A Novel Simple Fabrication Method for Mechanically Robust Superhydrophobic 2024 Aluminum Alloy Surfaces
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
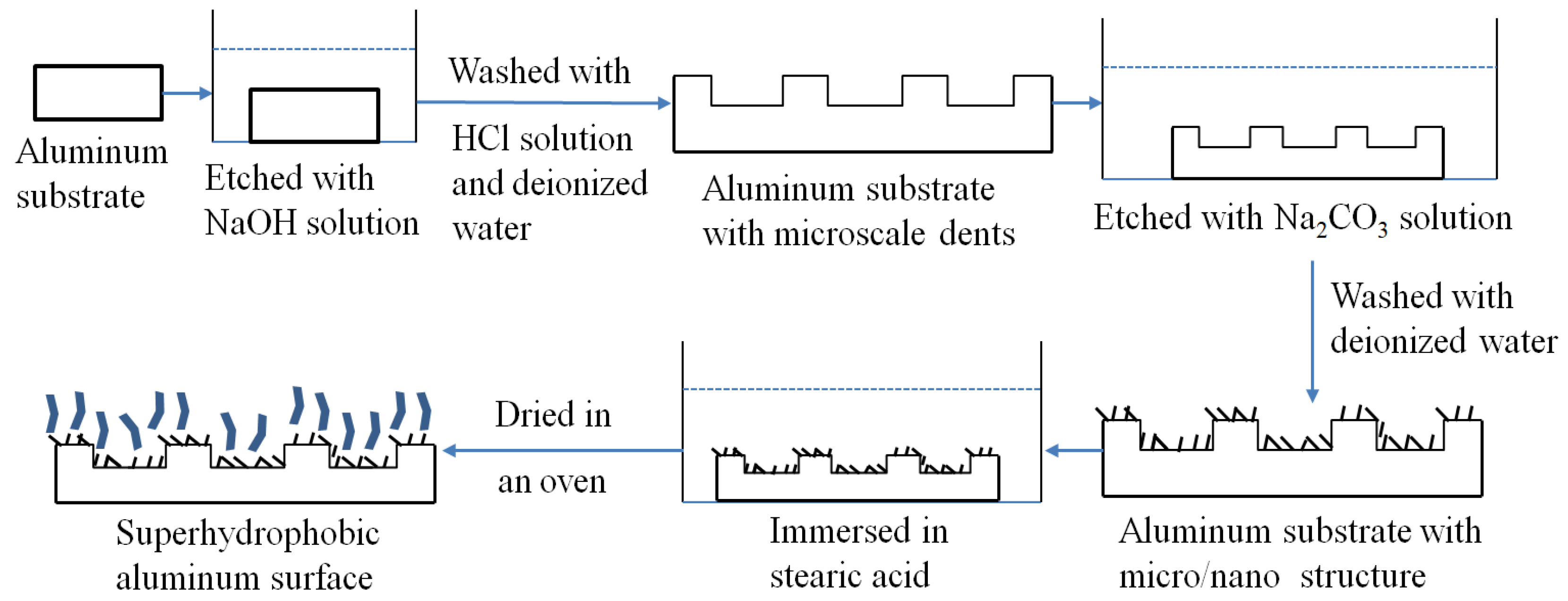
2.2. Preparation of the Superhydrophobic 2024 Aluminum Alloy Surface
2.3. Characterisation of As-Prepared Superhydrophobic Surface
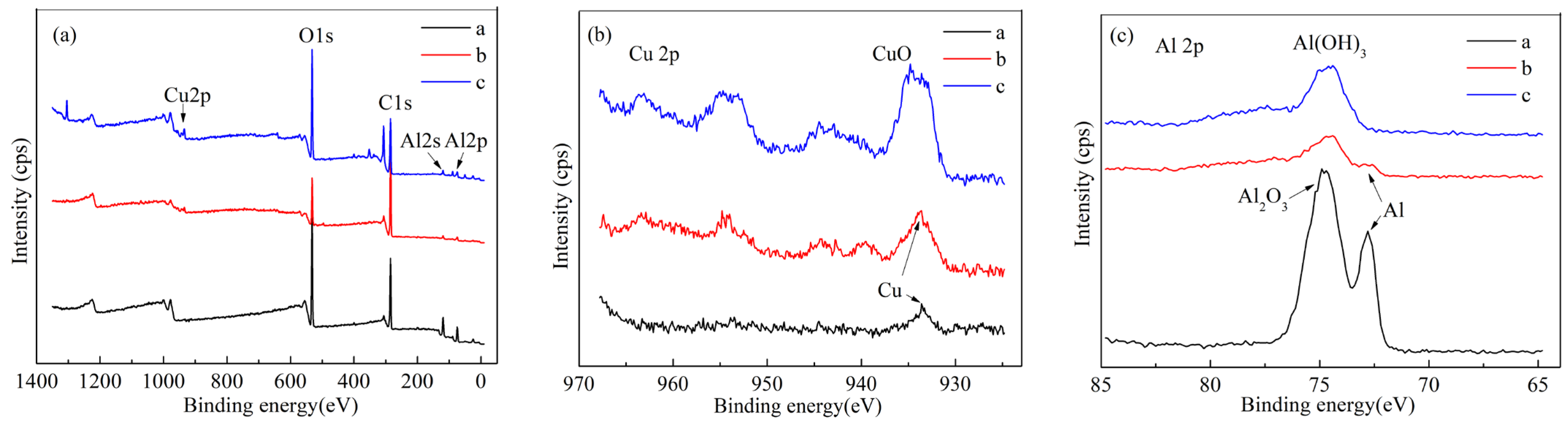
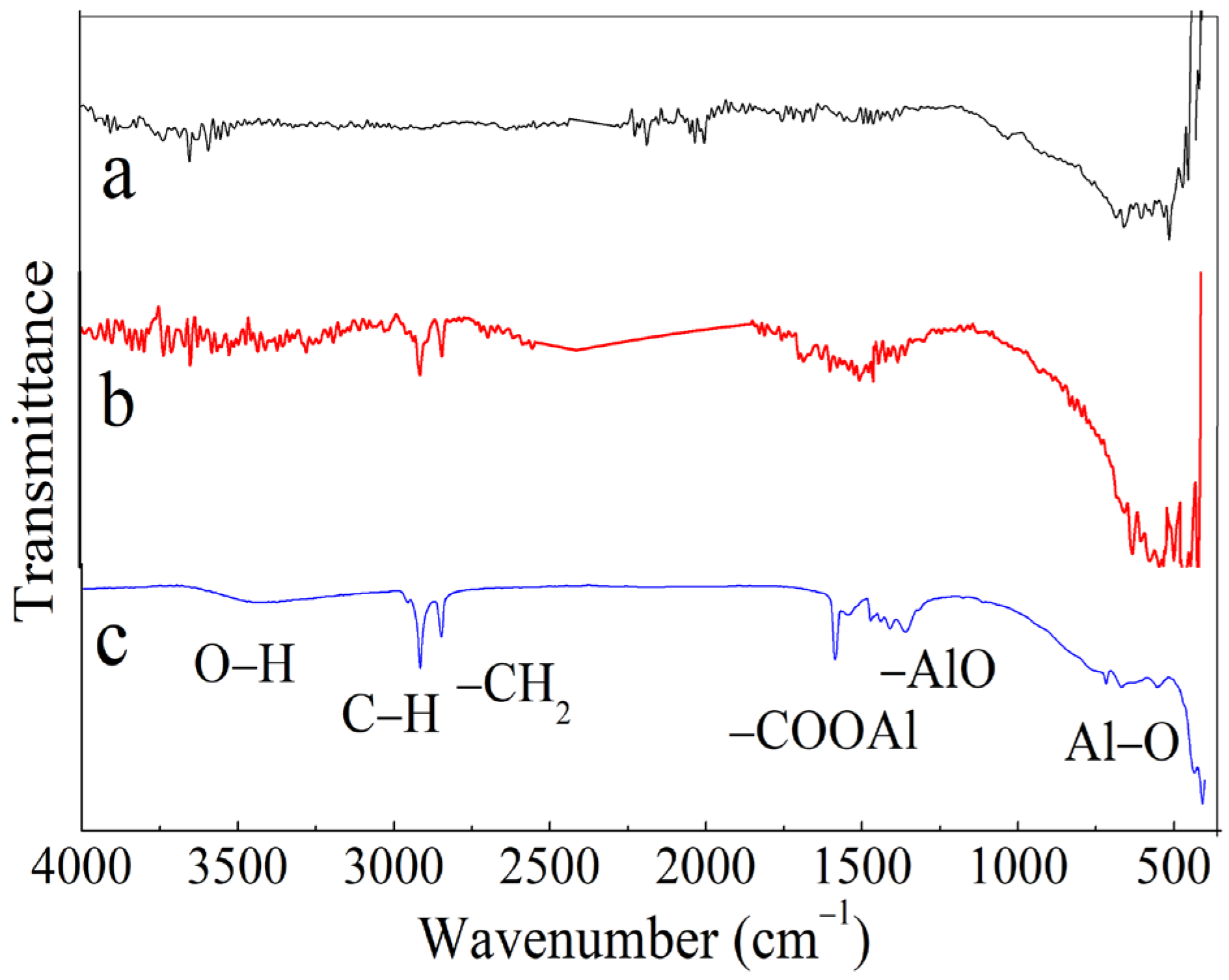
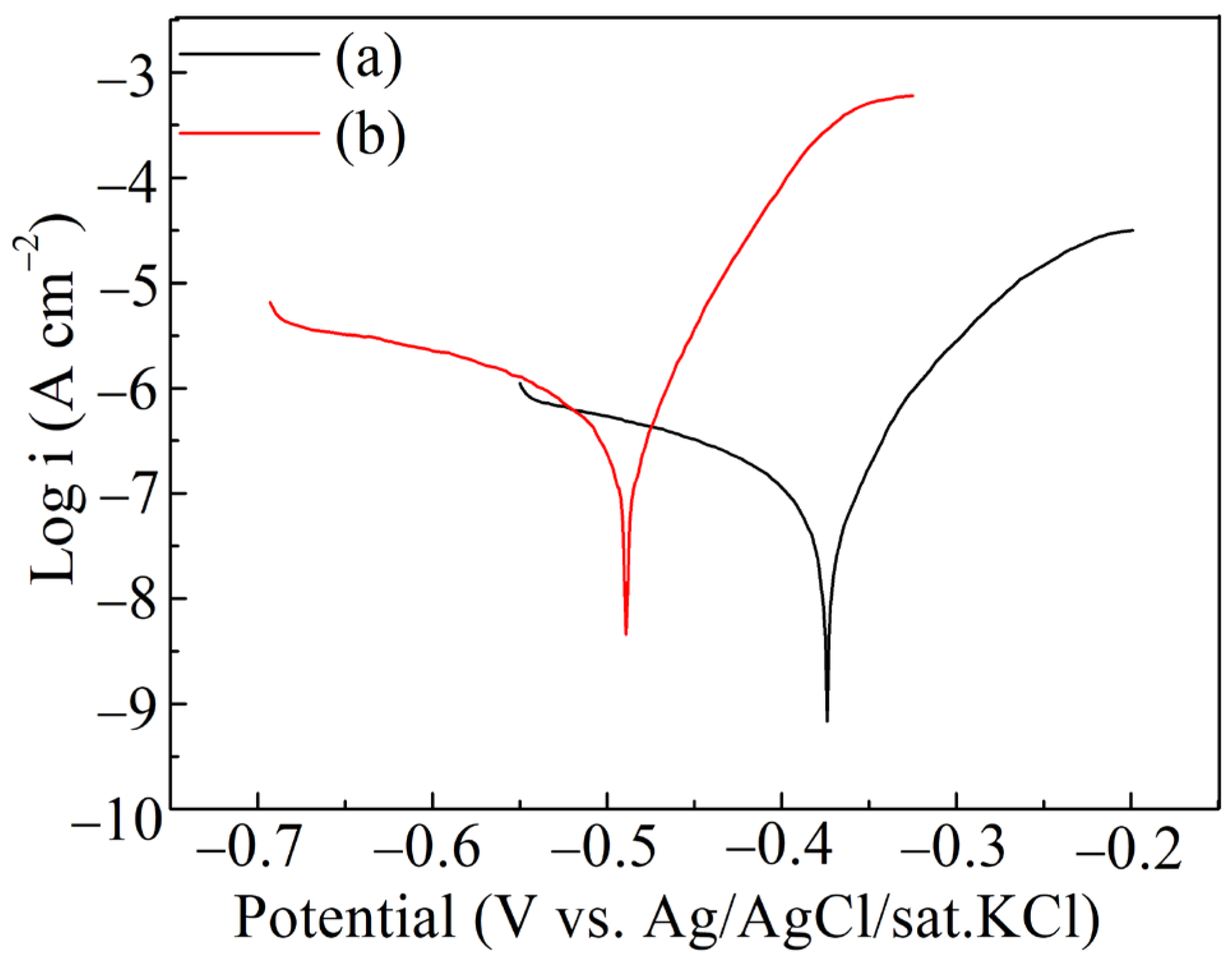
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Georgantzia, E.; Gkantou, M.; Kamaris, G.S. Aluminium alloys as structural material: A review of research. Eng. Struct. 2021, 227, 111372. [Google Scholar] [CrossRef]
- Canepa, E.; Stifanese, R.; Merotto, L.; Traverso, P. Corrosion behaviour of aluminium alloys in deep-sea environment: A review and the KM3NeT test results. Mar. Struct. 2018, 59, 271–284. [Google Scholar] [CrossRef]
- Critchlow, G.W.; Brewis, D.M. Review of surface pretreatments for aluminium alloys. Int. J. Adhes. Adhes. 1996, 16, 255–275. [Google Scholar] [CrossRef]
- Vazirinasab, E.; Jafari, R.; Momen, G. Application of superhydrophobic coatings as a corrosion barrier: A review. Surf. Coat. Technol. 2018, 34, 40–56. [Google Scholar] [CrossRef]
- Ran, M.R.; Zheng, W.Y.; Wang, H.M. Fabrication of superhydrophobic surfaces for corrosion protection: A review. Mater. Sci. Technol. 2019, 35, 313–326. [Google Scholar] [CrossRef]
- Sataeva, N.E.; Boinovich, L.B.; Emelyanenko, K.A.; Domantovsky, A.G.; Emelyanenko, A.M. Laser-assisted processing of aluminum alloy for the fabrication of superhydrophobic coatings withstanding multiple degradation factors. Surf. Coat. Technol. 2020, 397, 125993. [Google Scholar] [CrossRef]
- Milles, S.; Dahms, J.; Soldera, M.; Lasagni, A.F. Stable superhydrophobic aluminium surfaces based on laser-fabricated hierarchical textures. Materials 2021, 14, 184. [Google Scholar] [CrossRef]
- Li, X.J.; Yin, S.H.; Luo, H. Fabrication of robust superhydrophobic Ni-SiO2 composite coatings on aluminium alloy surfaces. Vacuum 2020, 181, 109674. [Google Scholar] [CrossRef]
- Zhu, G.; Zhao, Y.; Liu, L.; Wang, L.Y.; Wang, J.; Yu, S.R. Facile fabrication and evaluation of self-healing Zn-Al layered double hydroxide superhydrophobic coating on aluminum alloy. J. Mater. Sci. 2021, 56, 14803–14820. [Google Scholar] [CrossRef]
- Zhu, G.; Liu, E.Y.; Liu, L.; Wang, L.Y.; Wang, J.; Li, Q.; Yu, S.R.; Wang, B.Y.; Yang, X.Z. Preparation of gahnite (ZnAl2O4) superhydrophobic coating on aluminium alloy with self-healing and high stability properties. Colloid Surface A 2022, 634, 127977. [Google Scholar] [CrossRef]
- Sun, R.Y.; Zhao, J.; Li, Z.; Qin, N.; Mo, J.L.; Pan, Y.J.; Luo, D.B. Robust superhydrophobic aluminum alloy surfaces with anti-icing ability, thermostability, and mechanical durability. Prog. Org. Coat. 2020, 147, 105745. [Google Scholar] [CrossRef]
- Barthwal, S.; Lim, S.H. Robust and chemically stable superhydrophobic aluminum alloy surface with enhanced corrosion resistance properties. Int. J. Precis. Eng. Manuf.-Green Technol. 2020, 7, 481–492. [Google Scholar] [CrossRef]
- Cui, C.Y.; Cao, Y.F.; Qi, B.J.; Wei, J.J.; Yuan, J.; Wang, Y. Convenient and large-scale fabrication of cost-effective superhydrophobic aluminum alloy surface with excellent reparability. Langmuir 2021, 37, 7810–7820. [Google Scholar] [CrossRef]
- Wu, S.; Wu, R.M.; Jiang, H.Y.; Yuan, Z.Q.; Chen, Q.H. Preparation and characterization of superhydrophobic silane-based multilayer surface coatings on aluminum surface. J. Mater. Eng. Perform. 2022, 31, 3611–3620. [Google Scholar] [CrossRef]
- Cho, H.D.; Kim, D.S.; Lee, C.W.; Hwang, W.B. A simple fabrication method for mechanically robust superhydrophobic surface by hierarchical aluminum hydroxide structures. Curr. Appl. Phys. 2013, 13, 762–767. [Google Scholar] [CrossRef]
- Zhang, D.W.; Wang, L.T.; Qian, H.C.; Li, X.G. Superhydrophobic surfaces for corrosion protection: A review of recent progresses and future directions. J. Coat. Technol. Res. 2016, 13, 11–29. [Google Scholar] [CrossRef]
- Balitskii, O.I.; Pokhmurs’kii, V.I.; Tikhan, M.O. Laser treatment of plasma coatings. Sov. Mater. Sci. 1991, 27, 51–55. [Google Scholar] [CrossRef]
- Huang, C.; Ye, X.; Yang, X.H.; Yao, A.F.; Fan, Z.M. Preparation of a superhydrophobic aluminium alloy surface by uv-laser. Surf. Eng. 2020, 36, 558–564. [Google Scholar] [CrossRef]
- Guo, Y.G.; Zhang, X.; Wang, X.C.; Xu, Q.; Geng, T. Wetting and tribological properties of superhydrophobic aluminum surfaces with different water adhesion. J. Mater. Sci. 2020, 55, 11658–11668. [Google Scholar] [CrossRef]
- Milles, S.; Soldera, M.; Kuntze, T.; Lasagni, A.F. Characterization of self-cleaning properties on superhydrophobic aluminium surfaces fabricated by direct laser writing and direct laser interference patterning. Appl. Surf. Sci. 2020, 525, 146818. [Google Scholar] [CrossRef]
- Volpe, A.; Gaudiuso, C.; Di Venere, L.; Licciulli, F.; Giordano, F.; Ancona, A. Direct femtosecond laser fabrication of superhydrophobic aluminium alloy surfaces with anti-icing properties. Coatings 2020, 10, 587. [Google Scholar] [CrossRef]
- Kuznetsov, G.V.; Feoktistov, D.V.; Orlova, E.G.; Zykov, I.Y.; Bartuli, E.; Raudenský, M.; Zhuikov, A.V. Dynamic characteristics of water spreading over laser-textured aluminium alloy surfaces. Colloid Surf. A 2020, 603, 125253. [Google Scholar] [CrossRef]
- Volpe, A.; Covella, S.; Gaudiuso, C.; Ancona, A. Improving the laser texture strategy to get superhydrophobicaluminum alloy surfaces. Coatings 2021, 11, 369. [Google Scholar] [CrossRef]
- Tong, W.; Cui, L.L.; Qiu, R.X.; Yan, C.Q.; Liu, Y.T.; Wang, N.; Xiong, D.S. Laser textured dimple-patterns to govern the surface wettability of superhydrophobic aluminum plates. J. Mater. Sci. Technol. 2021, 89, 59–67. [Google Scholar] [CrossRef]
- Lian, Z.X.; Xu, J.K.; Yu, P.; Yu, Z.J.; Wang, Z.B.; Yu, H.D. Oil repellent and corrosion resistance properties of superhydrophobic and superoleophobic aluminium alloy surfaces based on nanosecond laser textured treatment. Met. Mater. Int. 2020, 26, 1603–1620. [Google Scholar] [CrossRef]
- Zaitsev, D.A.; Batishcheva, K.A.; Kuznetsov, G.V.; Orlova, E.G. Prediction of water droplet behavior on aluminum alloy surfaces modified by nanosecond laser pulses. Surf. Coat. Technol. 2020, 399, 126206. [Google Scholar] [CrossRef]
- Zhou, X.K.; Xue, W.; Liu, W.W.; Zhu, D.H.; Cao, Y. Quadri-directionally anisotropic droplets sliding surfaces fabricated by selective laser texturing of aluminum alloy plates. Appl. Surf. Sci. 2020, 509, 145406. [Google Scholar] [CrossRef]
- Rico, V.; Mora, J.; García, P.; Agüero, A.; Borrás, A.; González-Elipe, A.R.; López-Santos, C. Robust anti-icing superhydrophobic aluminum alloy surfaces by grafting fluorocarbon molecular chains. Appl. Mater. Today 2020, 21, 100815. [Google Scholar] [CrossRef]
- Qin, Y.K.; Li, Y.; Zhang, D.; Xu, N.; Zhu, X.C. Wettability, durability and corrosion properties of slippery laser-textured aluminum alloy surface under water impact. Surf. Coat. Technol. 2020, 394, 125856. [Google Scholar] [CrossRef]
- Feng, L.B.; Sultonzoda, F.; Wang, J.; Wang, H.T.; Xue, F.X. Superhydrophobic aluminum alloy surface with excellent universality, corrosion resistance, and antifouling performance prepared without prepolishing. Mater. Corros. 2021, 72, 652–659. [Google Scholar] [CrossRef]
- Zhang, Z.B.; Xue, F.X.; Bai, W.X.; Shi, X.T.; Liu, Y.H.; Feng, L.B. Superhydrophobic surface on al alloy with robust durability and excellentself-healing performance. Surf. Coat. Technol. 2021, 410, 126952. [Google Scholar] [CrossRef]
- Ivvala, J.; Mandal, P.; Arora, H.S.; Grewal, H.S. Eco-friendly and facile fabrication of superhydrophobic aluminum alloy. Proc. IMechE Part J J. Eng. Tribol. 2022, 236, 259–265. [Google Scholar] [CrossRef]
- Xu, N.; Sarkar, D.K.; Chen, X.G.; Tong, W.P. Corrosion performance of superhydrophobic nickel stearate/nickel hydroxide thin films on aluminum alloy by a simple one-step electrodeposition process. Surf. Coat. Technol. 2016, 302, 173–184. [Google Scholar] [CrossRef]
- Jiang, S.Z.; Guo, Z.N.; Deng, Y.; Dong, H.S.; Li, X.Y.; Liu, J.W. Effect of pulse frequency on the onestep preparation of superhydrophobic surface by pulse electrodeposition. Appl. Surf. Sci. 2018, 458, 603–611. [Google Scholar] [CrossRef]
- He, Z.H.; Zeng, Y.W.; Zhou, M.M.; Min, Y.L.; Shen, X.X.; Xu, Q.J. Superhydrophobic films with enhanced corrosion resistance and self-cleaning performance on an Al alloy. Langmuir 2021, 37, 524–541. [Google Scholar] [CrossRef]
- Yu, H.D.; Zhang, X.R.; Wan, Y.L.; Xu, J.K.; Yu, Z.J.; Li, Y.Q. Superhydrophobic surface prepared by micromilling and grinding on aluminium alloy. Surf. Eng. 2016, 32, 108–113. [Google Scholar] [CrossRef]
- Sebastian, D.; Yao, C.W.; Lian, I. Multiscale corrosion analysis of superhydrophobic coating on 2024 aluminum alloy in a 3.5 wt% NaCl solution. MRS Commun. 2020, 10, 305–311. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, J.; Zhang, B.B.; Zeng, Y.X.; Duan, J.Z.; Hou, B.R. Fabrication of anodized superhydrophobic 5083 aluminum alloy surface for marine anti-corrosion and anti-biofouling. J. Oceanol. Limnol. 2020, 38, 1246–1255. [Google Scholar] [CrossRef]
- Dong, X.J.; Meng, J.B.; Hu, Y.Z.; Wei, X.T.; Luan, X.S.; Zhou, H.A. Fabrication of self-cleaning superhydrophobic surfaces with improved corrosion resistance on 6061 aluminum alloys. Micromachines 2020, 11, 159. [Google Scholar] [CrossRef]
- Li, X.K.; Liu, X. Study on corrosion resistance of superhydrophobic film prepared on the surface of 2024 aluminum alloy. Int. J. Electrochem. Sci. 2022, 17, 1–10. [Google Scholar] [CrossRef]
- Tran, N.G.; Chun, D.M. Green manufacturing of extreme wettability contrast surfaces with superhydrophilic and superhydrophobic patterns on aluminium. J. Mater. Process. Technol. 2021, 297, 117245. [Google Scholar] [CrossRef]
- Khaskhoussi, A.; Calabrese, L.; Proverbio, E. Superhydrophobic self-assembled silane monolayers on hierarchical 6082 aluminum alloy for anti-corrosion applications. Appl. Sci. 2020, 10, 2656. [Google Scholar] [CrossRef]
- Zhang, B.B.; Zeng, Y.X.; Wang, J.; Sun, Y.Y.; Zhang, J.; Li, Y.T. Superamphiphobic aluminum alloy with low sliding angles and acid-alkali liquids repellency. Mater. Des. 2020, 188, 108479. [Google Scholar] [CrossRef]
- Shi, T.; Xue, S.; Ma, X.Y.; Peng, H.Q.; Du, J.; Zheng, B.Z.; Xia, Z.X. Fabrication of superhydrophobic micro nanostructured aluminium alloy surface via a cost effective processing using an ultra low concentration of fluoroalkylsilane. Appl. Phys. A-Mater. 2021, 27, 399. [Google Scholar] [CrossRef]
- Peng, H.Q.; Li, L.; Wang, Q.; Zhang, Y.B.; Wang, T.M.; Zheng, B.Z.; Zhou, H. Organic carbon dot coating for superhydrophobic aluminium alloy surfaces. J. Coat. Technol. Res. 2021, 18, 861–869. [Google Scholar] [CrossRef]
- Zheng, S.L.; Li, C.; Zhang, Y.P.; Xiang, T.F.; Cao, Y.; Li, Q.L.; Chen, Z. A general strategy towards superhydrophobic self-cleaning and anti-corrosion metallic surfaces: An example with aluminum alloy. Coatings 2021, 11, 788. [Google Scholar] [CrossRef]
- Noormohammed, S.; Sarkar, D.K. Rf-Sputtered Teflon®-modified superhydrophobic nanostructured titanium dioxide coating on aluminum alloy for icephobic applications. Coatings 2021, 11, 432. [Google Scholar] [CrossRef]
- Xiao, X.Y.; Xie, W.; Ye, Z.H. Preparation of corrosion-resisting superhydrophobic surface on aluminium substrate. Surf. Eng. 2019, 35, 411–417. [Google Scholar] [CrossRef]
- Barthwal, S.; Lim, S.H. A durable, fluorine-free, and repairable superhydrophobic aluminium surface with hierarchical micro/nanostructures and its application for continuous oil-water separation. J. Membr. Sci. 2021, 618, 118716. [Google Scholar] [CrossRef]
- Li, Y.Q.; Si, W.T.; Gao, R.J. Facile preparation of superamphiphobic aluminum alloy surfaces and their corrosion resistance. Surf. Coat. Technol. 2022, 430, 127997. [Google Scholar] [CrossRef]
- Guo, F.Q.; Duan, S.W.; Wu, D.T.; Matsuda, K.J.; Wang, T.; Zou, Y. Facile etching fabrication of superhydrophobic 7055 aluminum alloy surface towards chloride environment anticorrosion. Corros. Sci. 2021, 182, 109262. [Google Scholar] [CrossRef]
- Peng, H.Q.; Wang, Q.; Wang, T.M.; Li, L.; Xia, Z.X.; Du, J.; Zheng, B.Z.; Zhou, H.; Ye, L.W. Study on dynamics and freezing behaviors of water droplet on superhydrophobic aluminum surface. Appl. Phys. A-Mater. 2020, 126, 811. [Google Scholar] [CrossRef]
- Khajavian, E.; Attar, M.R.; Zahrani, E.M.; Liu, W.; Davoodi, A.; Hosseinpour, S. Tuning surface wettability of aluminum surface and its correlation with short and long term corrosion resistance in saline solutions. Surf. Coat. Technol. 2022, 429, 127950. [Google Scholar] [CrossRef]
- Liu, L.J.; Feng, X.R.; Guo, M.X. Eco-friendly fabrication of superhydrophobic bayerite array on Al foil via an etching and growth process. J. Phys. Chem. C 2013, 117, 25519–25525. [Google Scholar] [CrossRef]
- Kim, A.R.; Lee, C.; Kim, H.M.; Kim, J.W. Simple approach to superhydrophobic nanostructured Al for practical anti-frosting application based on enhanced self-propelled jumping droplets. ACS Appl. Mater. Interfaces 2015, 7, 7206–7213. [Google Scholar] [CrossRef]
- Xie, D.G.; Li, W. A novel simple approach to preparation of superhydrophobic surfaces of aluminum alloys. Appl. Surf. Sci. 2011, 258, 1004–1007. [Google Scholar] [CrossRef]
- Guo, Z.G.; Zhou, F.; Hao, J.C.; Liu, W.M. Effects of system parameters on making aluminum alloy lotus. J. Colloid Interface Sci. 2006, 303, 298–305. [Google Scholar] [CrossRef]
- Huang, Y.; Sarkar, D.K.; Chen, X.G. Superhydrophobic aluminum alloy surfaces prepared by chemical etching process and their corrosion resistance properties. Appl. Surf. Sci. 2015, 356, 1012–1024. [Google Scholar] [CrossRef]
- Saleema, N.; Sarkar, D.K.; Paynter, R.W.; Chen, X.G. Superhydrophobic aluminum alloy surfaces by a novel one-step process. ACS Appl. Mater. Interfaces 2010, 2, 2500–2502. [Google Scholar] [CrossRef]
- Guo, Z.G.; Zhou, F.; Hao, J.C.; Liu, W.M. Stable biomimetic super-hydrophobic engineering materials. J. Am. Chem. Soc. 2005, 127, 15670–15671. [Google Scholar] [CrossRef]
- Kim, J.H.; Puranik, R.; Shang, J.K.; Harris, D.M. Robust transferrable superhydrophobic surfaces. Surf. Eng. 2020, 36, 614–620. [Google Scholar] [CrossRef]
- Yang, H.J.; Gao, Y.M.; Frankel, G.S.; Qin, W.C.; Li, T.S.; Huang, Z.F.; Wu, L. Robust superhydrophobic surface with reinforced skeletons for corrosion protection. Appl. Surf. Sci. 2020, 499, 143916. [Google Scholar] [CrossRef]
- Rasitha, T.P.; Vanithakumari, S.C.; Krishna, D.N.G.; George, R.P.; Srinivasan, R.; Philip, J. Facile fabrication of robust superhydrophobic aluminum surfaces with enhanced corrosion protection and antifouling properties. Prog. Org. Coat. 2022, 162, 106560. [Google Scholar] [CrossRef]
- Lomga, J.; Varshney, P.; Nanda, D.; Satapathy, M.; Mohapatra, S.S.; Kumar, A. Fabrication of durable and regenerable superhydrophobic coatings with excellentself-cleaning and anti-fogging properties for aluminium surfaces. J. Alloys Compd. 2017, 702, 161–170. [Google Scholar] [CrossRef]
- Cornette, P.; Zanna, S.; Seyeux, A.; Costa, D.; Marcus, P. The native oxide film on a model aluminium-copper alloy studied by XPS and ToF-SIMS. Corros. Sci. 2020, 174, 108837. [Google Scholar] [CrossRef]
- Xing, M.; Lei, X.P.; Guan, X.L.; Song, Y.Y.; Zhou, Y.L. Research on construction of superhydrophobic surface of aluminum alloy and its stability and self-cleaning performance. Surf. Technol. 2021, 50, 152–161. [Google Scholar]

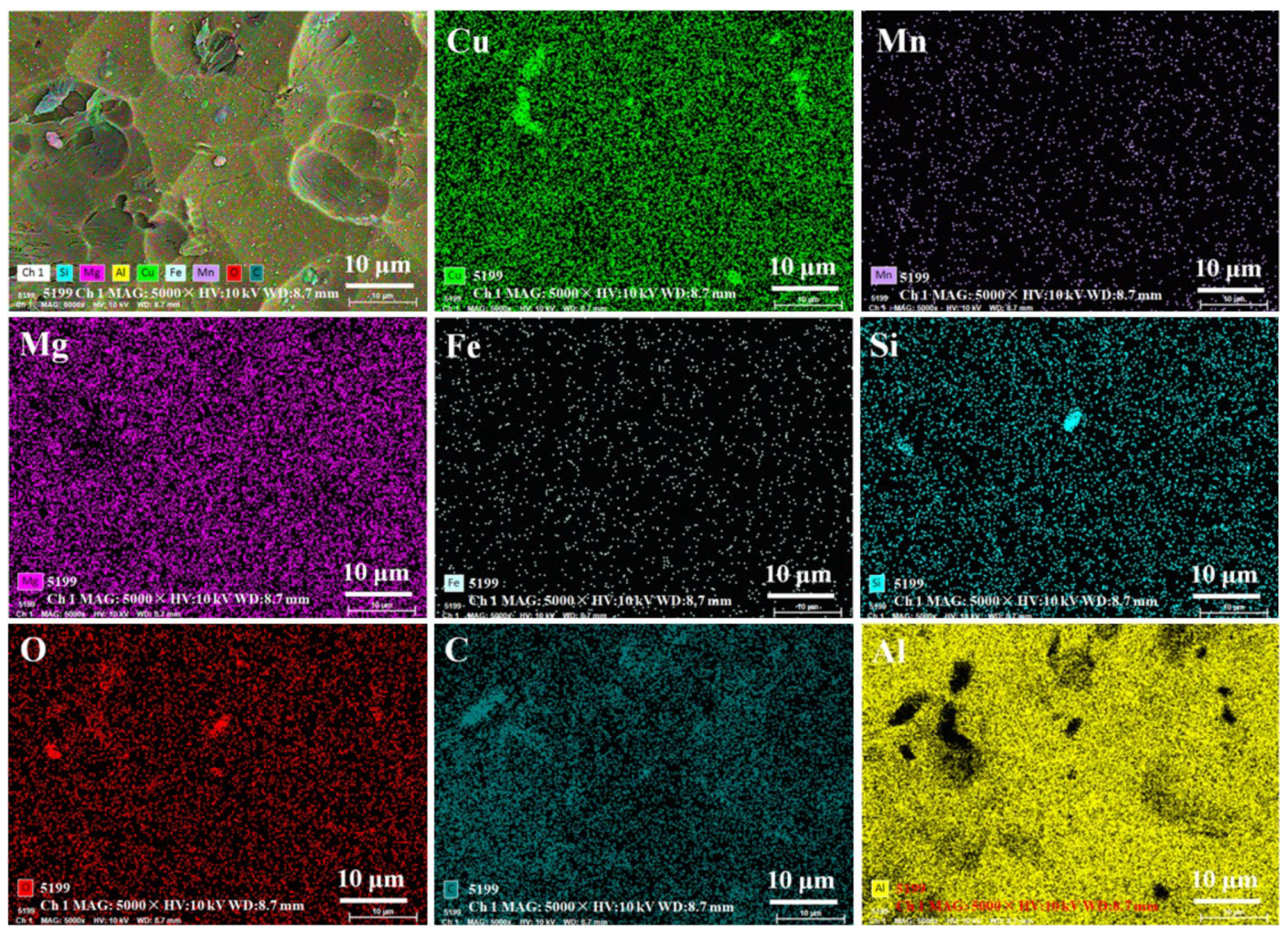


| Elements | Cu | Si | Fe | Mn | Mg | Cr | Zn | Ti | Al |
|---|---|---|---|---|---|---|---|---|---|
| wt% | 4.80 | 0.09 | 0.27 | 0.64 | 1.61 | 0.01 | 0.13 | 0.03 | 92.42 |
| Samples | (a) | (b) | (c) | (d) | (e) | (f) | (g) | (h) | (i) |
|---|---|---|---|---|---|---|---|---|---|
| SWCA *(°) | 60.5 | 21.8 | 108.7 | 108.1 | 161.7 | 58.9 | 164.7 | 144.6 | 133.9 |
| Samples | Cu wt% | Mn wt% | Mg wt% | Fe wt% | Si wt% | O wt% | C wt% | Al wt% |
|---|---|---|---|---|---|---|---|---|
| (a) | 4.63 | 0.82 | 1.19 | 0.46 | 0.46 | 1.47 | - | 90.98 |
| (b) | 35.97 | 3.82 | 11.55 | 0.68 | 1.93 | 37.30 | - | 8.76 |
| (c) | 5.74 | 0.32 | 1.64 | 0.01 | 0.34 | 3.01 | - | 88.95 |
| (d) | 8.29 | 0.46 | 1.55 | 0.79 | 0.08 | 5.11 | - | 83.72 |
| (e) | 4.87 | 0.30 | 1.23 | 0.06 | 0.42 | 2.39 | 21.36 | 69.36 |
| (f) | 31.33 | 5.15 | 11.35 | 0.77 | 0.44 | 35.56 | - | 15.42 |
| (g) | 27.31 | 2.87 | 9.06 | 0.80 | 4.41 | 35.84 | 8.22 | 11.48 |
| Sample | Ecorr (mV vs. Ag/AgCl/sat. KCl) | icorr (μA cm−2) | Rp (kΩ cm2) |
|---|---|---|---|
| (a) | 382 | 0.15 | 268.7 |
| (b) | 490 | 0.68 | 35.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shan, L.-M.; Liu, G.-B.; Tang, H.; Li, Z.-H.; Wu, J.-Y. A Novel Simple Fabrication Method for Mechanically Robust Superhydrophobic 2024 Aluminum Alloy Surfaces. Coatings 2022, 12, 1717. https://doi.org/10.3390/coatings12111717
Shan L-M, Liu G-B, Tang H, Li Z-H, Wu J-Y. A Novel Simple Fabrication Method for Mechanically Robust Superhydrophobic 2024 Aluminum Alloy Surfaces. Coatings. 2022; 12(11):1717. https://doi.org/10.3390/coatings12111717
Chicago/Turabian StyleShan, Li-Mei, Guo-Biao Liu, Hua Tang, Zhi-Hong Li, and Ju-Ying Wu. 2022. "A Novel Simple Fabrication Method for Mechanically Robust Superhydrophobic 2024 Aluminum Alloy Surfaces" Coatings 12, no. 11: 1717. https://doi.org/10.3390/coatings12111717
APA StyleShan, L.-M., Liu, G.-B., Tang, H., Li, Z.-H., & Wu, J.-Y. (2022). A Novel Simple Fabrication Method for Mechanically Robust Superhydrophobic 2024 Aluminum Alloy Surfaces. Coatings, 12(11), 1717. https://doi.org/10.3390/coatings12111717






