | 40 L/min |
| Auxiliary gas (H2) | 30 L/min |
| Substrate temperature | 400 °C |
| Liquid delivery speed | 80 rpm |
| Spraying distance | 60 mm |
| Cathode Types | Polarization Resistance (Ω·cm2) | ||||
|---|---|---|---|---|---|
| 800 °C | 850 °C | 900 °C | 950 °C | 1000 °C | |
| YSZ/LSM | 1.26 | 0.33 | 0.18 | 0.11 | 0.083 |
| LSM [27] | 2.55 | 1.04 | 0.4 | 0.21 | 0.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Tang, B.; Wen, P.; Dong, W.; Wang, L.; Wang, D. YSZ/LSM Composite Cathode Deposited by Solution Precursor Plasma Spraying. Coatings 2022, 12, 321. https://doi.org/10.3390/coatings12030321
Wang X, Tang B, Wen P, Dong W, Wang L, Wang D. YSZ/LSM Composite Cathode Deposited by Solution Precursor Plasma Spraying. Coatings. 2022; 12(3):321. https://doi.org/10.3390/coatings12030321
Chicago/Turabian StyleWang, Xiaoming, Boen Tang, Penghui Wen, Weiping Dong, Linlin Wang, and Dongyun Wang. 2022. "YSZ/LSM Composite Cathode Deposited by Solution Precursor Plasma Spraying" Coatings 12, no. 3: 321. https://doi.org/10.3390/coatings12030321
APA StyleWang, X., Tang, B., Wen, P., Dong, W., Wang, L., & Wang, D. (2022). YSZ/LSM Composite Cathode Deposited by Solution Precursor Plasma Spraying. Coatings, 12(3), 321. https://doi.org/10.3390/coatings12030321





