Flexible Free-Standing Graphene-Fe2O3 Hybrid Paper with Enhanced Electrochemical Performance for Rechargeable Lithium-Ion Batteries
Abstract
1. Introduction
2. Materials and Methods
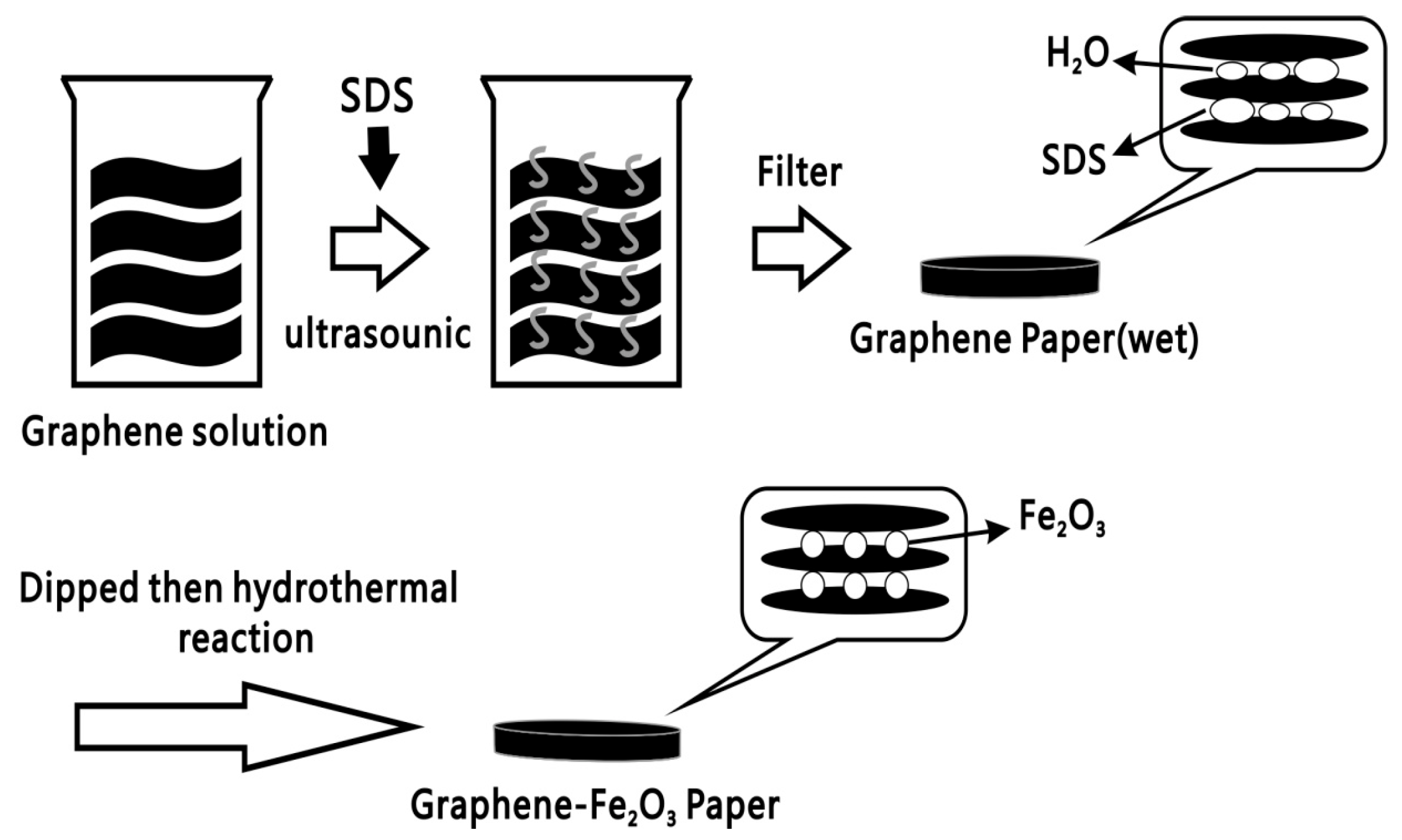
2.1. Materials Preparation
2.2. Materials Characterization
2.3. Electrochemical Analysis
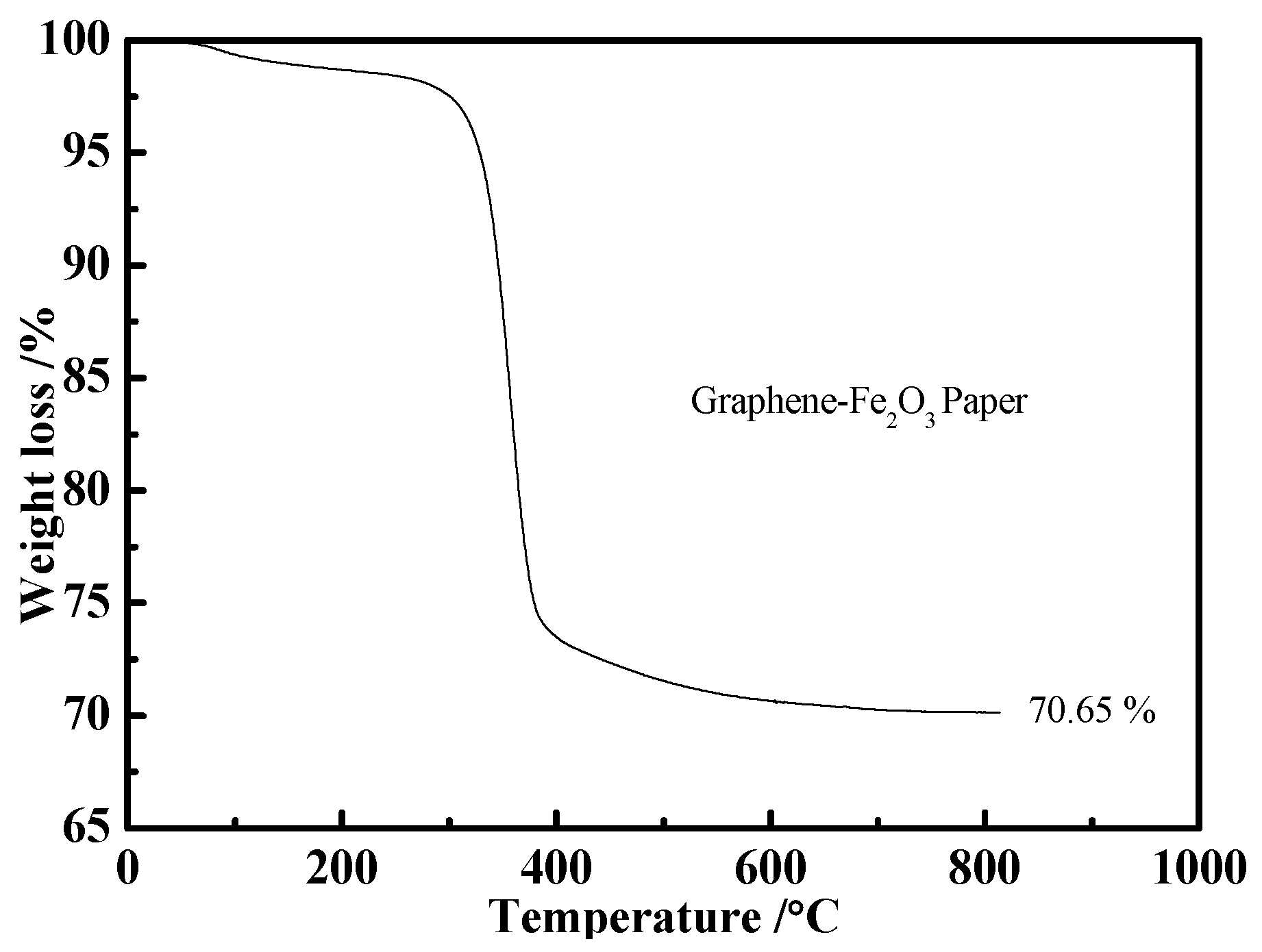
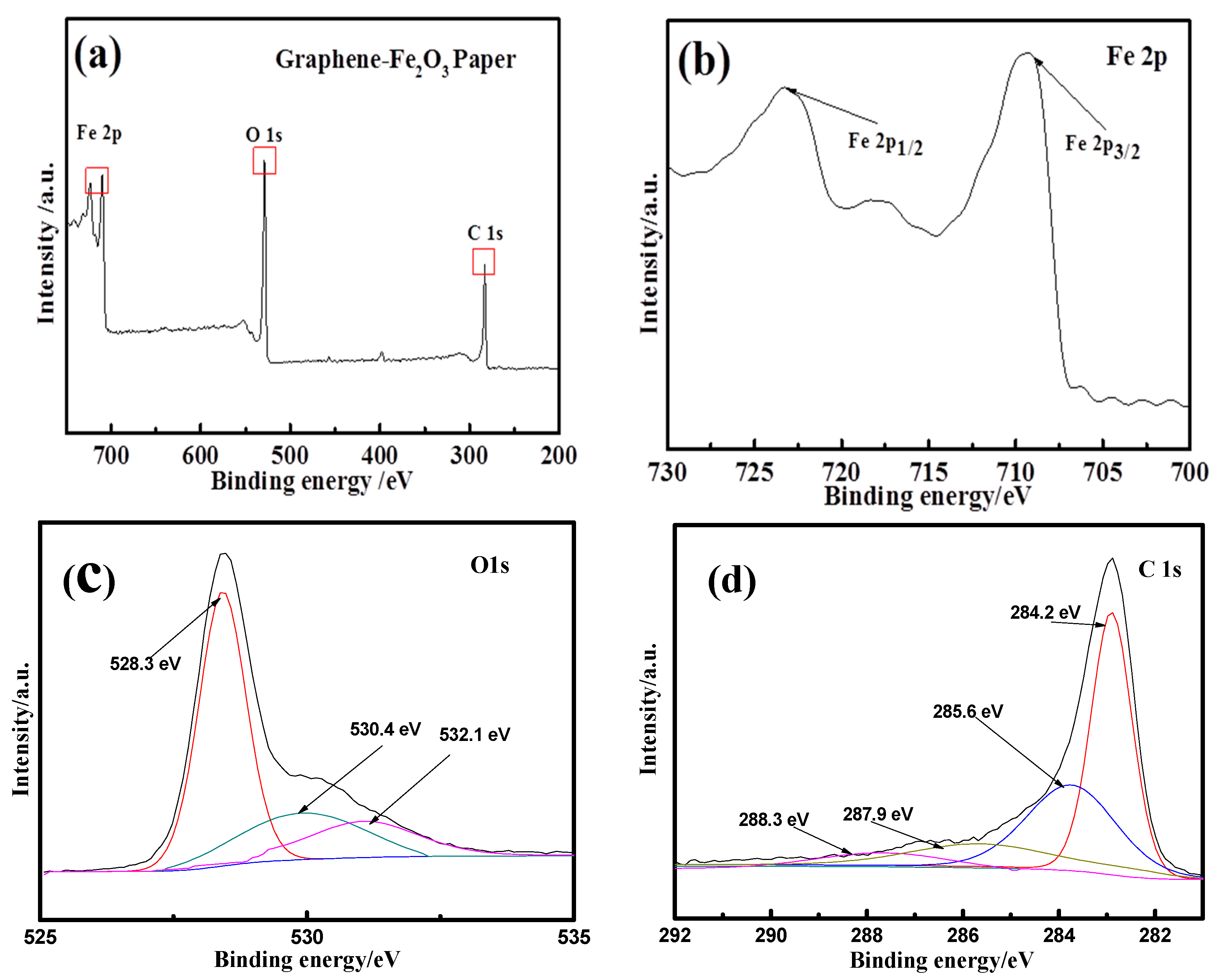
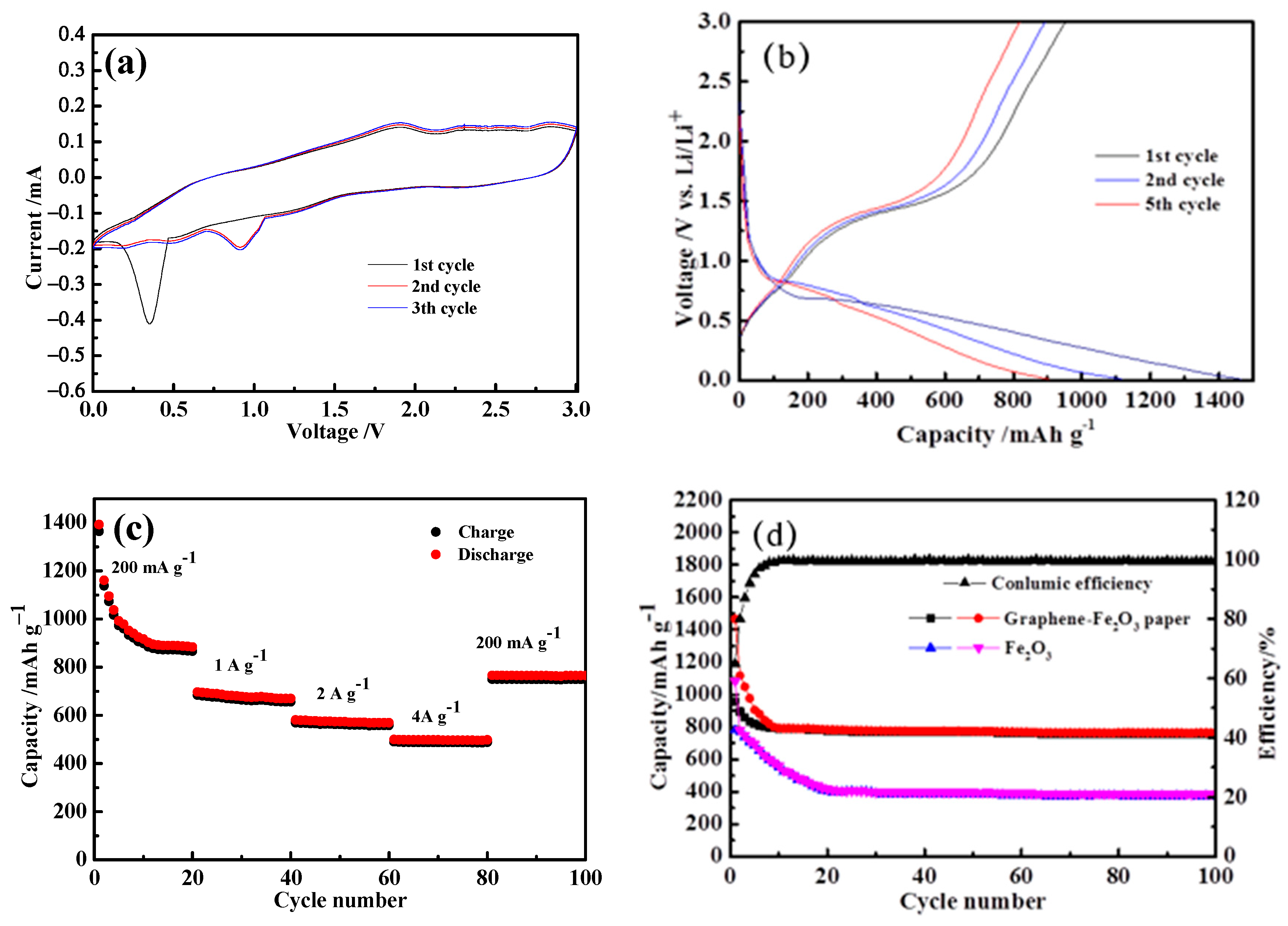
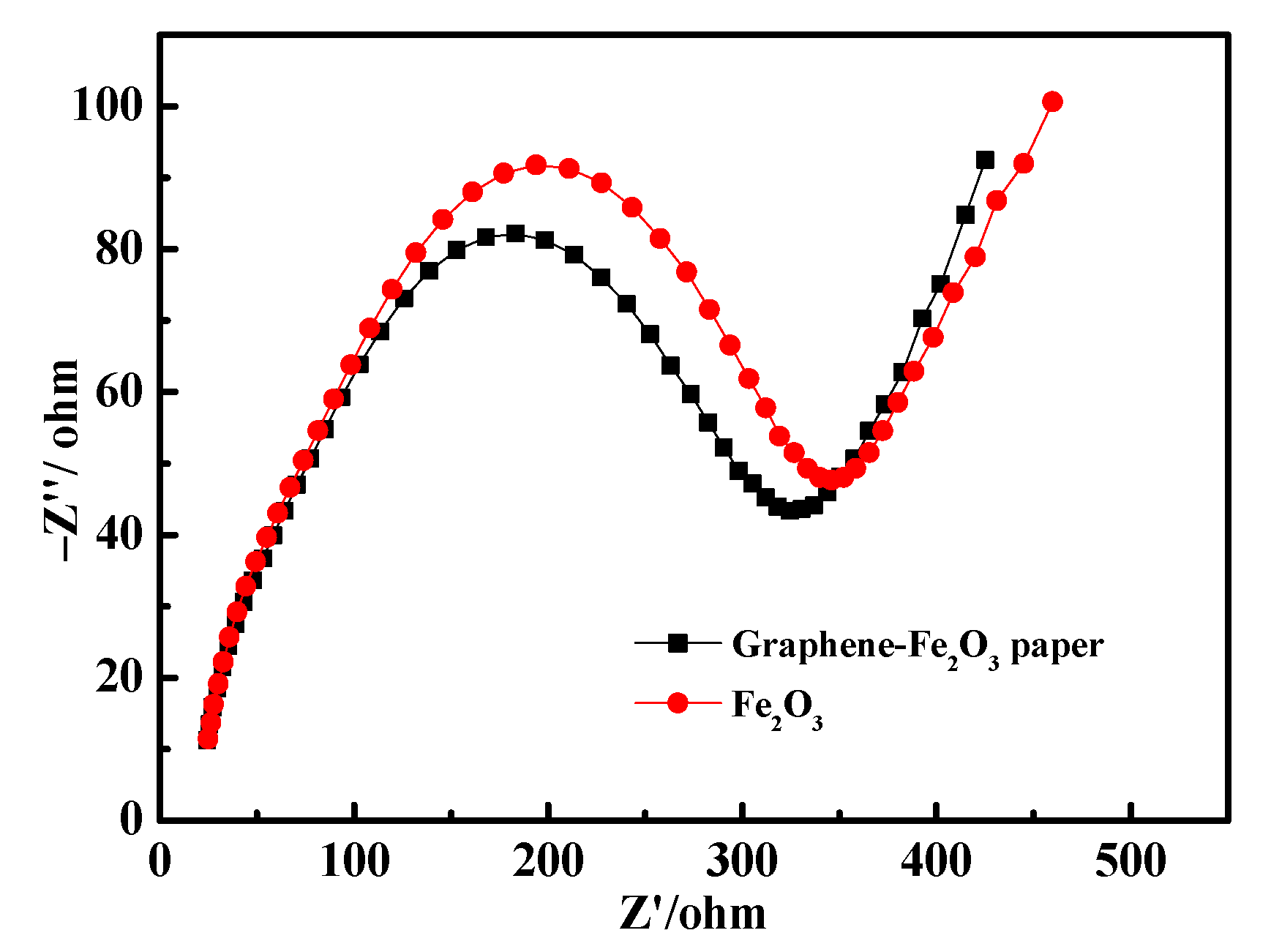
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wood, D.L.; Li, J.L.; Daniel, C. Prospects for reducing the processing cost of lithium ion batteries. J. Power Sources 2015, 275, 234–242. [Google Scholar] [CrossRef]
- Abazari, S.; Shamsipur, A. Graphene family nanomaterial reinforced magnesium-based matrix composites for biomedical application: A comprehensive review. Metals 2020, 10, 1002. [Google Scholar] [CrossRef]
- Roy, P.; Srivastava, S.K. Nanostructured anode materials for lithium ion batteries. J. Mater. Chem. A 2015, 3, 2454–2484. [Google Scholar] [CrossRef]
- Wang, Z.M.; Xu, X.T.; Kim, J.H.; Malgras, V.; Mo, R.; Li, C.L. Nanoarchitectured metal-organic framework/polypyrrole hybrid for brackish water desalination using capacitive deionization. Mater. Horiz. 2019, 6, 1433–1437. [Google Scholar] [CrossRef]
- Xu, X.T.; Allah, A.E.; Wang, C.; Tan, H.B.; Farghali, A.A.; Khedr, M.H. Capacitive deionization using nitrogen-doped mesostructured carbons for highly efficient brackish water desalination. Chem. Eng. J. 2019, 362, 887–896. [Google Scholar] [CrossRef]
- Adhami, T.; Kahrizsangi, R.E.; Bakhsheshi-Rad, H.R. Synthesis and electrochemical properties of TiNb2O7 and Ti2Nb10O29 anodes under various annealing atmospheres. Metals 2021, 11, 983. [Google Scholar] [CrossRef]
- Baghbaderani, M.Z.; Abazari, S. Dual synergistic effects of MgO-GO fillers on degradation behavior, biocompatibility and antibacterial activities of chitosan coated Mg alloy. Coatings 2022, 12, 63. [Google Scholar] [CrossRef]
- Ding, Z.B.; Xu, X.T.; Li, Y.Q.; Wang, K.; Lu, T.; Pan, L.K. Significantly improved stability of hybrid capacitive deionization using nickel hexacyanoferrate/reduced graphene oxide cathode at low voltage operation. Desalination 2019, 468, 114078. [Google Scholar] [CrossRef]
- Xu, X.T.; Tang, J.; Kaneti, Y.V.; Tan, H.B.; Chen, T.; Pan, L.K. Unprecedented capacitive deionization performance of interconnected iron–nitrogen-doped carbon tubes in oxygenated saline water. Mater. Horiz. 2020, 7, 1404–1412. [Google Scholar] [CrossRef]
- Wang, G.K.; Sun, X.; Lu, F.Y.; Sun, H.T.; Yu, M.P.; Jiang, W.L. Flexible pillared graphene-paper electrodes for high-performance electrochemical supercapacitors. Small 2012, 6, 452–461. [Google Scholar] [CrossRef]
- Tao, L.Q.; Zhang, K.N.; Tian, H.; Liu, Y.; Wang, D.Y.; Chen, Y.Q. Graphene-paper pressure sensor for detecting human motions. ACS Nano. 2017, 11, 8790–8795. [Google Scholar] [CrossRef] [PubMed]
- Chi, K.; Zhang, Z.Y.; Xi, J.B.; Huang, Y.G.; Xiao, F.; Wang, S. Freestanding graphene paper supported three-dimensional porous graphene–polyaniline nanocomposite synthesized by inkjet printing and in flexible all-solid-state supercapacitor. ACS Appl. Mater. Interfaces 2014, 6, 16312–16319. [Google Scholar] [CrossRef] [PubMed]
- Teng, C.; Xie, D.; Wang, J.F.; Yang, Z.; Ren, G.Y.; Zhu, Y. Ultrahigh conductive graphene paper based on ball-milling exfoliated graphene. Adv. Funct. Mater 2017, 27, 1700240. [Google Scholar] [CrossRef]
- Xu, X.T.; Yang, T.; Zhang, Q.W.; Xia, W.; Ding, Z.B.; Eid, K. Ultrahigh capacitive deionization performance by 3D interconnected MOF-derived nitrogen-doped carbon tubes. Chem. Eng. J. 2020, 390, 124493. [Google Scholar] [CrossRef]
- Huang, H.J.; Yan, M.M.; Yang, C.Z.; He, H.Y.; Jiang, Q.G.; Yang, L. Graphene nanoarchitectonics: Recent advances in graphene-based electrocatalysts for hydrogen evolution reaction. Adv. Mater 2019, 31, 1903415. [Google Scholar] [CrossRef]
- Liu, Y.H.; Zeng, J.; Han, D.; Wu, K.; Yu, B.W.; Chai, S.G. Graphene enhanced flexible expanded graphite film with high electric, thermal conductivities and EMI shielding at low content. Carbon 2018, 133, 435–445. [Google Scholar] [CrossRef]
- Xu, X.T.; Liu, Y.L.; Wang, M.; Zhu, C.; Lu, T.; Zhao, R.; Pan, L. Hierarchical hybrids with microporous carbon spheres decorated three-dimensional graphene frameworks for capacitive applications in supercapacitor and deionization. Electrochim. Acta. 2016, 193, 88–95. [Google Scholar] [CrossRef]
- Shi, Q.R.; Cha, Y.W.; Song, Y.; Lee, J.I.; Zhu, C.Z.; Li, X.Y. 3D graphene-based hybrid materials: Synthesis and applications in energy storage and conversion. Nanoscale 2016, 8, 15414–15447. [Google Scholar] [CrossRef]
- Peng, S.J.; Li, L.L.; Lee, J.K.Y.; Tian, L.L.; Srinivasan, M.; Adams, S. Electrospun carbon nanofibers and their hybrid composites as advanced materials for energy conversion and storage. Nano Energ. 2016, 22, 361–395. [Google Scholar] [CrossRef]
- Wang, R.H.; Xu, C.H.; Sun, J.; Gao, L. Three-dimensional Fe2O3 nanocubes/nitrogen-doped graphene aerogels: Nucleation mechanism and lithium storage properties. Sci. Rep. 2015, 4, 7171. [Google Scholar] [CrossRef]
- Wang, M.; Wang, G.; Wang, H. Flexible free-standing Fe2O3/graphene/carbon nanotubes hybrid filmsas anode materials for high performance lithium-ion batteries. Electrochimi. Acta. 2015, 182, 192–201. [Google Scholar] [CrossRef]
- Sahoo, M.; Sreena, K.P.; Vinayan, B.P.; Ramaprabhu, S. Green synthesis of boron doped graphene and its application as high performance anode material in Li ion battery. Mater. Res. Bull. 2015, 61, 383–390. [Google Scholar] [CrossRef]
- Liang, J.J.; Xu, Y.F.; Sui, D.; Zhang, L.; Huang, Y.; Ma, Y.F. Flexible, magnetic, and electrically conductive graphene/Fe3O4 paper and its application for magnetic-controlled switches. J. Phys. Chem. C 2010, 114, 17465–17471. [Google Scholar] [CrossRef]
- Yu, G.H.; Hu, L.B.; Vosgueritchian, M.; Wang, H.L.; Xie, X.; McDonough, J.R. Solution-processed graphene/MnO2 nanostructured textiles for high-performance electrochemical capacitors. Nano Lett. 2011, 11, 2905–2911. [Google Scholar] [CrossRef] [PubMed]
- Jaroonwatana, W.; Theerathanagorn, T.; Theerasilp, M.; Gobbo, S.; Yiamsawas, D. Nanoparticles of aromatic biopolymers catalyze CO2 cycloaddition to epoxides under atmospheric conditions. Sustain. Energ. Fuels 2021, 5, 5431–5444. [Google Scholar] [CrossRef]
- Hu, T.; Sun, X.; Sun, H.T.; Yu, M.P.; Lu, F.Y.; Liu, C.S. Flexible free-standing graphene-TiO2 hybrid paper for use as lithium ion battery anode materials. Carbon 2013, 51, 322–326. [Google Scholar] [CrossRef]
- Li, G.R.; Lei, W.; Luo, D.; Deng, Y.P.; Wang, D.L.; Chen, Z.W. 3D porous carbon sheets with multidirectional ion pathways for fast and durable lithium-sulfur batteries. Adv. Energy Mater. 2017, 8, 17023. [Google Scholar] [CrossRef]
- Hu, T.; Xie, M.; Zhong, J.; Sun, H.T.; Sun, X.; Scott, S. Porous Fe2O3 nanorods anchored onnitrogen-doped graphenes and ultrathin Al2O3 coating by atomic layer deposition for long-lived lithium ion battery anode. Carbon 2014, 76, 141–147. [Google Scholar] [CrossRef]
- Xiao, L.; Wu, D.Q.; Han, S.; Huang, Y.S.; Li, S.; He, M.Z. Self-assembled Fe₂O₃/graphene aerogel with high lithium storage performance. ACS Appl. Mater. Interfaces 2013, 5, 3764–3769. [Google Scholar] [CrossRef]
- Chen, D.Z.; Wei, W.; Wang, R.N.; Zhu, J.C.; Guo, L. α-Fe2O3 nanoparticles anchored on graphene with 3D quasi-laminated architecture: In situ wet chemistry synthesis and enhanced electrochemical performance for lithium ion batteries. New J. Chem. 2012, 36, 1589–1595. [Google Scholar] [CrossRef]
- Xue, X.Y.; Ma, C.H.; Cui, C.X.; Xing, L.L. High lithium storage performance of α-Fe2O3/graphene nanocomposites as lithium-ion battery anodes. Solid State Sci. 2011, 13, 1526–1530. [Google Scholar] [CrossRef]
- Xiao, W.; Wang, Z.X.; Guo, H.J.; Li, X.H.; Wang, J.X.; Huang, S.L. Fe2O3 particles enwrapped by graphene with excellent cyclability and rate capability as anode materials for lithium ion batteries. Appl. Surf. Sci. 2013, 266, 148–154. [Google Scholar] [CrossRef]
- Lian, X.B.; Cai, M.C.; Qin, L.L.; Cao, Y.; Wu, Q.H. Synthesis of hierarchical nanospheres Fe2O3 /graphene composite and its application in lithium-ion battery as a high-performance anode material. Ionics 2016, 22, 2015–2020. [Google Scholar] [CrossRef]
- Petnikota, S.; Marka, S.K.; Banerjee, A.; Reddy, M.V.; Srikanth, V.V.S.S.; Chowdari, B.V.R. Graphenothermal reduction synthesis of ‘exfoliated graphene oxide/iron (II) oxide’ composite for anode application in lithium ion batteries. J. Power Sources 2015, 293, 253–263. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Y.; Sun, C.C.; Liu, H.Z.; Li, L.Q.; Si, W.L. Graphene and cobalt phosphide nanowire composite as an anode material for high performance lithium-ion batteries. Nano. Res. 2016, 9, 612–621. [Google Scholar] [CrossRef]
- Ma, L.; Zhou, X.P.; Xu, L.M.; Xu, X.Y.; Zhang, L.L.; Chen, W.X. Ultrathin few-layered molybdenum selenide/graphene hybrid with superior electrochemical Li-storage performance. J. Power Sources 2015, 285, 274–280. [Google Scholar] [CrossRef]



| Active Material | Current Rate (mA·g−1) | Initial Capacity (mAh·g−1) | Reversible Capacity (mAh·g−1) | Cycle No. | Ref. |
|---|---|---|---|---|---|
| Graphene-Fe2O3 sheet anode of our work | 200 | 1466 | 765 | 100 | - |
| Micro-sized Fe2O3 spheres doped with graphene | 160 | 1800 | 660 | 100 | [28] |
| α-Fe2O3/graphene with 3D quasilaminated architecture | 100 | 1719 | 742 | 50 | [29] |
| α-Fe2O3/graphene nanocomposites | 200 | 1029 | 570 | 20 | [30] |
| Fe2O3 particles enwrapped by graphene | 50 | 1544 | 626 | 50 | [31] |
| Hierarchically nanospherical α-Fe2O3/graphene | 100 | 1442 | 1024 | 50 | [32] |
| Sample | Rs (Ω) | R1 (Ω) | CPE1 | R2 (Ω) | CPE2 | ||
|---|---|---|---|---|---|---|---|
| - | - | - | Y | n | - | Y | n |
| graphene-Fe2O3 paper | 2.54 | 22.89 | 9.6 × 10−5 | 0.84 | 45.36 | 4.6 × 10−5 | 0.65 |
| Fe2O3 | 4.58 | 89.26 | 1.1 × 10−4 | 0.92 | 67.58 | 5.4 × 10−5 | 0.67 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, C.; Xiao, W.; Ren, S.; Li, Q. Flexible Free-Standing Graphene-Fe2O3 Hybrid Paper with Enhanced Electrochemical Performance for Rechargeable Lithium-Ion Batteries. Coatings 2022, 12, 1726. https://doi.org/10.3390/coatings12111726
Yang C, Xiao W, Ren S, Li Q. Flexible Free-Standing Graphene-Fe2O3 Hybrid Paper with Enhanced Electrochemical Performance for Rechargeable Lithium-Ion Batteries. Coatings. 2022; 12(11):1726. https://doi.org/10.3390/coatings12111726
Chicago/Turabian StyleYang, Chuanning, Wangchuan Xiao, Shizhao Ren, and Qiyong Li. 2022. "Flexible Free-Standing Graphene-Fe2O3 Hybrid Paper with Enhanced Electrochemical Performance for Rechargeable Lithium-Ion Batteries" Coatings 12, no. 11: 1726. https://doi.org/10.3390/coatings12111726
APA StyleYang, C., Xiao, W., Ren, S., & Li, Q. (2022). Flexible Free-Standing Graphene-Fe2O3 Hybrid Paper with Enhanced Electrochemical Performance for Rechargeable Lithium-Ion Batteries. Coatings, 12(11), 1726. https://doi.org/10.3390/coatings12111726




