Effect of Antimony on Wetting Behavior and Interfacial Reaction between Zinc Liquid and X80 Steel
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
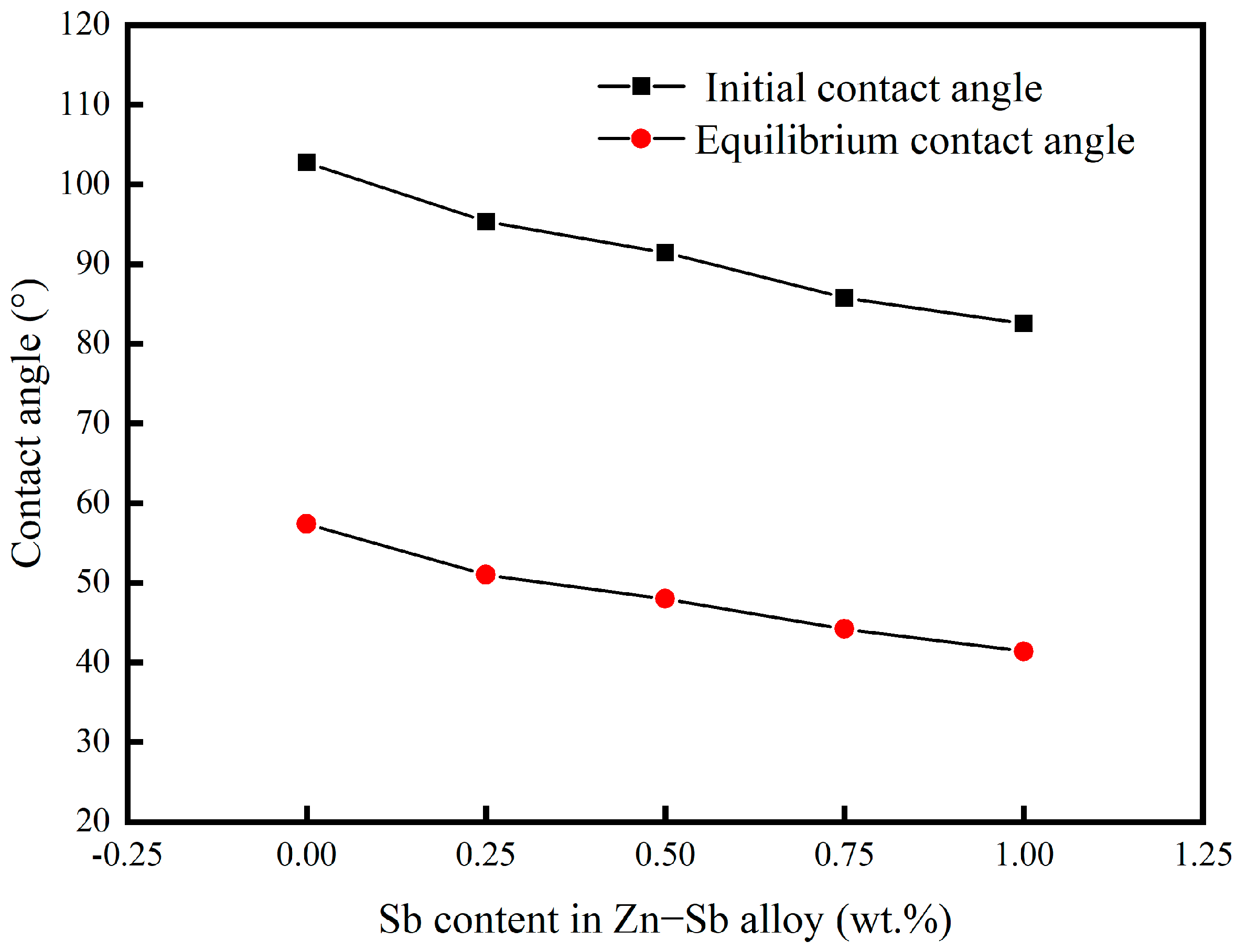
3.1. The Effect of Sb on the Wettability of Zinc Solution on X80 Steel
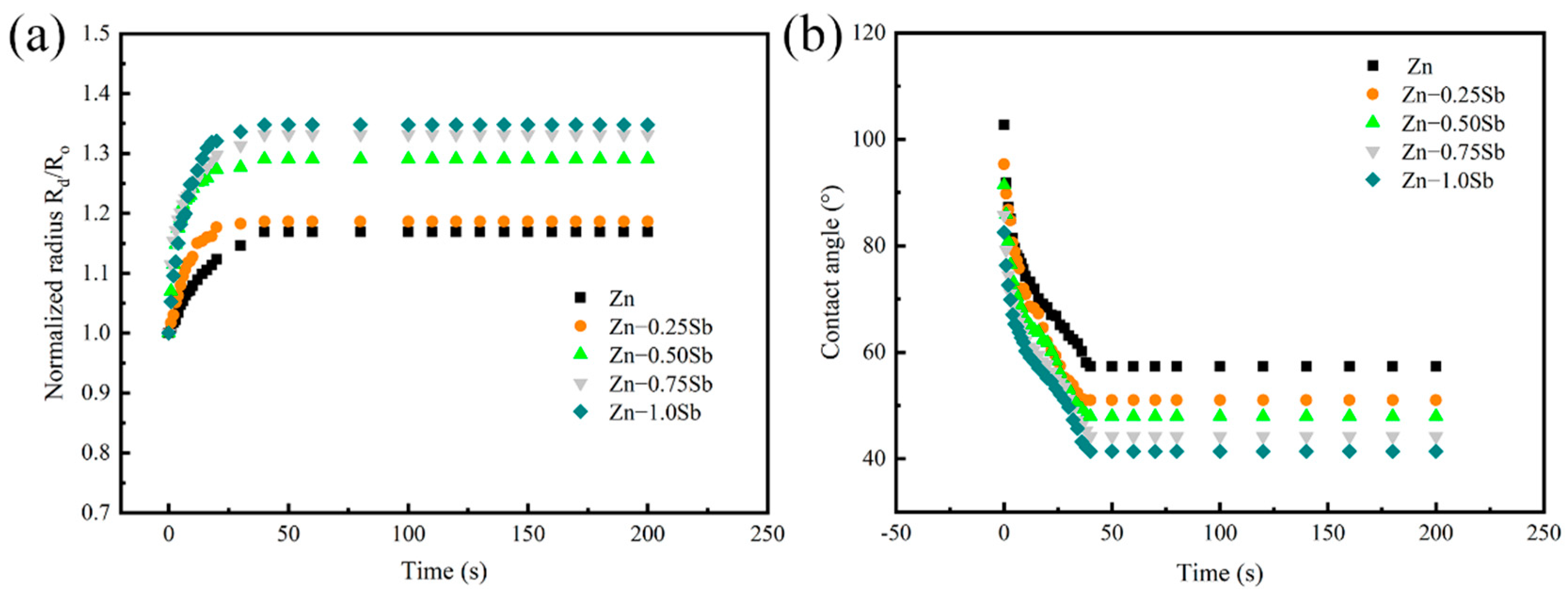
3.2. Spreading Kinetics of Zn-Sb Alloy on X80 Steel
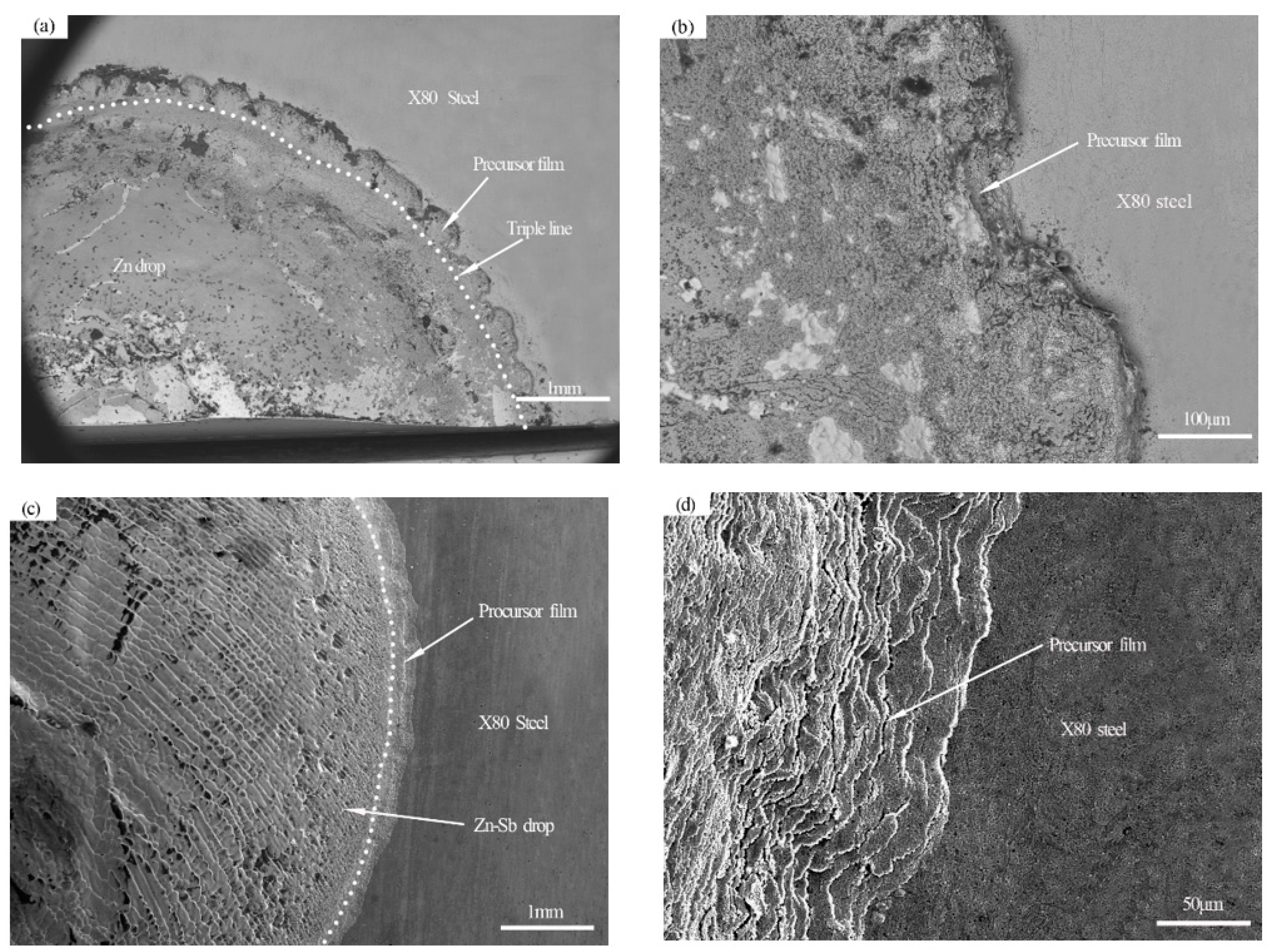
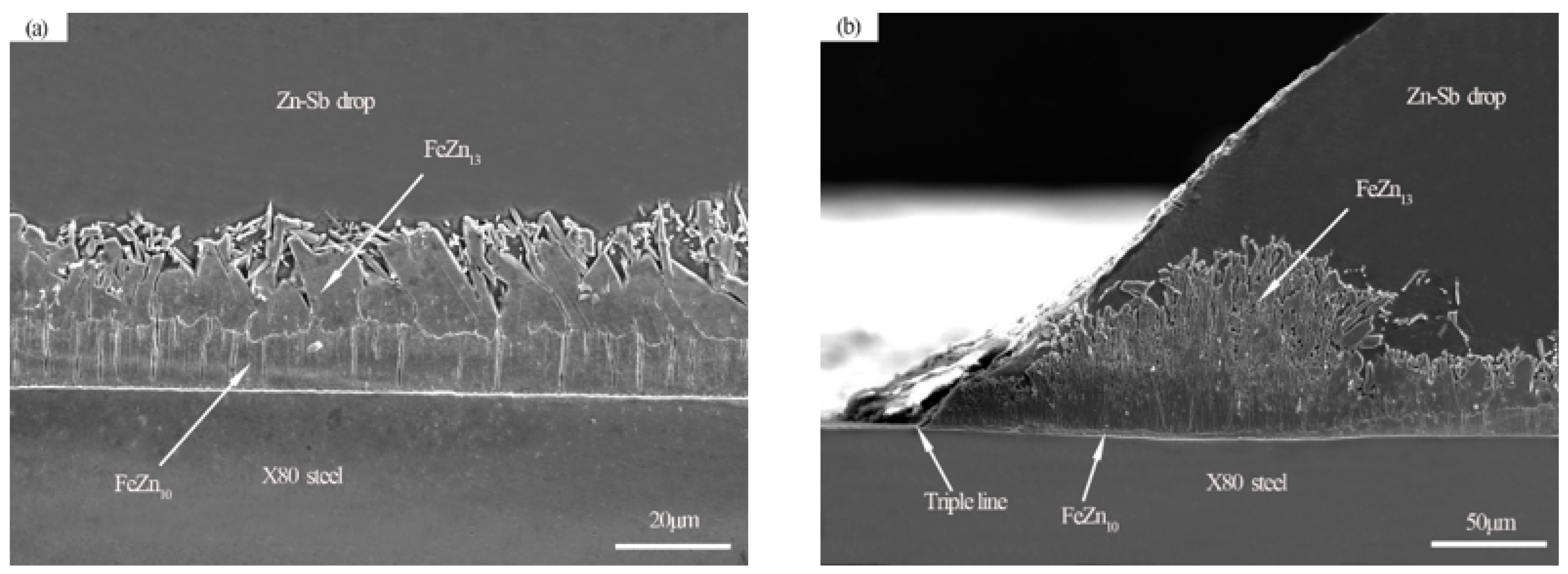
3.3. Wetting Morphology and Interface Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xin, Y.P. Current situation and development trend of oil and gas pipeline technology in China. Nat. Gas Oil 2020, 38, 26–31. [Google Scholar]
- Marder, A.R. The metallurgy of zinc-coated steel. Prog. Mater. Sci. 2000, 45, 191–271. [Google Scholar] [CrossRef]
- Li, Z.; Peng, H.; Xie, A.; Wu, C.; Kawi, S.; Wang, J. Effect of ball-milling pretreatment on microstructure and corrosion of hot-dip galvanized coating. Mater. Charact. 2022, 192, 112177. [Google Scholar] [CrossRef]
- Shibli, S.M.A.; Manu, R.; Dilimon, V.S. Effect of nickel-rich barrier layer on improvement of hot-dip zinc coating. Appl. Surf. Sci. 2005, 245, 179–185. [Google Scholar] [CrossRef]
- Guo, T.X.; Zhai, Z.G. The Summary of influence factors of hot-dip galvanization. Steel Roling 2000, 17, 48–51. [Google Scholar]
- Xu, Q.Y.; Zeng, Q.H. Progress in research and application of rare earth in zinc coatings. Corros. Prot. 2009, 30, 19–21. [Google Scholar]
- Wu, J.L.; Yu, Z.X.; Zhu, Y.D. Influence of adding some trace elements on performances of hot dipped zinc layer. Shanghai Nonferrous Met. 2001, 22, 54–58. [Google Scholar]
- Saiz, E.; Tomsia, A.P. Kinetics of high-temperature spreading. Curr. Opin. Insolid State Mater. Sci. 2005, 9, 167–173. [Google Scholar] [CrossRef]
- Saiz, E.; Cannon, R.M.; Tomsia, A.P. Reactive spreading: Adsorption, ridging and compound formation. Acta Mater. 2000, 48, 4449–4462. [Google Scholar] [CrossRef]
- Fratesi, R.; Ruffini, N.; Malavolta, M.; Bellezze, T. Contemporary use of Ni and Bi in hot-dip galvanizing. Surf. Coat. Technol. 2002, 157, 34–39. [Google Scholar] [CrossRef]
- Li, Z.; Peng, H.; Liu, Y.; Su, X.; Kawi, S.; Wang, J. Synergy of ball-milling and pre-oxidation on microstructure and corrosion of hot-dip zinc coating of nodular cast iron. J. Mater. Res. Technol. 2022, 16, 1402–1412. [Google Scholar] [CrossRef]
- Ferro, A.C.; Derby, B. Wetting behavior in the Al-Si/SiC system: Interface reactions and solubility effects. Acta Mater. 1995, 43, 3061–3073. [Google Scholar] [CrossRef]
- Barzilai, S.; Aizenshtein, M.; Lomberg, M.; Froumin, N.; Frage, N. Interface reaction and wetting in the CaF2/me systems. J. Alloys Compd. 2008, 452, 154–160. [Google Scholar] [CrossRef]
- Zhen, Q.I.; Liang, L.I.A.O.; Wang, R.Y.; Zhang, Y.G.; Yuan, Z.F. Roughness-dependent wetting and surface tension of molten lead on alumina. Trans. Nonferrous Met. Soc. China 2021, 31, 2511–2521. [Google Scholar]
- Li, Z.; Peng, H.; Liu, Y.; Wang, J.; Su, X. Effect of surface micromorphology and roughness of iron ingot on microstructure of hot-dip galvanized coating. Trans. Indian Inst. Met. 2022, 75, 397–406. [Google Scholar] [CrossRef]
- Zhu, D.; Liao, X.; Dai, P. Theoretical analysis of reactive solid-liquid interfacial energies. Chin. Sci. Bull. 2012, 57, 4517–4524. [Google Scholar] [CrossRef]
- Lin, Q.; Wang, J.; Sui, R.; Zhong, W. Wetting of Sn on Al-steel multilayered composite plate at 350–450 °C. Colloids Surf. A Physicochem. Eng. Asp. 2018, 555, 88–94. [Google Scholar] [CrossRef]
- Jin, P.; Zhong, W.Q.; Li, F.X.; Lin, Q.L. Reactive Wetting of Q235 steel and pure Ti by molten Al 6061 Alloys. Mater. Rep. 2017, 31, 59–63. [Google Scholar] [CrossRef]
- Kondoh, K.; Kawakami, M.; Imai, H.; Umeda, J.; Fujii, H. Wettability of pure Ti by molten pure Mg droplets. Acta Mater. 2010, 58, 606–614. [Google Scholar] [CrossRef]
- Zhao, H.; Debroy, T. Weld metal composition change during conduction mode laser welding of aluminum alloy 5182. Metall. Mater. Trans. B 2001, 32, 163–172. [Google Scholar] [CrossRef]
- Zhou, Y.; Lin, Q. Wetting of galvanized steel by Al4043 alloys in the first cycle of CMT process. J. Alloys Compd. 2014, 589, 307–313. [Google Scholar] [CrossRef]
- Chung, Y.; Wang, J.; Toguri, J.M.; Yao, M.X. Wetting of zinc alloys on steels containing silicon. In Proceedings of the 42nd Mechanical Working and Steel Processing Conference Proceedings, Toronto, ON, Canada, 22–25 October 2001; Volume 28, pp. 63–67. [Google Scholar]
- Giorgi, M.L.; Diawara, J.; Chen, S.; Koltsov, A.; Mataigne, J.M. Influence of annealing treatment on wetting of steels by zinc alloys. J. Mater. Sci. 2012, 47, 8483–8495. [Google Scholar] [CrossRef]
- Li, Z.; Ruan, R.; Xi, S.; Wang, J.; Lei, Y.; Deng, S.; Peng, H. The Influence of Al on the surface properties of the hot-dip galvanized melt. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2022, 37, 117–122. [Google Scholar] [CrossRef]
- Uchiyama, Y.; Koga, H.; Ohkubo, T.; Sasada, A. Effect of carbon and silicon in steel on the Fe-Zn reaction. Jpn. Inst. Met. Mater. 1984, 48, 173–180. [Google Scholar] [CrossRef]
- Yuan, Z.F.; Ke, J.J.; Li, J. Surface Tension of Metals and Alloys; Beijing Science Press: Beijing, China, 2006; Volume 4, pp. 26–32. [Google Scholar]
- Falke, W.L.; Schwaneke, A.E.; Nash, R.W. Surface tension of zinc: The positive temperature coefficient. Metall. Mater. Trans. B 1977, 8, 301–303. [Google Scholar] [CrossRef]
- Nogi, K.; Ogino, K.; McLean, A.; Miller, W.A. The temperature coefficient of the surface tension of pure liquid metals. Metall. Mater. Trans. B 1986, 17, 163–170. [Google Scholar] [CrossRef]
- Gremillard, L.; Saiz, E.; Radmilovic, V.R.; Tomsia, A.P. Role of titanium on the reactive spreading of lead-free solders on alumina. J. Mater. Res. 2006, 21, 3222–3233. [Google Scholar] [CrossRef]
- Zhu, D.Y.; Dai, P.Q.; Luo, X.B.; Zhang, Y.C. Novel characterization of wetting properties and the calculation of liquid-solid interface tension(I). Sci. Technol. Eng. 2007, 7, 3057–3062. Available online: http://en.cnki.com.cn/Article_en/CJFDTOTAL-KXJS200713003.htm (accessed on 20 November 2022).
- Zhu, D.Y.; Zhang, Y.C.; Dai, P.Q.; Zhang, Y.C. Novel characterization of wetting properties and the calculation of liquid-solid interface tension(II). Sci. Technol. Eng. 2007, 7, 3063–3069. Available online: http://en.cnki.com.cn/Article_en/CJFDTOTAL-KXJS200713004.htm (accessed on 20 November 2022).

| Composition | C | Si | Mn | P | S | Cr | Ni | Mo | Nb |
|---|---|---|---|---|---|---|---|---|---|
| Mass Fraction (wt. %) | 0.054 | 0.30 | 1.84 | 0.011 | 0.0041 | 0.33 | 0.10 | 0.091 | 0.075 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, K.; Li, Z.; Zhou, N.; Su, X.; Lei, Y.; Liu, Y.; Peng, H. Effect of Antimony on Wetting Behavior and Interfacial Reaction between Zinc Liquid and X80 Steel. Coatings 2022, 12, 1890. https://doi.org/10.3390/coatings12121890
Xie K, Li Z, Zhou N, Su X, Lei Y, Liu Y, Peng H. Effect of Antimony on Wetting Behavior and Interfacial Reaction between Zinc Liquid and X80 Steel. Coatings. 2022; 12(12):1890. https://doi.org/10.3390/coatings12121890
Chicago/Turabian StyleXie, Kunlun, Zhiwei Li, Nianyong Zhou, Xuping Su, Yun Lei, Ya Liu, and Haoping Peng. 2022. "Effect of Antimony on Wetting Behavior and Interfacial Reaction between Zinc Liquid and X80 Steel" Coatings 12, no. 12: 1890. https://doi.org/10.3390/coatings12121890
APA StyleXie, K., Li, Z., Zhou, N., Su, X., Lei, Y., Liu, Y., & Peng, H. (2022). Effect of Antimony on Wetting Behavior and Interfacial Reaction between Zinc Liquid and X80 Steel. Coatings, 12(12), 1890. https://doi.org/10.3390/coatings12121890








