The Preparation of a Superhydrophobic Fluorine Rubber Surface
Abstract
:1. Introduction
2. Experiment
2.1. Materials
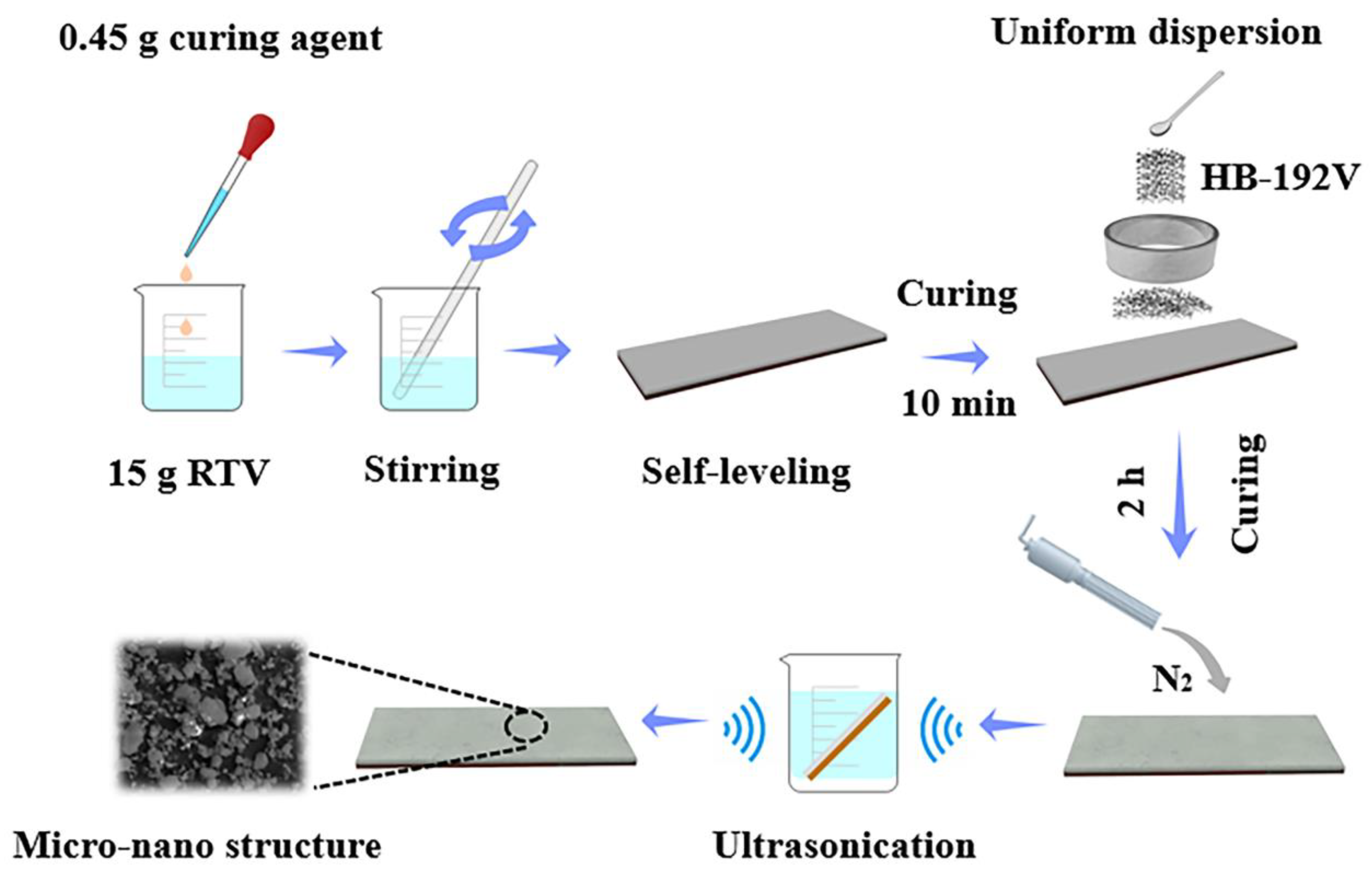
2.2. Preparation of Superhydrophobic Surfaces
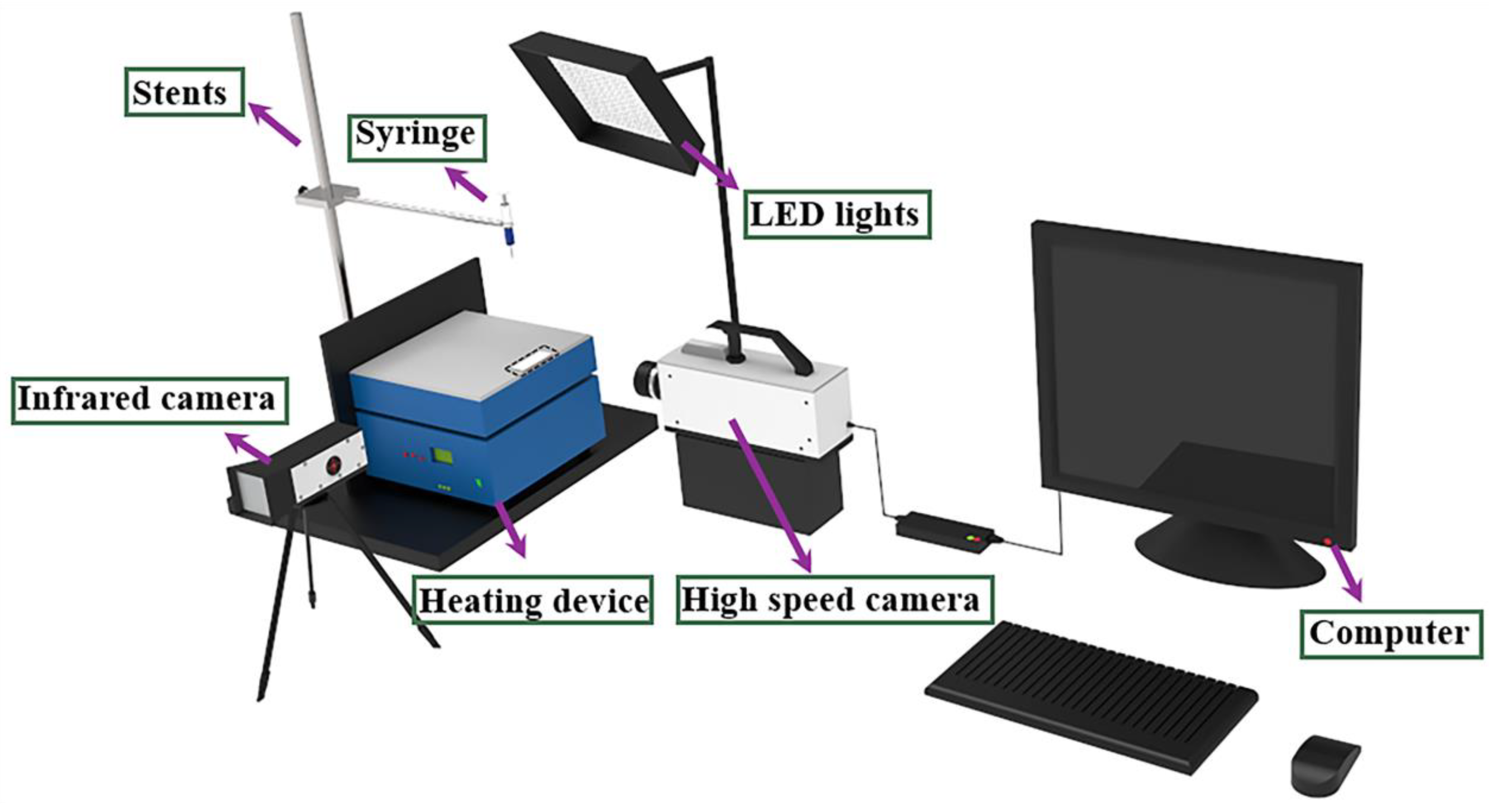
2.3. Sample Characterization and Performance Test
3. Results and Discussion
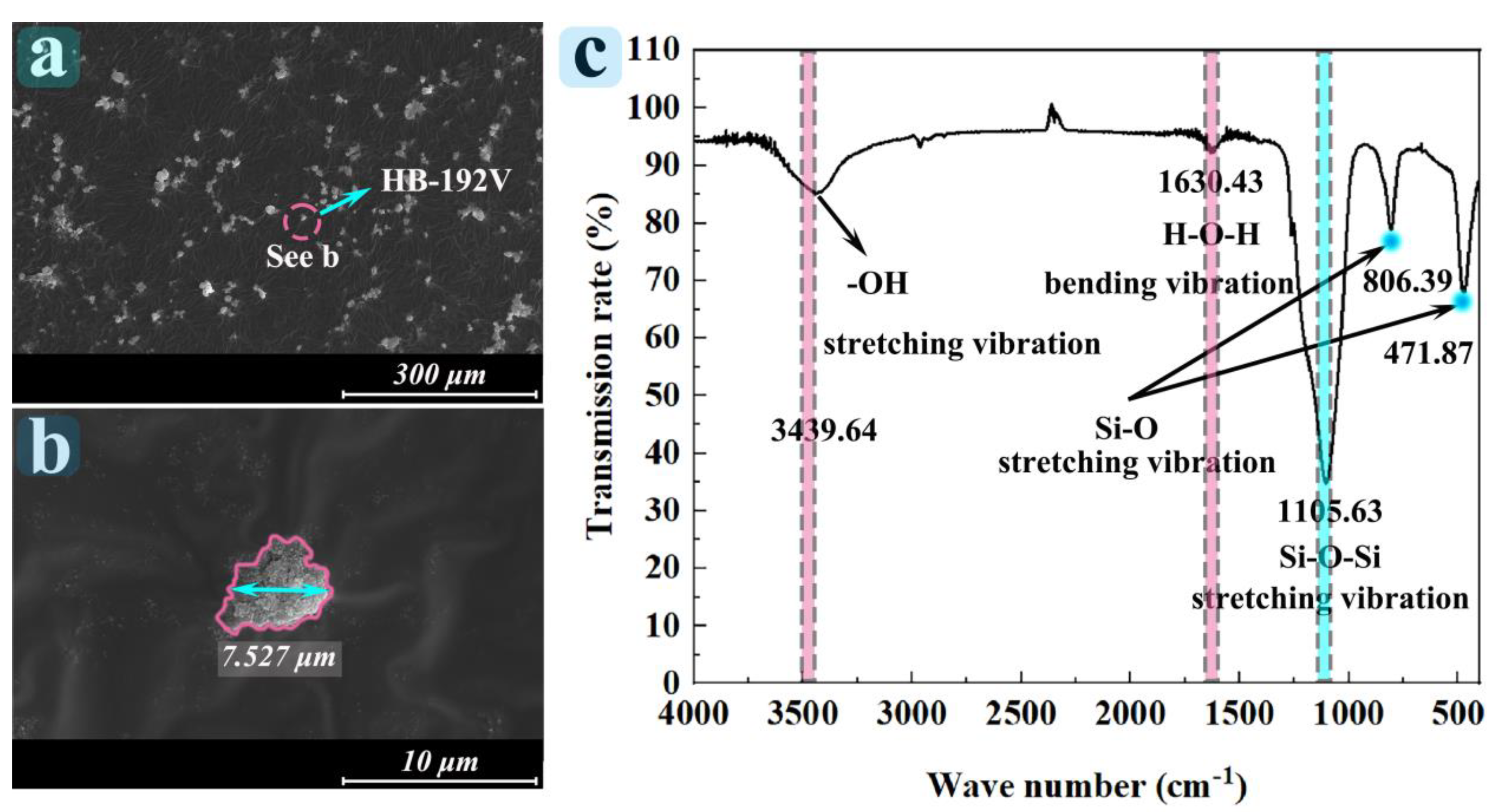
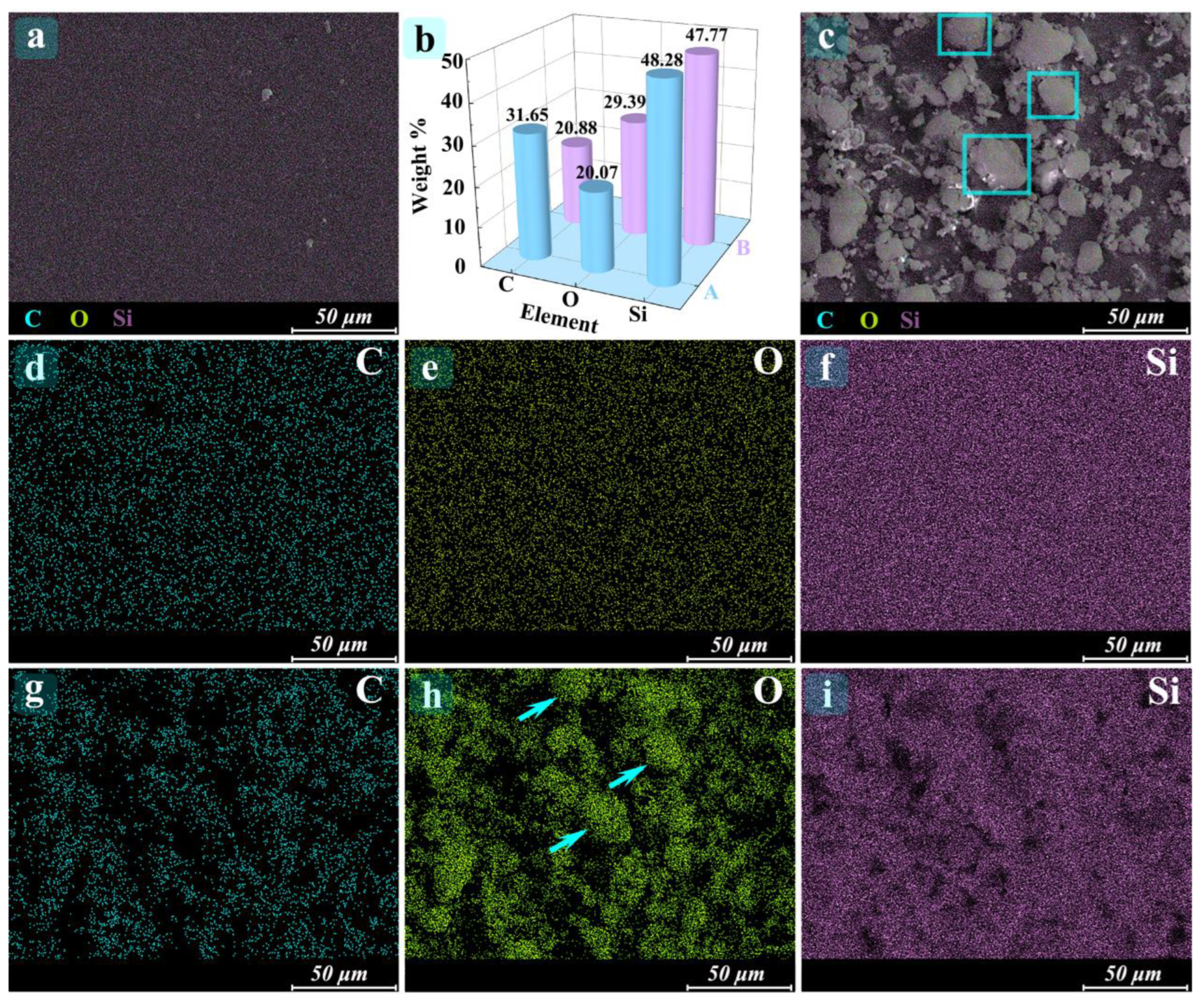
3.1. Characterization Analysis of Particles
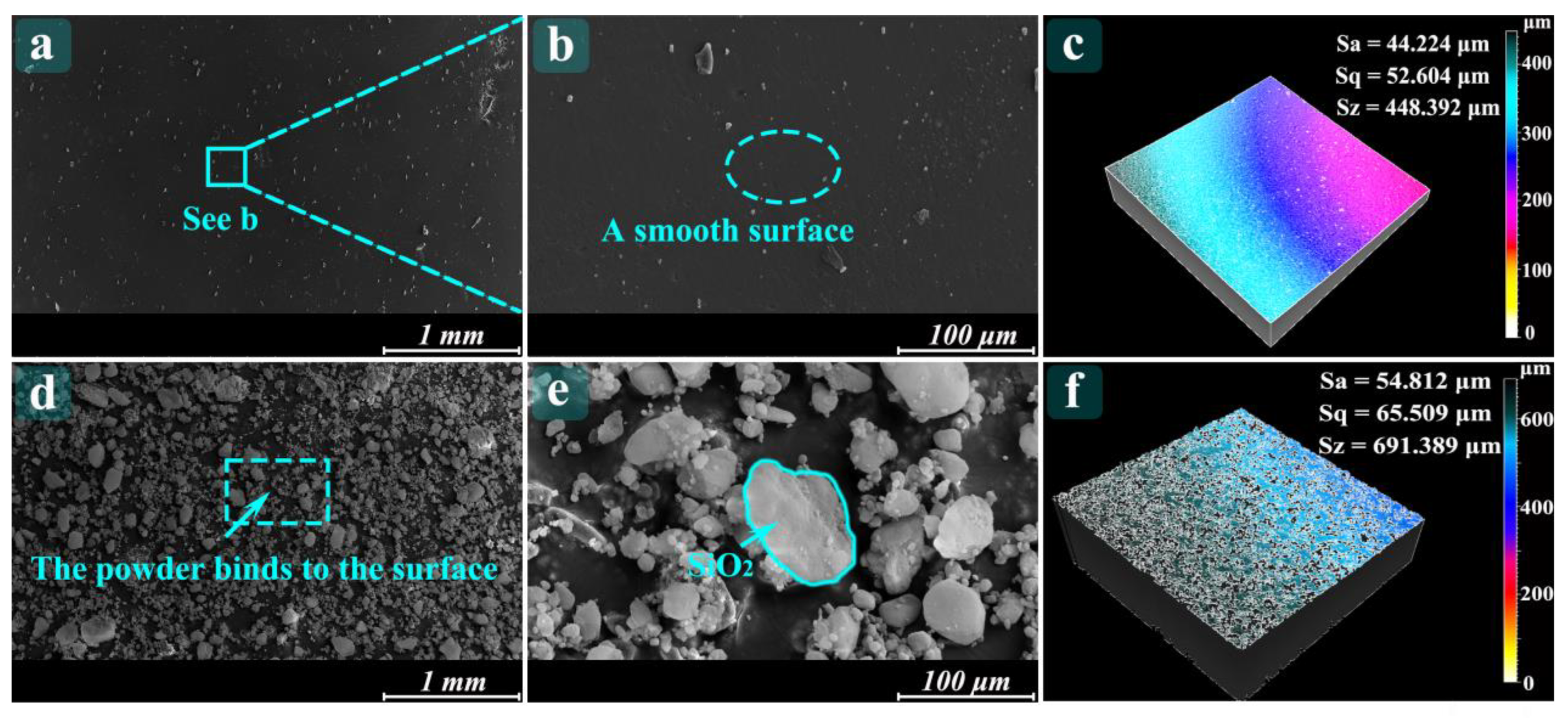
3.2. Characterization of Samples
3.3. Hydrophobicity and Mechanism Analysis
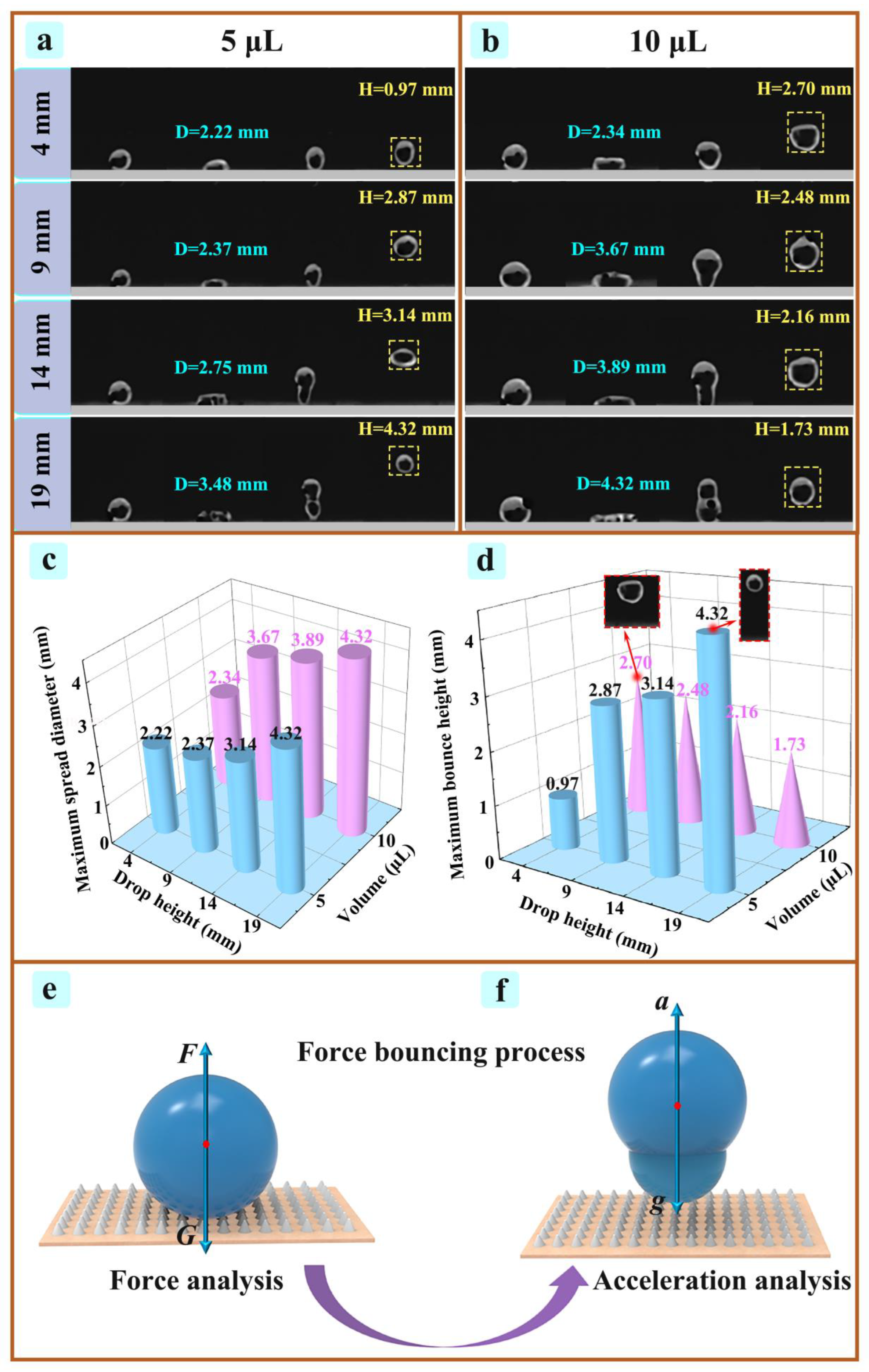
3.4. Analysis of Bounce Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qin, Y.; Fang, Z.; Chai, X.; Cui, X. A Superhydrophobic Alkali Activated Materials Coating by Facile Preparation. Coatings 2022, 12, 864. [Google Scholar] [CrossRef]
- Barthlott, W.; Neinhuis, C. Purity of the sacred lotus, or escape from contamination in biological surfaces. Planta 1997, 202, 1–8. [Google Scholar] [CrossRef]
- Bains, N.S.; Vaishya, R.O.; Kant, S.; Gupta, A. Bioinspired hydrophobic surfaces: An overview. Mater. Today Proc. 2022, 64, 1486–1489. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, X.; Wang, R.; Long, F.; Zhao, P.; Liu, L. A mosquito-eye-like superhydrophobic coating with super robustness against abrasion. Mater. Des. 2021, 203, 109552. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Z.; Xu, J.; Yu, H. One-step method using laser for large-scale preparation of bionic superhydrophobic & drag-reducing fish-scale surface. Surf. Coat. Technol. 2021, 409, 126801. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, Z.; Wang, Y.; Xu, D.; Zhao, Y. Butterfly inspired functional materials. Mater. Sci. Eng. R Rep. 2021, 144, 100605. [Google Scholar] [CrossRef]
- Zhang, K.; Xu, F.; Gao, Y. Superhydrophobic and oleophobic dual-function coating with durablity and self-healing property based on a waterborne solution. Appl. Mater. Today 2021, 22, 100970. [Google Scholar] [CrossRef]
- He, Q.; Wang, G.; Zhang, F.; Li, A. Study on mechanical and friction properties of nano-Al2O3 as fluororubber additive. Vacuum 2022, 206, 111550. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, Y.; He, Q. Study on properties of fish scale biomimetic fluororubber prepared by template method. J. Polym. Res. 2022, 29, 368. [Google Scholar] [CrossRef]
- Ma, Y.; He, Q. Preparation of superhydrophobic conductive CNT/PDMS film on paper by foam spraying method. Colloids Surf. A Physicochem. Eng. Asp. 2022, 648, 129327. [Google Scholar] [CrossRef]
- Li, A.; Jia, Y.; Zhang, F.; Zhao, Y.; Zhang, F. The Effects of Zinc Oxide/Silicon Dioxide Composite Coating on Surface Wettability and the Mechanical Properties of Paper Mulching Film. Coatings 2022, 12, 555. [Google Scholar] [CrossRef]
- Yim, J.H.; Rodriguez-Santiago, V.; Williams, A.A.; Gougousi, T.; Pappas, D.D.; Hirvonen, J.K. Atmospheric pressure plasma enhanced chemical vapor deposition of hydrophobic coatings using fluorine-based liquid precursors. Surf. Coat. Technol. 2013, 234, 21–32. [Google Scholar] [CrossRef]
- Jin, Y.; Huang, L.; Zheng, K.; Zhou, S. Blending Electrostatic Spinning Fabrication of Superhydrophilic/Underwater Superoleophobic Polysulfonamide/Polyvinylpyrrolidone Nanofibrous Membranes for Efficient Oil–Water Emulsion Separation. Langmuir 2022, 38, 8241–8251. [Google Scholar] [CrossRef]
- Shan, L.M.; Liu, G.B.; Tang, H.; Li, Z.H.; Wu, J.Y. A novel simple fabrication method for mechanically robust superhydrophobic 2024 aluminum alloy surfaces. Coatings 2022, 12, 1717. [Google Scholar] [CrossRef]
- Liu, J.F.; Xiao, X.Y.; Shi, Y.L.; Wan, C. Fabrication of a superhydrophobic surface from porous polymer using phase separation. Appl. Surf. Sci. 2014, 297, 33–39. [Google Scholar] [CrossRef]
- Li, A.; Wang, G.; Zhang, Y.; Zhang, J.; He, W.; Ren, S.; Xu, Z.; Wang, J.; Ma, Y. Preparation methods and research progress of superhydrophobic paper. Coord. Chem. Rev. 2021, 449, 214207. [Google Scholar] [CrossRef]
- He, Q.; He, W.; Zhang, F.; Zhao, Y.; Li, L.; Yang, X.; Zhang, F. Research Progress of Self-Cleaning, Anti-Icing, and Aging Test Technology of Composite Insulators. Coatings 2022, 12, 1224. [Google Scholar] [CrossRef]
- Su, F.H.; Yao, K. Facile fabrication of superhydrophobic surface with excellent mechanical abrasion and corrosion resistance on copper substrate by a novel method. ACS Appl. Mater. Interfaces 2014, 6, 8762–8770. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, Y.; Luo, S.; Cheng, C.; Wang, S.; Liu, B. Fabrication of Superhydrophobic Composite Membranes with Honeycomb Porous Structure for Oil/Water Separation. Coatings 2022, 12, 1698. [Google Scholar] [CrossRef]
- Zhang, C.Q.; Li, Y.M.; Bai, N.N.; Tan, C.; Cai, P.; Li, Q. Fabrication of robust 3D superhydrophobic material by a simple and low-cost method for oil-water separation and oil absorption—ScienceDirect. Mater. Sci. Eng. B 2017, 224, 117–124. [Google Scholar] [CrossRef]
- Tang, Y.; Yang, X.; Li, Y.; Lu, Y.; Zhu, D. Robust micro-nanostructured superhydrophobic surfaces for long-term dropwise condensation. Nano Lett. 2021, 21, 9824–9833. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.R.; Cui, X.; Jiang, H.; Wu, N.; Peng, C.; Hu, Z.; Liang, X.; Yan, Y.; Huang, J.; Li, D. A superhydrophobic coating harvesting mechanical robustness, passive anti-icing and active de-icing performances. J. Colloid Interface Sci. 2021, 590, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Gong, Q.; Zhan, S.; Jiang, L.; Zheng, Y. Robust anti-icing performance of a flexible superhydrophobic surface. Adv. Mater. 2016, 28, 7729–7735. [Google Scholar] [CrossRef] [PubMed]
- Xiao, N. Application and development of fluorine rubber. China Pet. Chem. Ind. 2005, 26–29. [Google Scholar]
- Park, J.; Lee, B.; Kim, H.J. A comparative study on the physical properties of a fluororubber complex. Mater. Sci. Technol. 2022, 38, 1597–1604. [Google Scholar] [CrossRef]
- Zhou, H.; Li, S.; Zhang, Z.; Cai, R.; Wang, F.; Wang, H.; Li, Z. Preparation of fluororubber/carbon nanotube composites and the effect of carbon nanotubes on aging resistance and solvent resistance of fluororubber. J. Macromol. Sci. Part A 2022, 59, 689–697. [Google Scholar] [CrossRef]
- Ren, H.F.; Jiang, J.F.; Chen, W.F. Production technology and application prospect of fluorine rubber. Organo-Fluor. Ind. 2017, 4, 36–40. [Google Scholar]
- Zhang, L.; Wang, S.X. Application of rubber products in oil drilling and production engineering. China Rubber 2011, 27, 18–20. [Google Scholar]
- He, Q.; Xu, Z.H.; Li, A.; Wang, J.; Zhang, J.; Zhang, Y. Study on hydrophobic properties of fluororubber prepared by template method under high temperature conditions. Colloids Surf. A Physicochem. Eng. Asp. 2020, 612, 125837. [Google Scholar] [CrossRef]
- Zhou, W.Y.; Phule, A.D.; Ma, L.Y.; Shan, T.K.; Guo, J.; Zhang, Z.X. Fluororubber superhydrophobic coating: Preparation, characterisation, and EMI shielding performance. Surf. Eng. 2021, 37, 1308–1319. [Google Scholar] [CrossRef]
- Li, A.; Wang, G.; Ma, Y.; Zhao, C.; Zhang, F.; He, Q.; Zhang, F. Study on preparation and properties of superhydrophobic surface of RTV silicone rubber. J. Mater. Res. Technol. 2020, 11, 135–143. [Google Scholar] [CrossRef]
- Wang, G.; Li, A.; Li, K.; Zhao, Y.; Ma, Y.; He, Q. A fluorine-free superhydrophobic silicone rubber surface has excellent self-cleaning and bouncing properties. J. Colloid Interface Sci. 2021, 4, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Li, A.; Zhao, W.; Xu, Z.; Ma, Y.; Zhang, F.; Zhang, Y.; Zhou, J.; He, Q. A Review on Fabrication Methods and Research Progress of Superhydrophobic Silicone Rubber Materials Adv. Mater. Interfaces 2020, 8, 2001460. [Google Scholar] [CrossRef]
- Wang, G.; Zhou, J.; Wang, M.; Zhang, Y.; Zhang, Y.; He, Q. A superhydrophobic surface with aging resistance, excellent mechanical restorablity and droplet bounce properties. Soft Matter 2020, 16, 5514–5524. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhou, W.; Zhou, J.; Wang, M.; Zhang, Y.; Qiang, H. Superhydrophobic silicone rubber surface prepared by direct replication. Surf. Eng. 2020, 37, 278–287. [Google Scholar] [CrossRef]
- Cheng, Y.; Zuo, X.; Yuan, X.; Zhu, H.; Huang, H.; Wang, Y.; Zhang, Y. Preparation of fluorine silicon copolymer superhydrophobic anticorrosive coating on copper aluminium composite by one step spraying. Mater. Lett. 2021, 304, 130496. [Google Scholar] [CrossRef]
- Lee, E.; Kim, D.H. Simple fabrication of asphalt-based superhydrophobic surface with controllable wetting transition from Cassie-Baxter to Wenzel wetting state. Colloids Surf. A Physicochem. Eng. Asp. 2021, 625, 126927. [Google Scholar] [CrossRef]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Cassie, A.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Yin, L.; Zhang, H.F.; Shi, S.Y.; Lu, Y.; Wang, Y.; Liu, X.W. Numerical investigation of relationship between water contact angle and drag reduction ratio of superhydrophobic surfaces. Front. Phys. 2016, 11, 114701. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, X.; Ren, S.; Tong, R. The Preparation of a Superhydrophobic Fluorine Rubber Surface. Coatings 2022, 12, 1878. https://doi.org/10.3390/coatings12121878
He X, Ren S, Tong R. The Preparation of a Superhydrophobic Fluorine Rubber Surface. Coatings. 2022; 12(12):1878. https://doi.org/10.3390/coatings12121878
Chicago/Turabian StyleHe, Xinyang, Shuaichang Ren, and Ruiting Tong. 2022. "The Preparation of a Superhydrophobic Fluorine Rubber Surface" Coatings 12, no. 12: 1878. https://doi.org/10.3390/coatings12121878
APA StyleHe, X., Ren, S., & Tong, R. (2022). The Preparation of a Superhydrophobic Fluorine Rubber Surface. Coatings, 12(12), 1878. https://doi.org/10.3390/coatings12121878







