Effect of Dispersing Agents on the Stability of Recycled Paints
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Samples Preparation
2.1.1. Recycled Paints
2.1.2. Dispersing Agents
2.1.3. Addition of Dispersing Agents in Paints
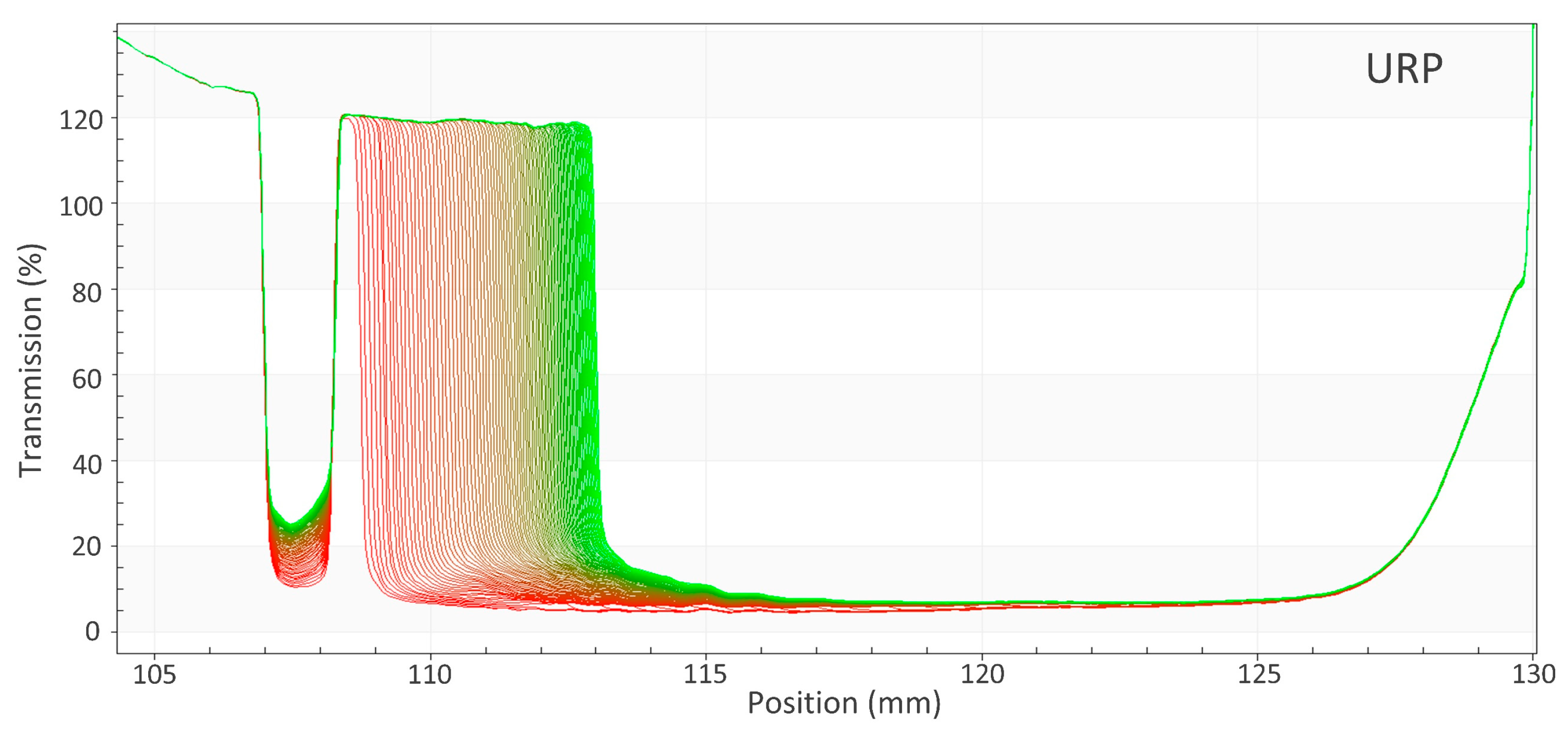
2.2. Measurement of the Instability Index
- 10 measurements with an interval of 10 s between each measurement;
- 10 measurements with an interval of 30 s between each measurement;
- 101 measurements with an interval of 210 s between each measurement.
2.3. Evaluation of Supernatant Height
3. Results
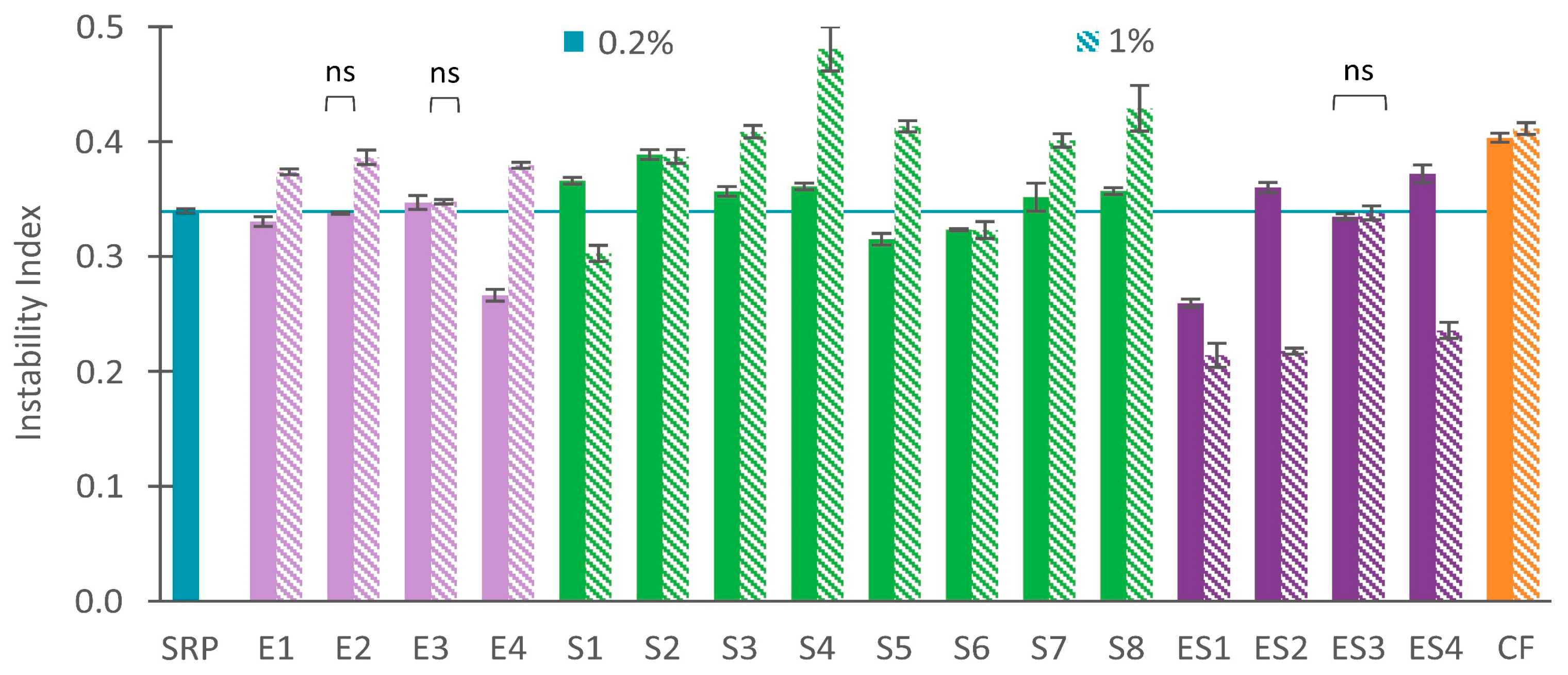
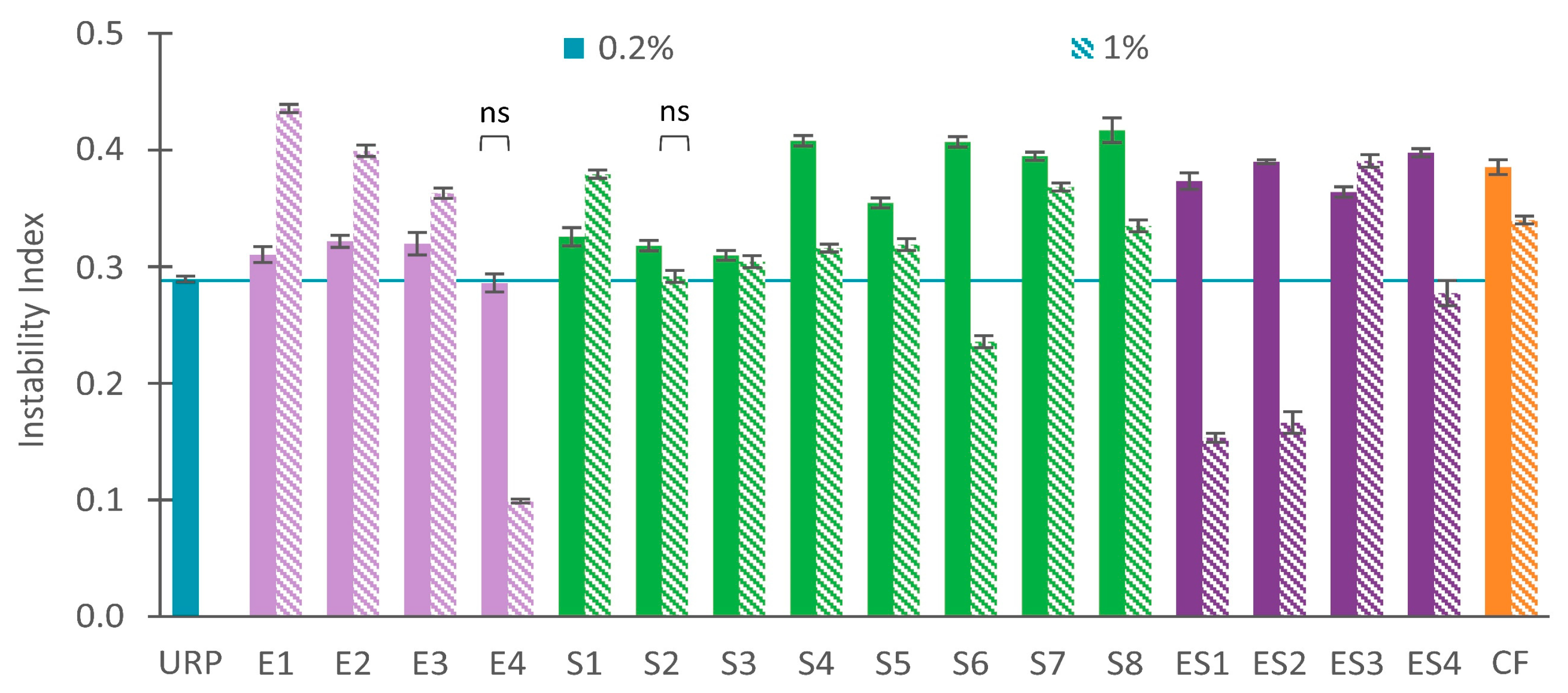
3.1. Instability Index
3.1.1. Stable Recycled Paint with Dispersing Agents
3.1.2. Unstable Recycled Paint with Dispersing Agents
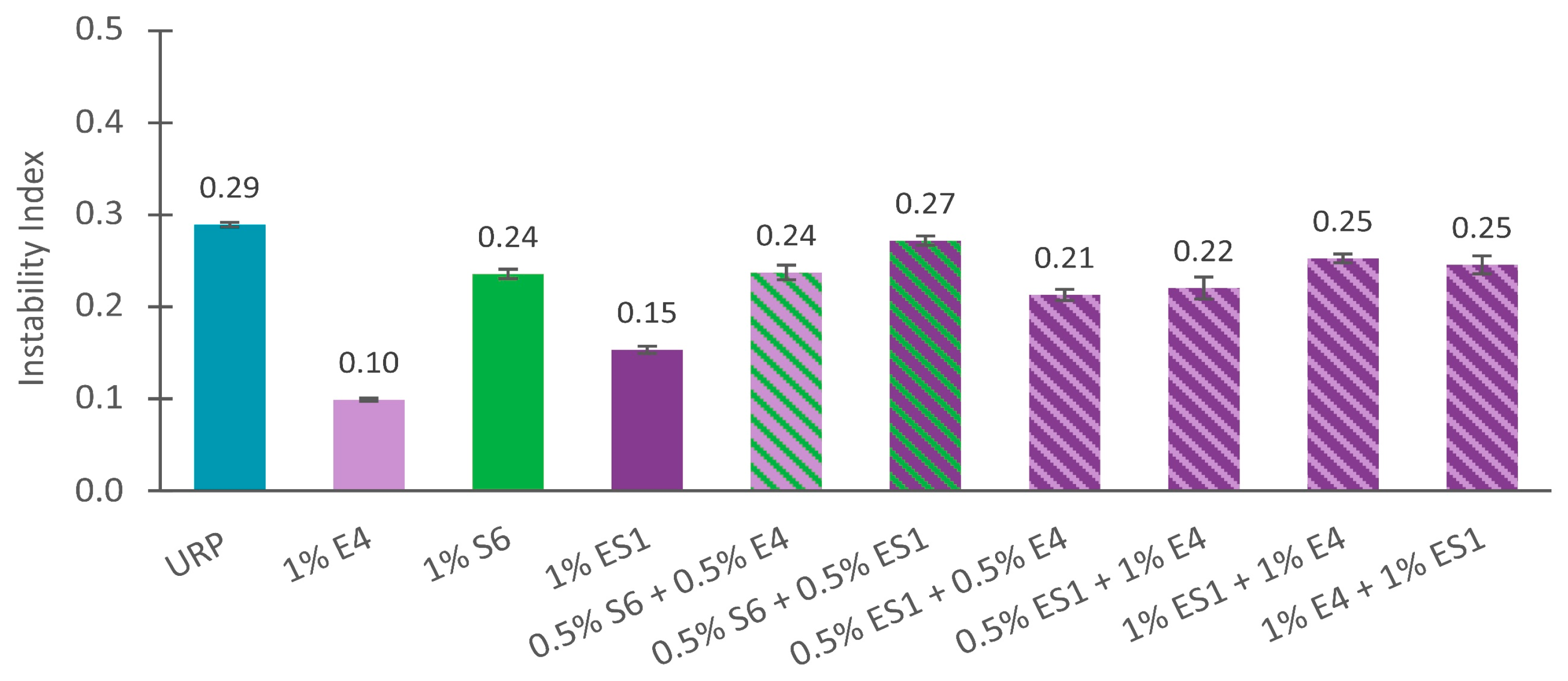
3.1.3. Combining Dispersing Agents
3.2. Evaluation of the Supernatant at 25 °C
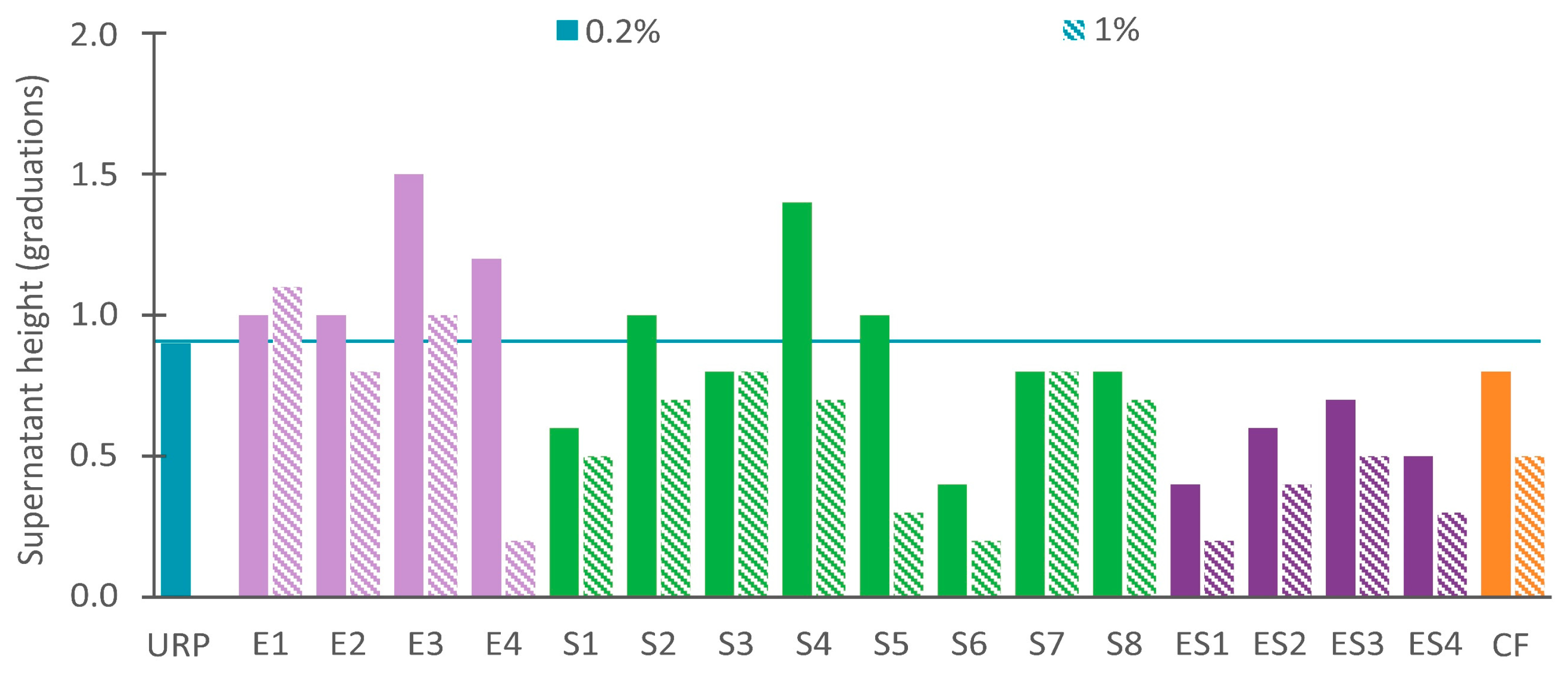
3.2.1. Stable Recycled Paint with Dispersing Agents
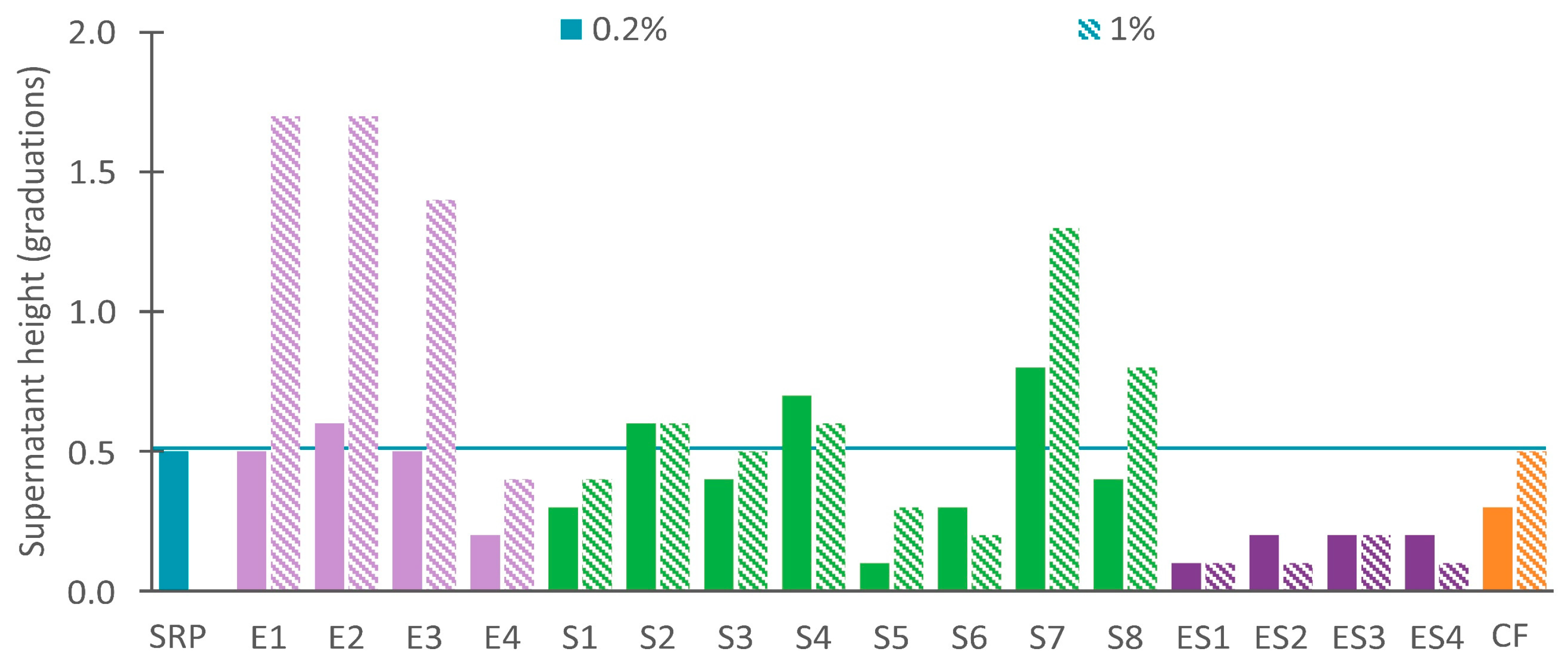
3.2.2. Unstable Recycled Paint with Dispersing Agents
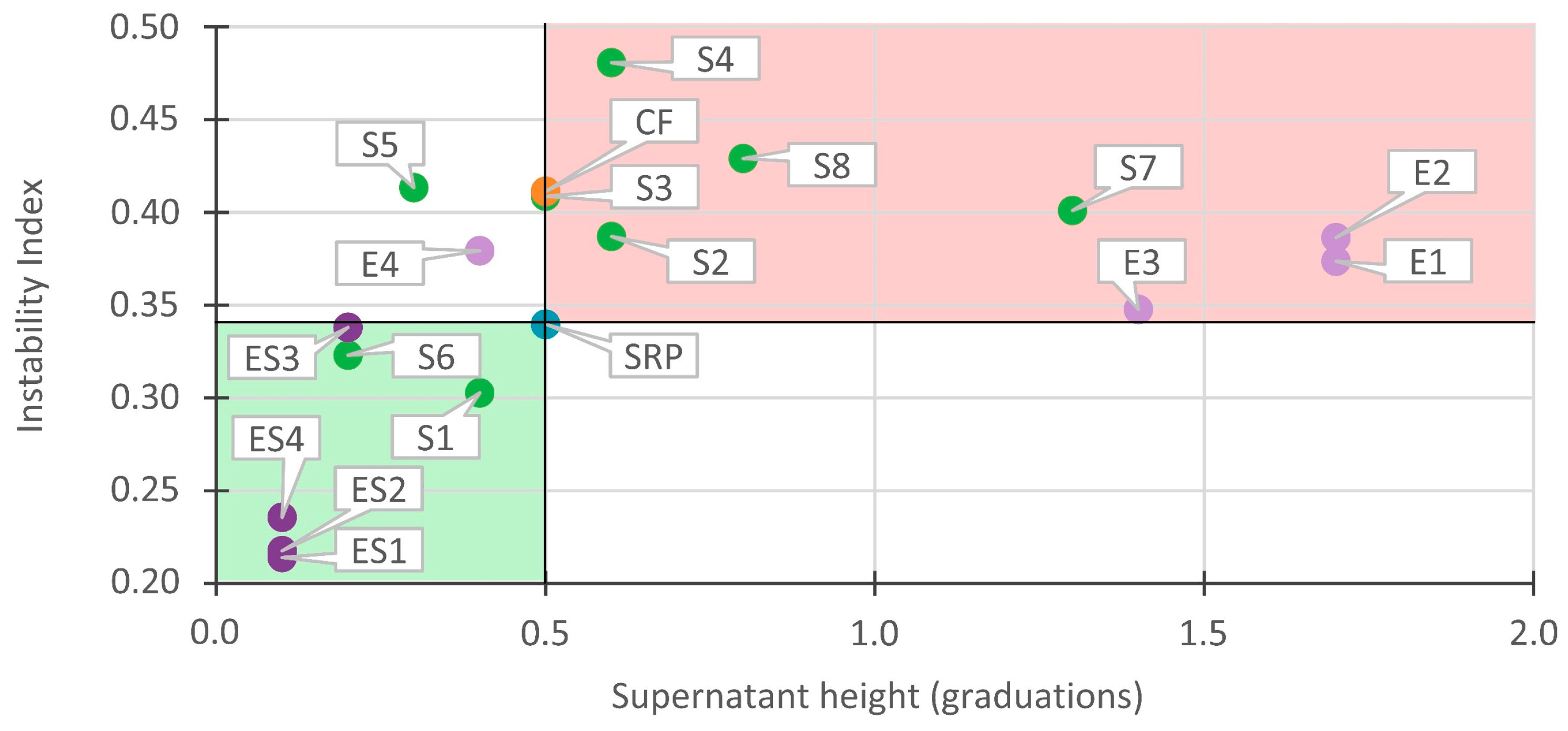
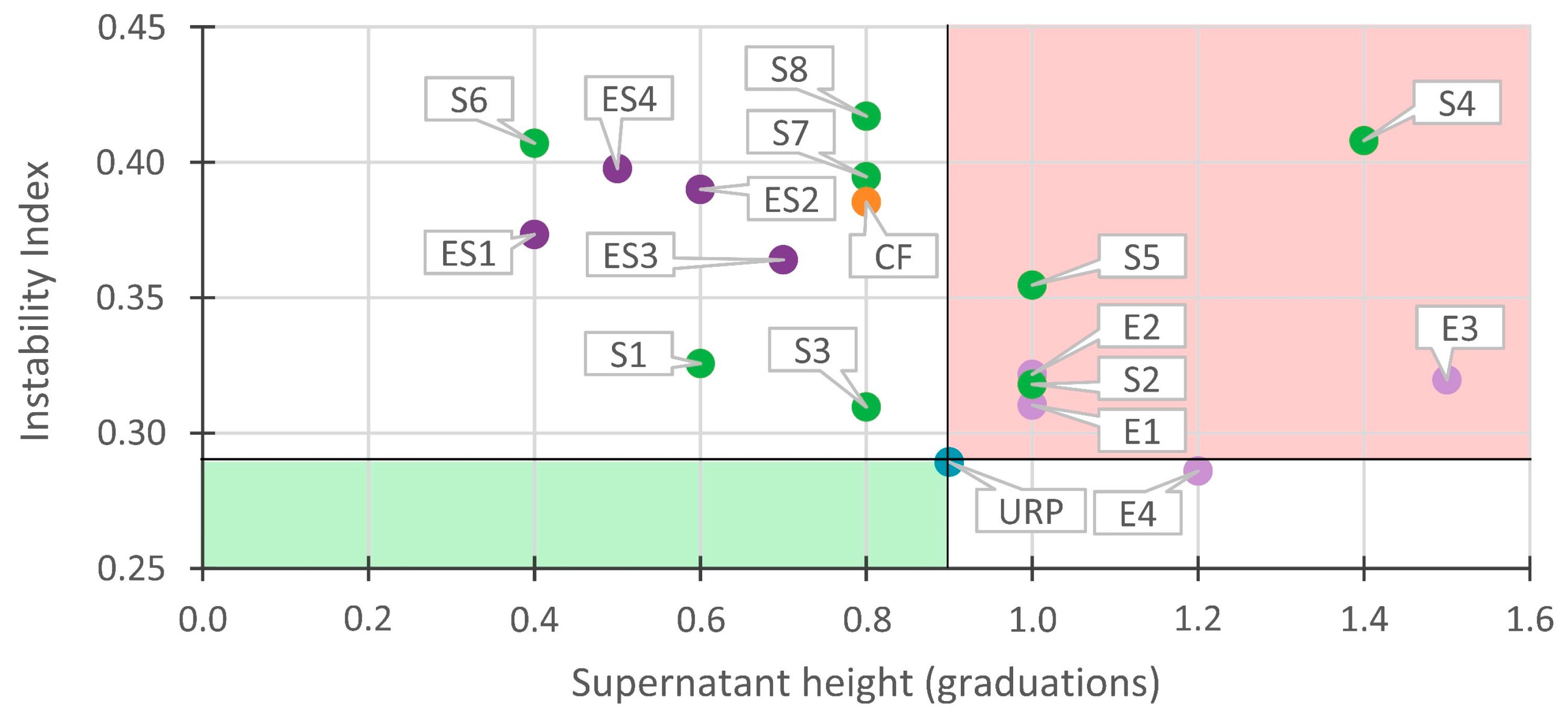
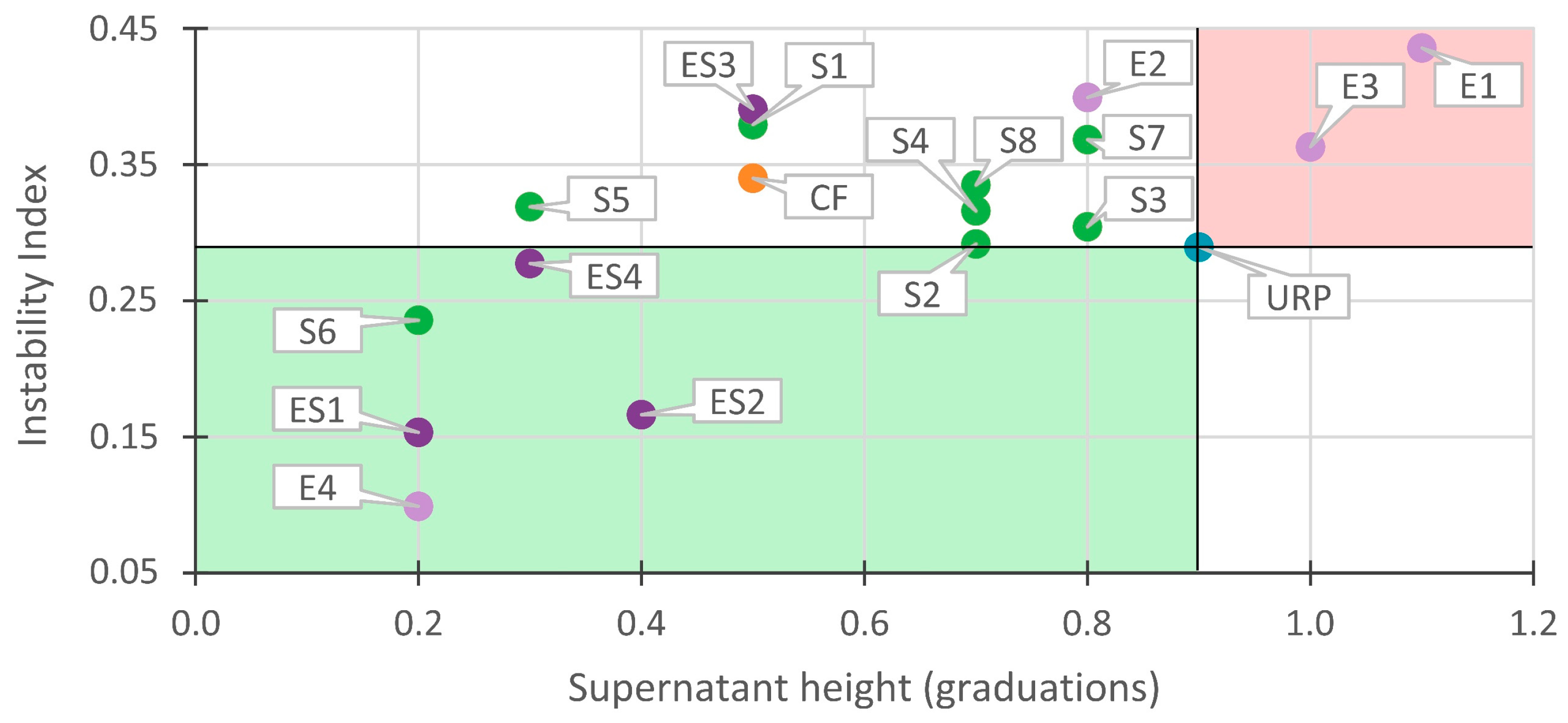
3.3. Correlations between Instability Index and Supernatant Height
3.3.1. Correlations in Stable Recycled Paint
3.3.2. Correlations in Unstable Recycled Paint
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A

References
- A Circular Economy for Paints. Available online: https://www.rsc.org/new-perspectives/sustainability/recycling-paints/ (accessed on 30 September 2022).
- Royal Society of Chemistry. Polymers in Liquid Formulations Technical Report: A Landscape View of the Global PLFs Market; RSC: London, UK, 2021. [Google Scholar]
- Tadros, T.F. Emulsion Formation, Stability, and Rheology. In Emulsion Formation and Stability; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2013; pp. 1–75. [Google Scholar]
- Mc Clements, D.J. Emulsion Stability. In Food Emulsions Principles, Practices, and Techniques; CRC Press: Boca Raton, MA, USA, 2005; pp. 269–340. [Google Scholar]
- Dorst, K.; Stewart, S.; Staudinger, I.; Paton, B. Food Emulsions, 4th ed.; Marcel Dekker: New York, NY, USA, 2004. [Google Scholar]
- Goldschmidt, A.; Streitberger, H.-J. Basics of Coating Technology, 3rd ed.; Vincentz Network: Münster, Germany, 2018. [Google Scholar]
- Schramm, L.L. Emulsions, Foams, and Suspensions: Fundamentals and Applications; Wiley-VCH: Weinheim, Germany, 2005. [Google Scholar]
- Eastman, J. Colloid Stability. In Colloid Science—Principles, Methods and Applications; Blackwell Publishing: Bristol, UK, 2005; pp. 36–49. [Google Scholar]
- Holmberg, K. Applications of Surfactants in Paints. In Surfactants in Polymers, Coatings, Inks and Adhesives; Blackwell Publishing: Boca Raton, MA, USA, 2003; pp. 152–179. [Google Scholar]
- Müller, B. Wetting and Dispersing Agents. In Understanding Additives; Vincentz Network: Hanover, Germany, 2010; pp. 11–41. [Google Scholar]
- Evonik Industries. AG The Big TEGO; Evonik Industries: Essen, Germany, 2012. [Google Scholar]
- Kleinsteinberg, K. Wetting and Dispersing Additives. In Additives for Waterborne Coatings; Vincentz Network: Hanover, Germany, 2009; pp. 20–38. [Google Scholar]
- BYK Additives & Instruments. Wetting and Dispersing Additives; BYK-Chemie GmbH: Wesel, Germany, 2009. [Google Scholar]
- Goodarzi, F.; Zendehboudi, S. A Comprehensive Review on Emulsions and Emulsion Stability in Chemical and Energy Industries. Can. J. Chem. Eng. 2019, 97, 281–309. [Google Scholar] [CrossRef]
- Tadros, T.F. Rheology of Paints. In Colloids in Paints; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2010; pp. 211–241. [Google Scholar]
- Lerche, D.; Sobisch, T. Direct and Accelerated Characterization of Formulation Stability. J. Dispers. Sci. Technol. 2011, 32, 1799–1811. [Google Scholar] [CrossRef]
- Chevalier, Y. Granulométrie, Morphologie et Structure. In Les Latex Synthétiques; Lavoisier: Paris, France, 2006; pp. 47–81. [Google Scholar]
- Lerche, D. Characterization of Nano-and Microparticles, and Dispersion Stability by in-Situ Visualization of Separation Behaviour. Dispers. Lett. 2019, 10, 1–15. [Google Scholar]
- Detloff, T.; Sobisch, T.; Lerche, D. Particle Size Distribution by Space or Time Dependent Extinction Profiles Obtained by Analytical Centrifugation. Part. Part. Syst. Charact. 2006, 23, 184–187. [Google Scholar] [CrossRef]
- Detloff, T.; Sobisch, T.; Lerche, D. Instability Index. Dispers. Lett. Tech. 2013, 4, 1–4. [Google Scholar]
- Petzold, G.; Goltzsche, C.; Mende, M.; Schwarz, S.; Jaeger, W. Monitoring the Stability of Nanosized Silica Dispersions in Presence of Polycations by a Novel Centrifugal Sedimentation Method. J. Appl. Polym. Sci. 2009, 114, 696–704. [Google Scholar] [CrossRef]
- Schwarz, S.; Petzold, G.; Bhatti, Q. Stability for Dispersions. Eur. Coat. J. 2010, 8, 33–37. [Google Scholar]
- ISO/TR 13097; International Organization for Standardization Guidelines for the Characterization of Dispersion Stability. 2013. Available online: https://www.iso.org/standards.html (accessed on 11 October 2022).
- Mikkonen, K.S.; Kirjoranta, S.; Xu, C.; Hemming, J.; Pranovich, A.; Bhattarai, M.; Peltonen, L.; Kilpeläinen, P.; Maina, N.; Tenkanen, M.; et al. Industrial Crops & Products Environmentally-Compatible Alkyd Paints Stabilized by Wood Hemicelluloses. Ind. Crop. Prod. 2019, 133, 212–220. [Google Scholar] [CrossRef]
- Piech, M.; Walz, J.Y. Depletion Interactions Produced by Nonadsorbing Charged and Uncharged Spheroids. J. Colloid Interface Sci. 2000, 232, 86–101. [Google Scholar] [CrossRef] [PubMed]
- Croll, S. DLVO Theory Applied to TiO2 Pigments and Other Materials in Latex Paints. Prog. Org. Coat. 2002, 44, 131–146. [Google Scholar] [CrossRef]
- Wijting, W.K.; van Reenen, A.; Laven, J.; van Benthem, R.A.T.M.; de With, G. Competitive Adsorption of (Phosphorylated) Ethoxylated Styrene Oxide Polymer and Polyacrylic Acid on Silica Coated Iron Oxide Pigment. Colloids Surf. A Physicochem. Eng. Asp. 2014, 449, 19–30. [Google Scholar] [CrossRef]
- Lerche, D. Dispersion Stability and Particle Characterization by Sedimentation Kinetics in a Centrifugal Field. J. Dispers. Sci. Technol. 2002, 23, 699–709. [Google Scholar] [CrossRef]
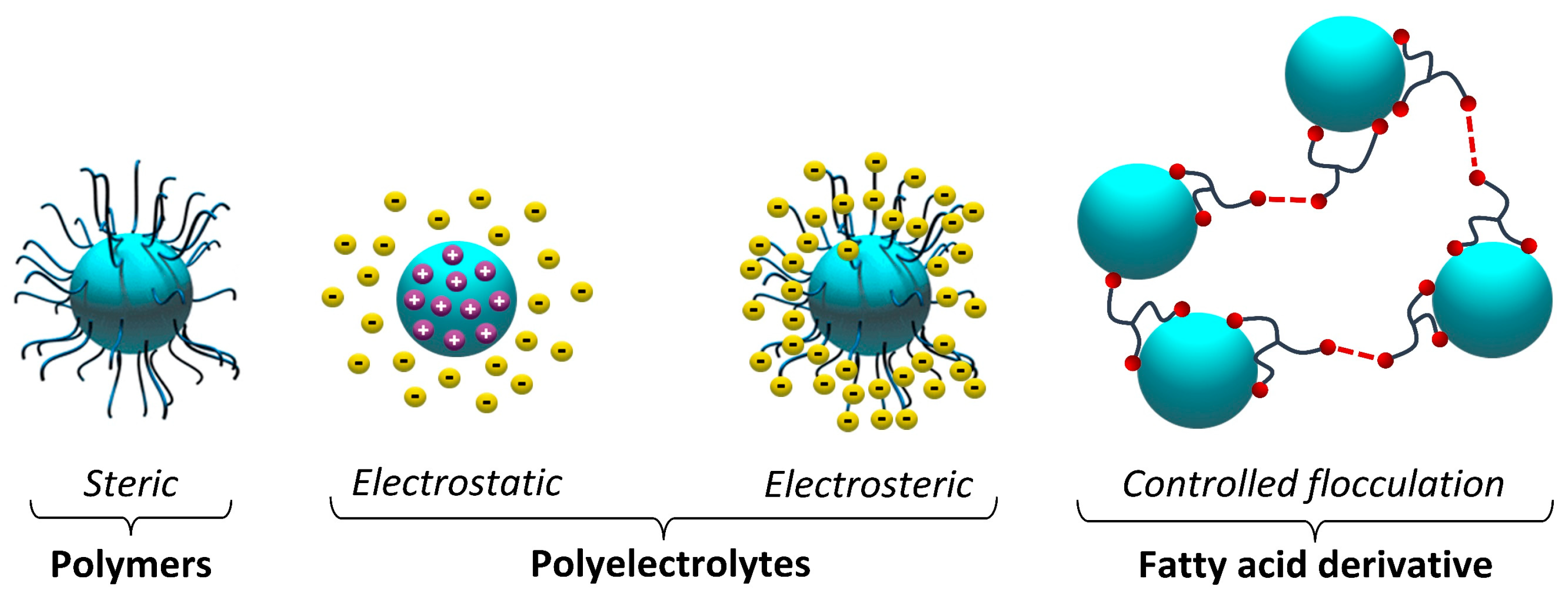


| Electrostatic Dispersing Agents | Chemical Structures | Pigment-Affinic Groups |
|---|---|---|
| E1 | Sodium polyacrylate | No |
| E2 | Ammonium polyacrylate | No |
| E3 | Copolymer of potassium acrylate and acrylic ester | No |
| E4 | Hydrophobically modified potassium polyacrylate | Yes |
| Steric Dispersing Agents | Chemical Structures | Pigment-Affinic Groups |
|---|---|---|
| S1 | Polyether functionalized by phosphoric acid | Yes |
| S2 | Copolymer based on polyether | Yes |
| S3 | Copolymer based on polyether | Yes |
| S4 | Copolymer based on polyether | Yes |
| S5 | Phenol ethoxylate | Yes |
| S6 | Alcohol ethoxylate | Yes |
| S7 | Nonionic polymer | No |
| S8 | Copolymer based on polyether | Yes |
| Electrosteric Dispersing Agents | Chemical Structures | Pigment-Affinic Groups |
|---|---|---|
| ES1 | Polyfunctional acrylate copolymer | Yes |
| ES2 | Acrylate copolymer | Yes |
| ES3 | Acrylate copolymer | Yes |
| ES4 | Acrylate copolymer | Yes |
| Controlled Flocculation Dispersing Agent | Chemical Structure | Pigment-Affinic Group |
|---|---|---|
| FC | Fatty acid derivative | Yes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jacob, J.; Grelier, S.; Grau, M.; Chorein, B. Effect of Dispersing Agents on the Stability of Recycled Paints. Coatings 2022, 12, 1722. https://doi.org/10.3390/coatings12111722
Jacob J, Grelier S, Grau M, Chorein B. Effect of Dispersing Agents on the Stability of Recycled Paints. Coatings. 2022; 12(11):1722. https://doi.org/10.3390/coatings12111722
Chicago/Turabian StyleJacob, Jessie, Stéphane Grelier, Maïlys Grau, and Blandine Chorein. 2022. "Effect of Dispersing Agents on the Stability of Recycled Paints" Coatings 12, no. 11: 1722. https://doi.org/10.3390/coatings12111722
APA StyleJacob, J., Grelier, S., Grau, M., & Chorein, B. (2022). Effect of Dispersing Agents on the Stability of Recycled Paints. Coatings, 12(11), 1722. https://doi.org/10.3390/coatings12111722






