Evaluating the Corrosion Inhibition Efficiency of Pyridinium-Based Cationic Surfactants for EN3B Mild Steel in Acidic-Chloride Media
Abstract
1. Introduction
2. Experimentation
2.1. Chemicals and Reagents
2.2. Sample and Solution Preparation
2.3. Electrochemical Measurements
2.4. Surface Monitoring
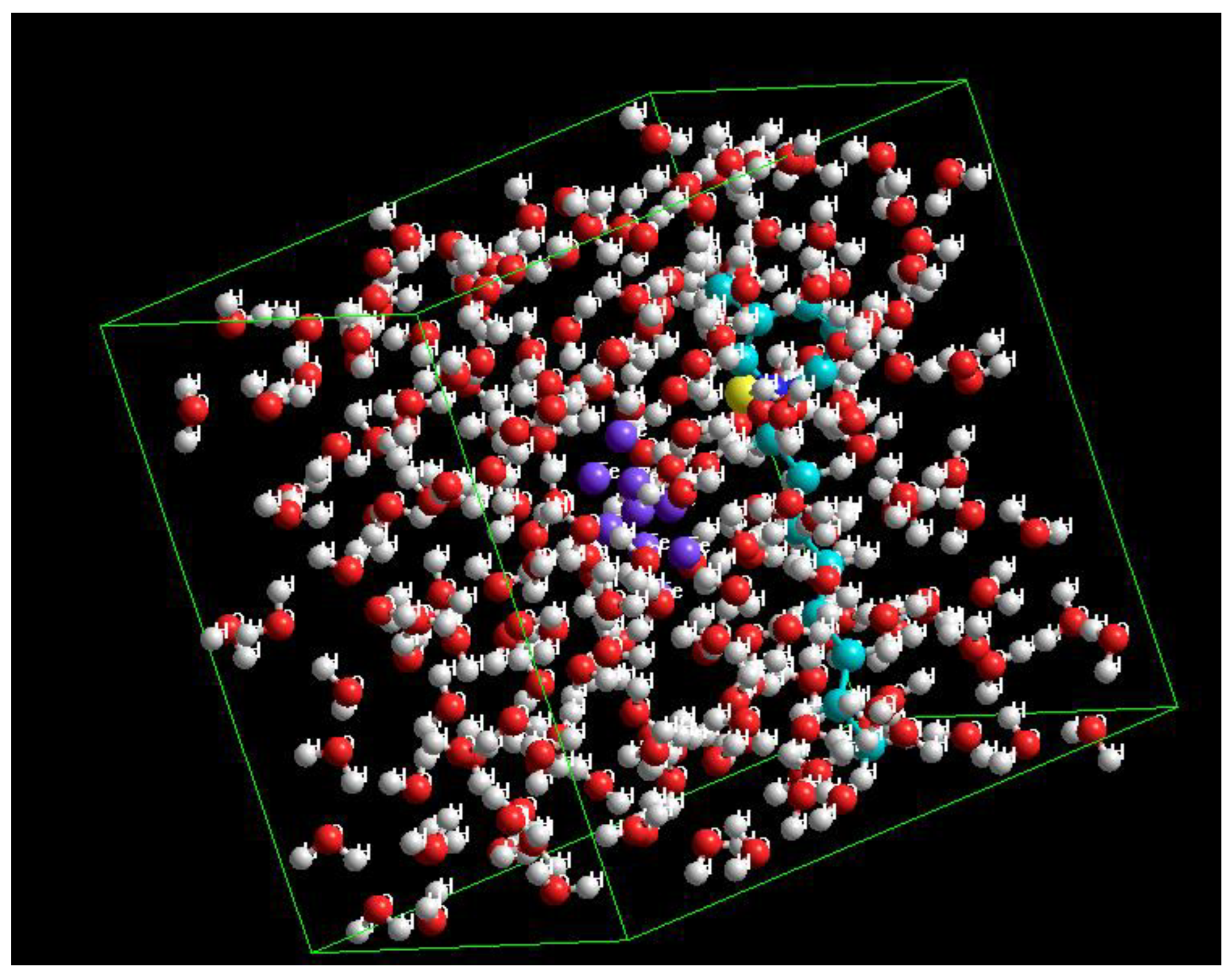
2.5. Computational Details
3. Results and Discussion
3.1. Open Circuit Potential (OCP)
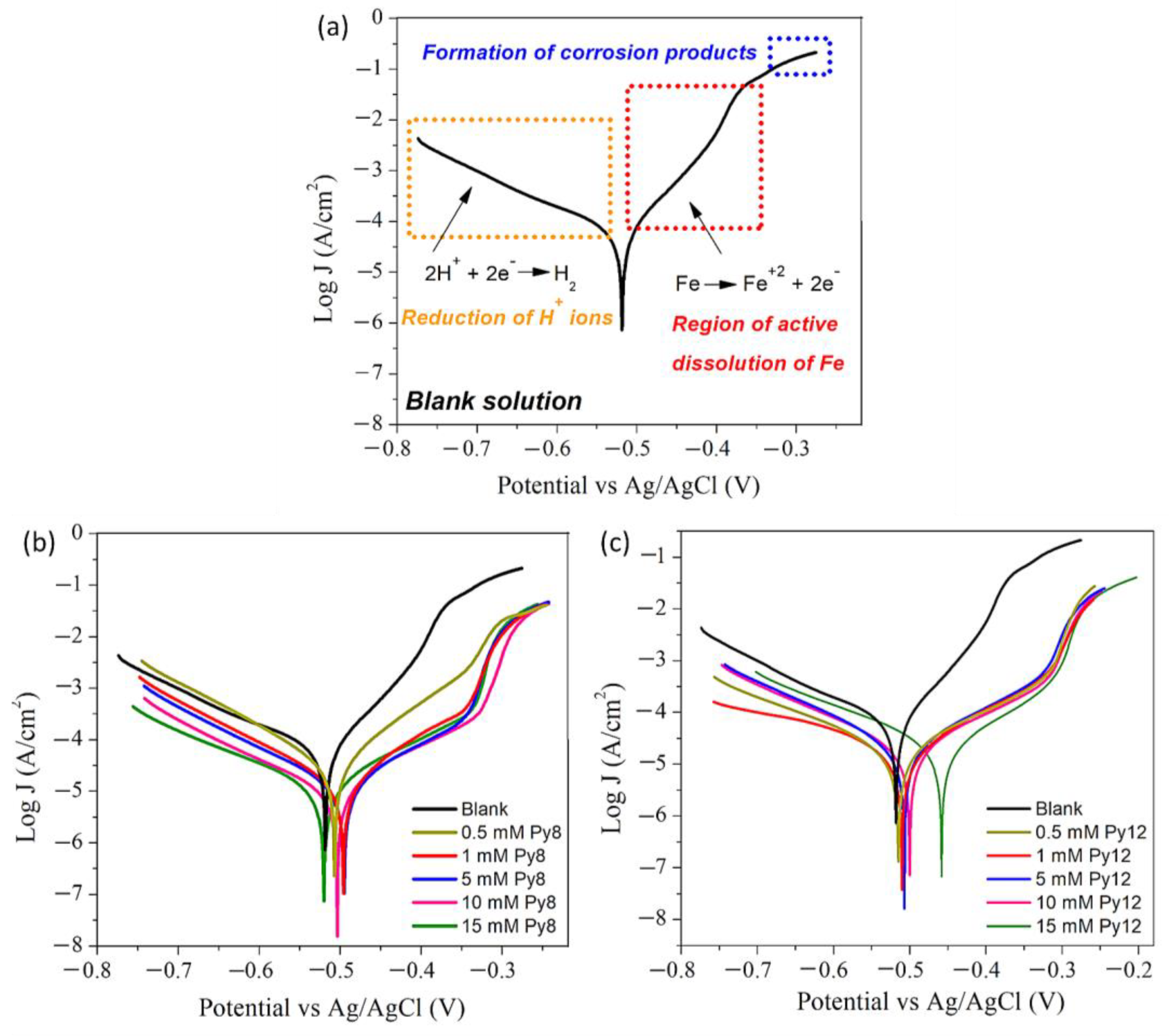
3.2. Potentiodynamic Polarization
3.2.1. Tafel Polarization
3.2.2. Linear Polarization
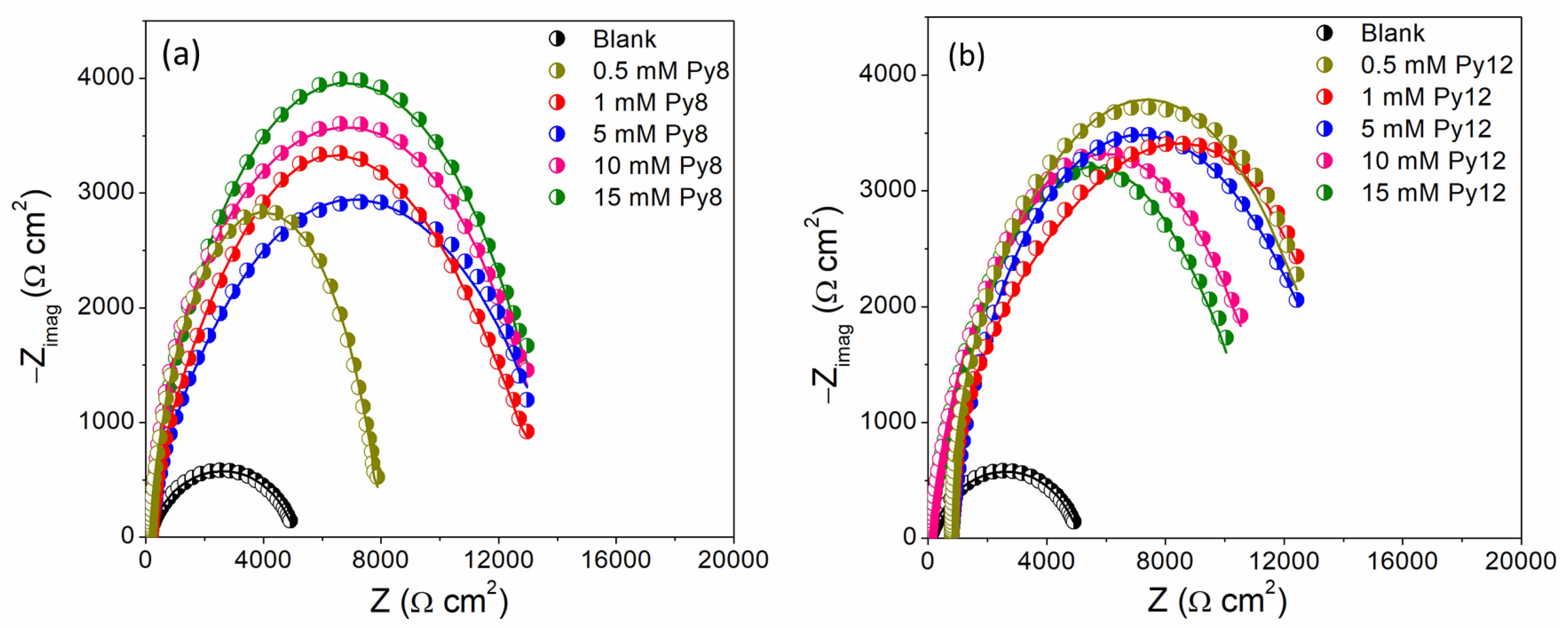
3.3. Electrochemical Impedance Spectroscopy (EIS)
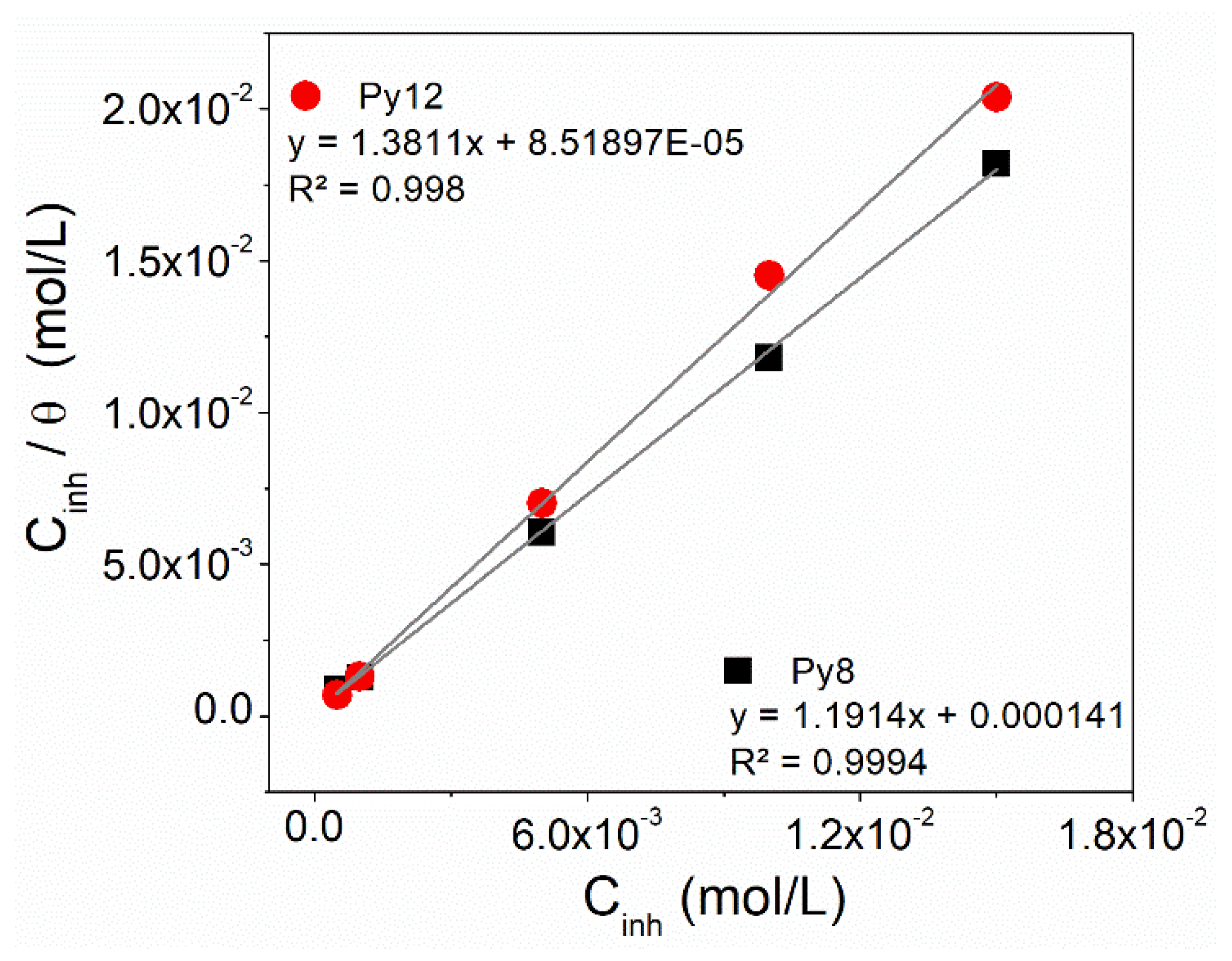
3.4. Adsorption Isotherm
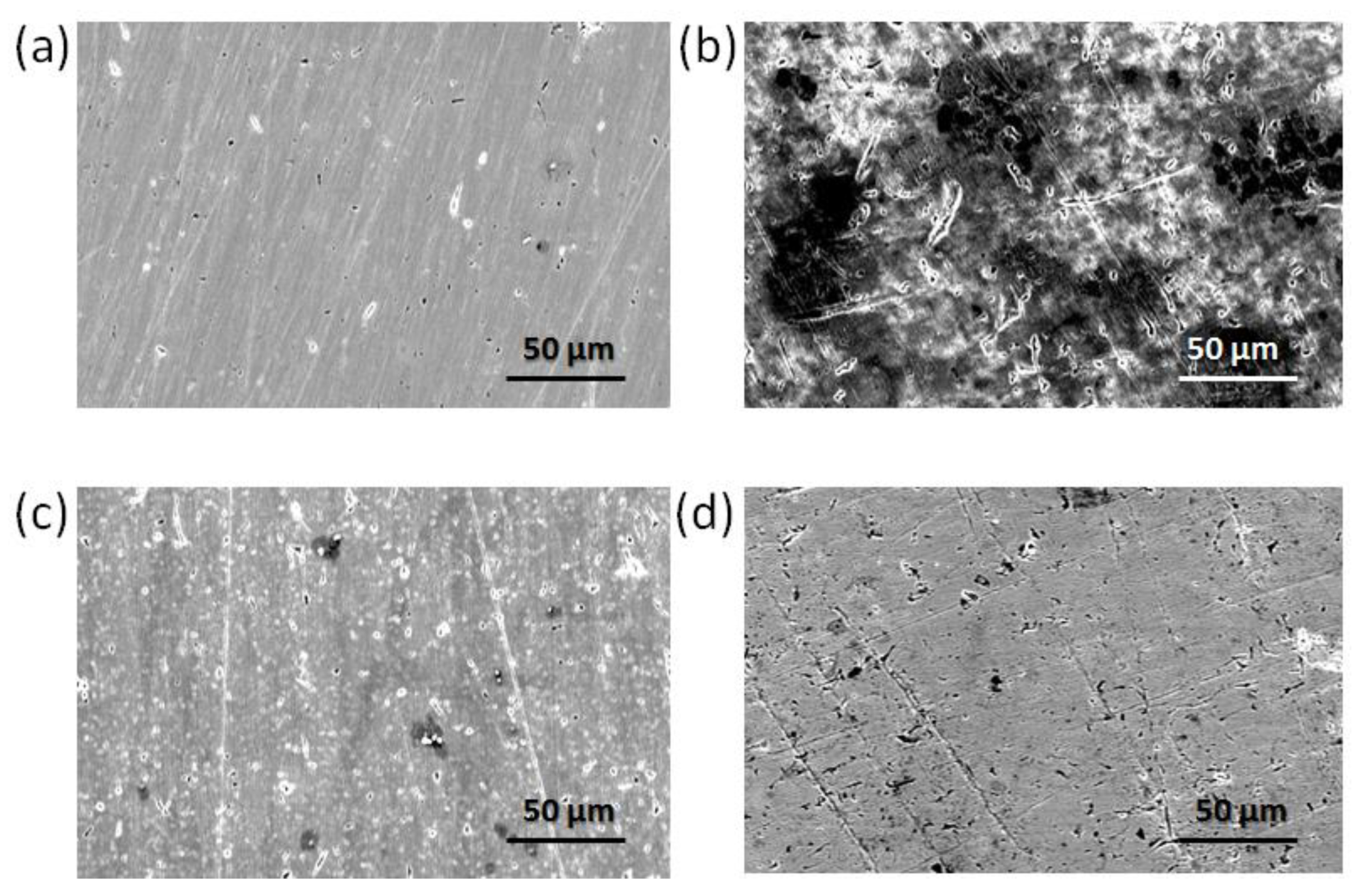
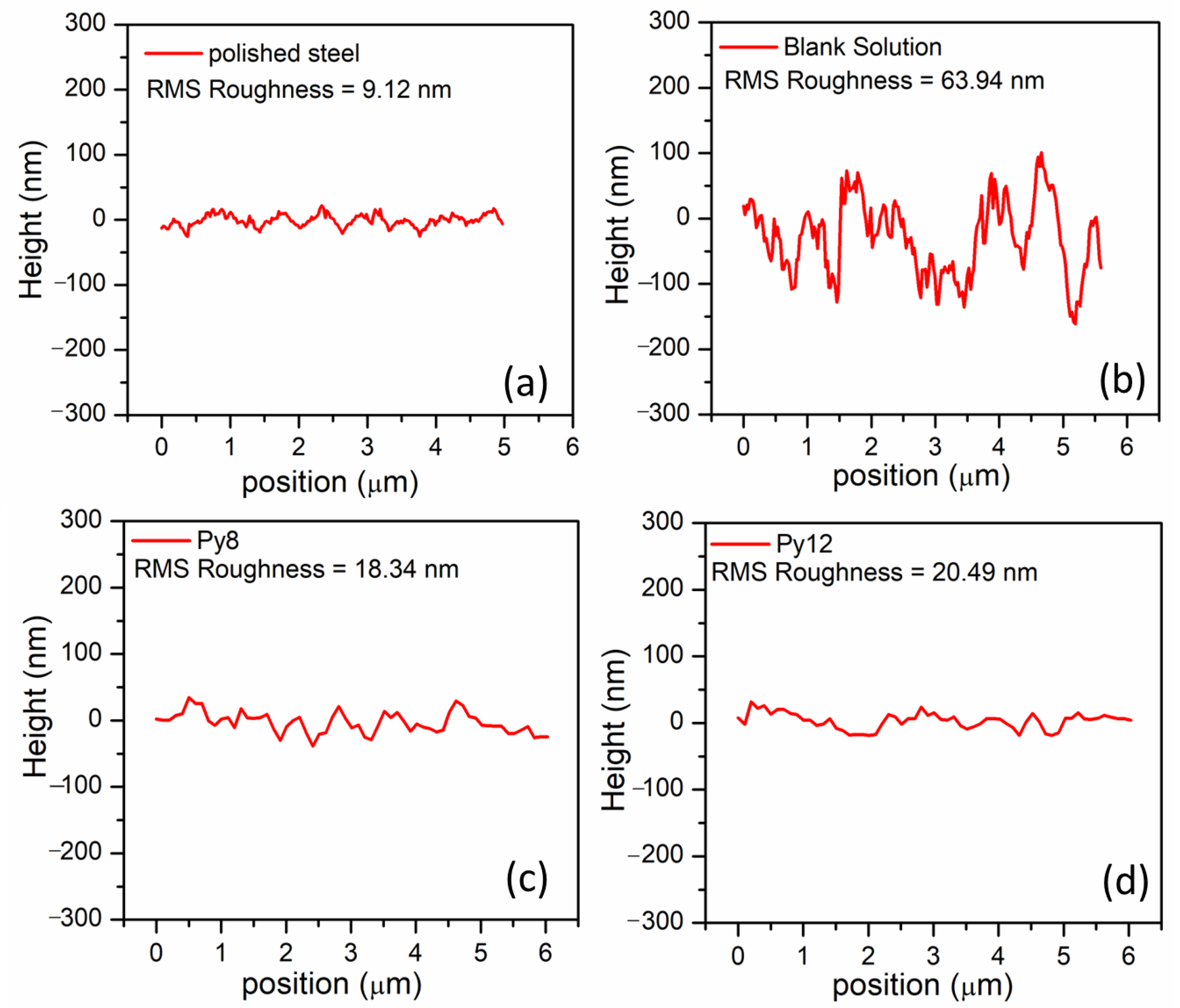
3.5. SEM and AFM Analysis
3.6. Quantum Chemical Calculations
3.6.1. Mulliken Charge Analysis
3.6.2. Interaction Energies
3.7. Mechanism of Corrosion of EN3B Mild Steel and Its Inhibition by Py8 and Py12
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McCafferty, E. Introduction to Corrosion Science; Springer Science & Business Media: New York, NY, USA, 2010. [Google Scholar]
- Wood, M.H.; Wood, T.J.; Welbourn, R.J.; Poon, J.; Madden, D.C.; Clarke, S.M. An X-ray and Neutron Reflectometry Study of Iron Corrosion in Seawater. Langmuir 2018, 34, 5990–6002. [Google Scholar] [CrossRef] [PubMed]
- Koch, G.H.; Brongers, M.P.; Thompson, N.G.; Virmani, Y.P.; Payer, J.H. Corrosion Cost and Preventive Strategies in the United States; US Federal Highway Administration: Tallahassee, FL, USA, 2002.
- El-Lateef, H.M.A.; Ismael, M.; Mohamed, I.M. Novel Schiff base amino acid as corrosion inhibitors for carbon steel in CO2-saturated 3.5% NaCl solution: Experimental and computational study. Corros. Rev. 2015, 33, 77–97. [Google Scholar] [CrossRef]
- El-Tabei, A.; Hegazy, M.; Bedair, A.; Sadeq, M. Synthesis and inhibition effect of a novel tri-cationic surfactant on carbon steel corrosion in 0.5 M H2SO4 solution. J. Surfactants Deterg. 2014, 17, 341–352. [Google Scholar] [CrossRef]
- Sherif, E.-S.M. A comparative study on the electrochemical corrosion behavior of iron and X-65 steel in 4.0 wt % sodium chloride solution after different exposure intervals. Molecules 2014, 19, 9962–9974. [Google Scholar] [CrossRef] [PubMed]
- Negm, N.A.; El Farargy, A.F.; Al Sabagh, A.M.; Abdelrahman, N.R. New schiff base cationic surfactants: Surface and thermodynamic properties and applicability in bacterial growth and metal corrosion prevention. J. Surfactants Deterg. 2011, 14, 505–514. [Google Scholar] [CrossRef]
- Wood, M.H.; Clarke, S.M. Neutron reflectometry for studying corrosion and corrosion inhibition. Metals 2017, 7, 304. [Google Scholar] [CrossRef]
- Dawson, J. Chemical Treating in Oil and Gas Production; Elsevier: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Usman, B.J.; Ali, S.A. Carbon dioxide corrosion inhibitors: A review. Arab. J. Sci. Eng. 2018, 43, 1–22. [Google Scholar] [CrossRef]
- El-Lateef, H.M.A.; Abbasov, V.M.; Aliyeva, L.I.; Ismayilov, T.A. Corrosion protection of steel pipelines against CO2 corrosion—A review. Chem. J. 2012, 2, 52–63. [Google Scholar]
- Usman, B.J.; Gasem, Z.M.; Umoren, S.A.; Solomon, M.M. Eco-friendly 2-Thiobarbituric acid as a corrosion inhibitor for API 5L X60 steel in simulated sweet oilfield environment: Electrochemical and surface analysis studies. Sci. Rep. 2019, 9, 830. [Google Scholar] [CrossRef]
- Fuchs-Godec, R.; Doleček, V. A effect of sodium dodecylsulfate on the corrosion of copper in sulphuric acid media. Colloids Surf. A Physicochem. Eng. 2004, 244, 73–76. [Google Scholar] [CrossRef]
- Negm, N.; Kandile, N.; Aiad, I.; Mohammad, M. New eco-friendly cationic surfactants: Synthesis, characterization and applicability as corrosion inhibitors for carbon steel in 1N HCl. Colloids Surf. A Physicochem. Eng. 2011, 391, 224–233. [Google Scholar] [CrossRef]
- Raja, P.B.; Sethuraman, M.G. Natural products as corrosion inhibitor for metals in corrosive media—A review. Mater. Lett. 2008, 62, 113–116. [Google Scholar] [CrossRef]
- Ji, G.; Shukla, S.K.; Dwivedi, P.; Sundaram, S.; Prakash, R. Inhibitive effect of Argemone mexicana plant extract on acid corrosion of mild steel. Ind. Eng. Chem. Res. 2011, 50, 11954–11959. [Google Scholar] [CrossRef]
- Gerengi, H.; Sahin, H.I. Schinopsis lorentzii extract as a green corrosion inhibitor for low carbon steel in 1 M HCl solution. Ind. Eng. Chem. Res. 2012, 51, 780–787. [Google Scholar] [CrossRef]
- Ji, G.; Dwivedi, P.; Sundaram, S.; Prakash, R. Inhibitive effect of chlorophytum borivilianum root extract on mild steel corrosion in HCl and H2SO4 solutions. Ind. Eng. Chem. Res. 2013, 52, 10673–10681. [Google Scholar] [CrossRef]
- Kamal, C.; Sethuraman, M.G. Caulerpin—A bis-indole alkaloid as a green inhibitor for the corrosion of mild steel in 1 M HCl solution from the marine alga caulerpa racemosa. Ind. Eng. Chem. Res. 2012, 51, 10399–10407. [Google Scholar] [CrossRef]
- Umoren, S.A.; Gasem, Z.M.; Obot, I.B. Natural products for material protection: Inhibition of mild steel corrosion by date palm seed extracts in acidic media. Ind. Eng. Chem. Res. 2013, 52, 14855–14865. [Google Scholar] [CrossRef]
- Fu, J.-J.; Li, S.-N.; Wang, Y.; Cao, L.-H. Computational and electrochemical studies of some amino acid compounds as corrosion inhibitors for mild steel in hydrochloric acid solution. J. Mater. Sci. 2010, 45, 6255–6265. [Google Scholar] [CrossRef]
- Gece, G.; Bilgiç, S. A theoretical study on the inhibition efficiencies of some amino acids as corrosion inhibitors of nickel. Corros. Sci. 2010, 52, 3435–3443. [Google Scholar] [CrossRef]
- Helal, N.; Badawy, W. Environmentally safe corrosion inhibition of Mg–Al–Zn alloy in chloride free neutral solutions by amino acids. Electrochim. Acta 2011, 56, 6581–6587. [Google Scholar] [CrossRef]
- Amin, M.A.; Khaled, K.; Mohsen, Q.; Arida, H. A study of the inhibition of iron corrosion in HCl solutions by some amino acids. Corros. Sci. 2010, 52, 1684–1695. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, S.; Gong, M.; Li, W. Experimental and theoretical study on the corrosion inhibition of mild steel by 1-octyl-3-methylimidazolium L-prolinate in sulfuric acid solution. Ind. Eng. Chem. Res. 2014, 53, 16349–16358. [Google Scholar] [CrossRef]
- Aoun, S.B. On the corrosion inhibition of carbon steel in 1 M HCl with a pyridinium-ionic liquid: Chemical, thermodynamic, kinetic and electrochemical studies. RSC Adv. 2017, 7, 36688–36696. [Google Scholar] [CrossRef]
- Koundal, M.; Singh, A.; Sharma, C. Study on the effect of imidazolium ionic liquid as a modulator of corrosion inhibition of anionic surfactant sodium dodecyl sulfate (SDS) on mild steel in sodium chloride solution. J. Mol. Liq. 2022, 350, 118561. [Google Scholar] [CrossRef]
- Jin, X.; Wang, J.; Zheng, S.; Li, J.; Ma, X.; Feng, L.; Zhu, H.; Hu, Z. The study of surface activity and anti-corrosion of novel surfactants for carbon steel in 1 M HCl. J. Mol. Liq. 2022, 353, 118747. [Google Scholar] [CrossRef]
- Fatima, S.; Sharma, R.; Asghar, F.; Kamal, A.; Badshah, A.; Kraatz, H.-B. Study of new amphiphiles based on ferrocene containing thioureas as efficient corrosion inhibitors: Gravimetric, electrochemical, SEM and DFT studies. J. Ind. Eng. Chem. 2019, 76, 374–387. [Google Scholar] [CrossRef]
- Talat, R.; Fayyaz, S.; Ali, S.; Khalid, N.; Haider, A.; Shah, A.; Ullah, F. Designing of new cationic surfactant based micellar systems as drug carriers: An investigation into the drug cell membrane interactions. J. Dispers. Sci. Technol. 2019, 40, 958–968. [Google Scholar] [CrossRef]
- Akhter, K.; Ullah, K.; Talat, R.; Haider, A.; Khalid, N.; Ullah, F.; Ali, S. Synthesis and characterization of cationic surfactants and their interactions with drug and metal complexes. Heliyon 2019, 5, e01885. [Google Scholar] [CrossRef]
- Fayyaz, S.; Talat, R.; Ali, S.; Khalid, N.; Shah, A.; Ullah, F.; Haider, A. 2-Methylpyridinium based surfactants: Synthesis, characterization and potential application as drug carrier systems. Proc. Pakistan Acad. Sci. A 2019, 56, 47. [Google Scholar]
- Fayyaz, S.; Ali, S.; Khalid, N.; Shah, A.; Ullah, F. One pot synthesis and properties of cationic surfactants: N-alkyl-3-methylpyridinium bromide. J. Surfactants Deterg. 2016, 19, 841–848. [Google Scholar] [CrossRef]
- Fayyaz, S.; Talat, R.; Ali, S.; Khalid, N.; Shah, A.; Ullah, F. Synthesis, characterization, and micellization behavior of cationic surfactants: N-alkyl-3-methylpyridinium bromides and their drug interaction study by UV–Visible spectroscopy and conductometry. J. Surfactants Deterg. 2019, 22, 625–632. [Google Scholar] [CrossRef]
- Heakal, F.E.-T.; Rizk, S.; Elkholy, A.E. Characterization of newly synthesized pyrimidine derivatives for corrosion inhibition as inferred from computational chemical analysis. J. Mol. Struct. 2018, 1152, 328–336. [Google Scholar] [CrossRef]
- Nazir, U.; Akhter, Z.; Ali, N.Z.; Hussain, R.; Liaqat, F.; Tahir, A.; Qamar, S. Corrosion inhibition studies of ferrocenyl Schiff bases in a mild acidic medium through experimental methods and DFT calculations. New J. Chem. 2022, 46, 3925–3938. [Google Scholar] [CrossRef]
- Ahlrichs, R.; Baer, M.; Haeser, M.; Horn, H.; Koelmel, C. Electronic structure calculations on workstation computers: The program system turbomole. Chem. Phys. Lett. 1989, 162, 165–169. [Google Scholar] [CrossRef]
- Emzerhof, M.; Scuseria, G.E. An accurate density functional method for the study of magnetic properties: The PBE0 model. J. Mol. Struct. Theochem 1999, 493, 145–157. [Google Scholar] [CrossRef]
- Casaletto, M.P.; Figà, V.; Privitera, A.; Bruno, M.; Napolitano, A.; Piacente, S. Inhibition of Cor-Ten steel corrosion by “green” extracts of Brassica campestris. Corros. Sci. 2018, 136, 91–105. [Google Scholar] [CrossRef]
- Oguzie, E.; Li, Y.; Wang, F. Corrosion inhibition and adsorption behavior of methionine on mild steel in sulfuric acid and synergistic effect of iodide ion. J. Colloid Interface Sci. 2007, 310, 90–98. [Google Scholar] [CrossRef]
- Al-Otaibi, M.; Al-Mayouf, A.; Khan, M.; Mousa, A.; Al-Mazroa, S.; Alkhathlan, H. Corrosion inhibitory action of some plant extracts on the corrosion of mild steel in acidic media. Arab. J. Chem. 2014, 7, 340–346. [Google Scholar] [CrossRef]
- Ahmed, A.; Al-Amiery, A.A.; Kadhum, A.A.H.; Alobaidy, A.H.M.; Mohamad, A.B.; Hoon, P.S. Novel Corrosion Inhibitor for Mild Steel in HCl. Materials 2014, 7, 662–672. [Google Scholar] [CrossRef]
- Wei, H.; Ding, D.; Wei, S.; Guo, Z. Anticorrosive conductive polyurethane multiwalled carbon nanotube nanocomposites. J. Mater. Chem. A 2013, 1, 10805–10813. [Google Scholar] [CrossRef]
- Valentim, I.B.; Joekes, I. Adsorption of sodium dodecylsulfate on chrysotile. Colloids Surf. A Physicochem. Eng. 2006, 290, 106–111. [Google Scholar] [CrossRef]
- Jayaperumal, D. Effects of alcohol-based inhibitors on corrosion of mild steel in hydrochloric acid. Mater. Chem. Phys. 2010, 119, 478–484. [Google Scholar] [CrossRef]
- Olivares-Xometl, O.; López-Aguilar, C.; Herrastí-González, P.; Likhanova, N.V.; Lijanova, I.; Martínez-Palou, R.; Rivera-Márquez, J.A. Adsorption and corrosion inhibition performance by three new ionic liquids on API 5L X52 steel surface in acid media. Ind. Eng. Chem. Res. 2014, 53, 9534–9543. [Google Scholar] [CrossRef]
- Atta, A.M.; El-Mahdy, G.A.; Al-Lohedan, H.A.; Ezzat, A.R.O. A new green ionic liquid-based corrosion inhibitor for steel in acidic environments. Molecules 2015, 20, 11131–11153. [Google Scholar] [CrossRef]
- Döner, A.; Kardaş, G. N-Aminorhodanine as an effective corrosion inhibitor for mild steel in 0.5 M H2SO4. Corros. Sci. 2011, 53, 4223–4232. [Google Scholar] [CrossRef]
- Hegazy, M. Novel cationic surfactant based on triazole as a corrosion inhibitor for carbon steel in phosphoric acid produced by dihydrate wet process. J. Mol. Liq. 2015, 208, 227–236. [Google Scholar] [CrossRef]
- Xu, B.; Ji, Y.; Zhang, X.; Jin, X.; Yang, W.; Chen, Y. Experimental and theoretical studies on the corrosion inhibition performance of 4-amino-N, N-di-(2-pyridylmethyl)-aniline on mild steel in hydrochloric acid. RSC Adv. 2015, 5, 56049–56059. [Google Scholar] [CrossRef]
- Singh, T.; Kumar, A. Aggregation behavior of ionic liquids in aqueous solutions: Effect of alkyl chain length, cations, and anions. J. Phys. Chem. B 2007, 111, 7843–7851. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, H.; Zhang, X. Tuning the amphiphilicity of building blocks: Controlled self-assembly and disassembly for functional supramolecular materials. Adv. Mater. 2009, 21, 2849–2864. [Google Scholar] [CrossRef]
- Song, Q.; Couzis, A.; Somasundaran, P.; Maldarelli, C. A transport model for the adsorption of surfactant from micelle solutions onto a clean air/water interface in the limit of rapid aggregate disassembly relative to diffusion and supporting dynamic tension experiments. Colloids Surf. A Physicochem. Eng. Asp. 2006, 282, 162–182. [Google Scholar] [CrossRef]
- Ansari, K.R.; Quraishi, M.A.; Singh, A.; Ramkumar, S.; Obote, I.B. Corrosion inhibition of N80 steel in 15% HCl by pyrazolone derivatives: Electrochemical, surface and quantum chemical studies. RSC Adv. 2016, 6, 24130–24141. [Google Scholar] [CrossRef]
- Torres, V.V.; Rayol, V.A.; Magalhaes, M.; Viana, G.M.; Aguiar, L.C.S.; Machado, S.P.; Orofino, H.; D’Elia, E. Study of thiourea derivatives synthesized from a green route as corrosion inhibitors for mild steel in HCl solution. Corros. Sci. 2014, 79, 108–118. [Google Scholar] [CrossRef]
- Lgaz, H.; Salghi, R.; Jodeh, S.; Hammouti, B. Effect of clozapine on inhibition of mild steel corrosion in 1.0 M HCl medium. J. Mol. Liq. 2017, 225, 271–280. [Google Scholar] [CrossRef]
- Espinoza-Vázquez, A.; Rodriguez-Gomez, F.; Mata, R.; Madariaga-Mazon, A.; Ángeles-Beltran, D. Perezone as corrosion inhibitor for AISI 1018 steel immersed in NaCl saturated with CO2. J. Solid State Electrochem. 2017, 21, 1687–1697. [Google Scholar] [CrossRef]
- El-Lateef, H.M.A. Experimental and computational investigation on the corrosion inhibition characteristics of mild steel by some novel synthesized imines in hydrochloric acid solutions. Corros. Sci. 2015, 92, 104–117. [Google Scholar] [CrossRef]
- Muralidharan, S.; Phani, K.; Pitchumani, S.; Ravichandran, S.; Iyer, S. Polyamino-benzoquinone polymers: A new class of corrosion inhibitors for mild steel. J. Electrochem. Soc. 1995, 142, 1478. [Google Scholar] [CrossRef]
- Zhang, G.; Chen, C.; Lu, M.; Chai, C.; Wu, Y. Evaluation of inhibition efficiency of an imidazoline derivative in CO2-containing aqueous solution. Mater. Chem. Phys. 2007, 105, 331–340. [Google Scholar] [CrossRef]
- El-Lateef, H.M.A.; Tantawy, A.H. Synthesis and evaluation of novel series of Schiff base cationic surfactants as corrosion inhibitors for carbon steel in acidic/chloride media: Experimental and theoretical investigations. RSC Adv. 2016, 6, 8681–8700. [Google Scholar] [CrossRef]
- Bentiss, F.; Lebrini, M.; Lagrenee, M. Thermodynamic characterization of metal dissolution and inhibitor adsorption processes in mild steel/2, 5-bis (n-thienyl)-1, 3, 4-thiadiazoles/hydrochloric acid system. Corros. Sci. 2005, 47, 2915–2931. [Google Scholar] [CrossRef]
- Popova, A. Temperature effect on mild steel corrosion in acid media in presence of azoles. Corros. Sci. 2007, 49, 2144–2158. [Google Scholar] [CrossRef]
- Singh, A.; Lin, Y.; Liu, W.; Kuanhai, D.; Pan, J.; Huang, B.; Ren, C.; Zeng, D. A study on the inhibition of N80 steel in 3.5% NaCl solution saturated with CO2 by fruit extract of Gingko biloba. J. Taiwan Inst. Chem. Eng. 2014, 45, 1918–1926. [Google Scholar] [CrossRef]
- Heydari, M.; Javidi, M. Corrosion inhibition and adsorption behaviour of an amido-imidazoline derivative on API 5L X52 steel in CO2-saturated solution and synergistic effect of iodide ions. Corros. Sci. 2012, 61, 148–155. [Google Scholar] [CrossRef]
- Reddy, M.J.; Verma, C.B.; Ebenso, E.; Singh, K.; Quaraishi, M. Electrochemical and thermodynamic investigation of nitrofurantoin as effective corrosion inhibitor for mild steel in 1 M hydrochloric acid solution. Int. J. Electrochem. Sci. 2014, 9, 4884–4899. [Google Scholar]
- Aljourani, J.; Raeissi, K.; Golozar, M. Benzimidazole and its derivatives as corrosion inhibitors for mild steel in 1M HCl solution. Corros. Sci. 2009, 51, 1836–1843. [Google Scholar] [CrossRef]
- Abdel-Gaber, A.; Khamis, E.; Abo-ElDahab, H.; Adeel, S. Inhibition of aluminium corrosion in alkaline solutions using natural compound. Mater. Chem. Phys. 2008, 109, 297–305. [Google Scholar] [CrossRef]
- Ahamad, I.; Prasad, R.; Quraishi, M. Inhibition of mild steel corrosion in acid solution by Pheniramine drug: Experimental and theoretical study. Corros. Sci. 2010, 52, 3033–3041. [Google Scholar] [CrossRef]
- Benzakour, J.; Berrekhis, F.; Bakasse, M.; Villemin, D. Investigation of the effect of piperidin-1-yl-phosphonic acid on corrosion of iron in sulfuric acid. Arab. J. Chem. 2016, 9, S1218–S1224. [Google Scholar] [CrossRef]
- Aoun, S.B. Erratum to “Highly Efficient Corrosion Inhibition of Carbon Steel in Aggressive Acidic Media with a Pyridazinium-based Ionic Liquid. Int. J. Electrochem. Sci. 2014, 9, 8476. [Google Scholar]
- Martinez, S.; Metikoš-Huković, M. A nonlinear kinetic model introduced for the corrosion inhibitive properties of some organic inhibitors. J. Appl. Electrochem. 2003, 33, 1137–1142. [Google Scholar] [CrossRef]
- Negm, N.A. Solubilization, surface active and thermodynamic parameters of Gemini amphiphiles bearing nonionic hydrophilic spacers. J. Surfactants Deterg. 2007, 10, 71–80. [Google Scholar] [CrossRef]
- Negm, N.; Al Sabagh, A.; Migahed, M.; Bary, H.A.; El Din, H. Effectiveness of some diquaternary ammonium surfactants as corrosion inhibitors for carbon steel in 0.5 M HCl solution. Corros. Sci. 2010, 52, 2122–2132. [Google Scholar] [CrossRef]
- Ghailane, T.; Balkhmima, R.; Ghailane, R.; Souizi, A.; Touir, R.; Touhami, M.E.; Marakchi, K.; Komiha, N. Experimental and theoretical studies for mild steel corrosion inhibition in 1 M HCl by two new benzothiazine derivatives. Corros. Sci. 2013, 76, 317–324. [Google Scholar] [CrossRef]
- Tazouti, A.; Errahmany, N.; Rbaa, M.; Galai, M.; Rouifi, Z.; Touir, R.; Zarrouk, A.; Kaya, S.; Touhami, M.E.; El Ibrahimi, B. Effect of hydrocarbon chain length for acid corrosion inhibition of mild steel by three 8-(n-bromo-R-alkoxy) quinoline derivatives: Experimental and theoretical investigations. J. Mol. Struct. 2021, 1244, 130976. [Google Scholar] [CrossRef]
- Verma, C.; Quraishi, M.; Rhee, K. Hydrophilicity and hydrophobicity consideration of organic surfactant compounds: Effect of alkyl chain length on corrosion protection. Adv. Colloid Interface Sci. 2022, 306, 102723. [Google Scholar] [CrossRef]
- Touafri, L.; Hellal, A.; Chafaa, S.; Khelifa, A.; Kadri, A. Synthesis, characterisation and DFT studies of three Schiff bases derived from histamine. J. Mol. Struct. 2017, 1149, 750–760. [Google Scholar] [CrossRef]
- Khaled, K. Monte Carlo simulations of corrosion inhibition of mild steel in 0.5 M sulphuric acid by some green corrosion inhibitors. J. Solid State Electrochem. 2009, 13, 1743–1756. [Google Scholar] [CrossRef]
- Khaled, K.; Amin, M.A. Computational and electrochemical investigation for corrosion inhibition of nickel in molar nitric acid by piperidines. J. Appl. Electrochem. 2008, 38, 1609–1621. [Google Scholar] [CrossRef]
- Obot, I.B.; Obi-Egbedi, N.O. Theoretical study of benzimidazole and its derivatives and their potential activity as corrosion inhibitors. Corros. Sci. 2010, 52, 657–660. [Google Scholar] [CrossRef]
- Yadav, M.; Behera, D.; Kumar, S.; Sinha, R.R. Experimental and quantum chemical studies on the corrosion inhibition performance of benzimidazole derivatives for mild steel in HCl. Ind. Eng. Chem. Res. 2013, 52, 6318–6328. [Google Scholar] [CrossRef]
- Kokalj, A. On the HSAB based estimate of charge transfer between adsorbates and metal surfaces. Chem. Phys. 2012, 393, 1–12. [Google Scholar] [CrossRef]
- Mrani, S.A.; Ech-chihbi, E.; Arrousse, N.; Rais, Z.; El Hajjaji, F.; El Abiad, C.; Radi, S.; Mabrouki, J.; Taleb, M.; Jodeh, S. DFT and Electrochemical Investigations on the Corrosion Inhibition of Mild Steel by Novel Schiff’s Base Derivatives in 1 M HCl Solution. Arab. J. Sci. Eng. 2021, 46, 5691–5707. [Google Scholar] [CrossRef]
- Hong, S.; Chen, W.; Luo, H.Q.; Li, N.B. Inhibition effect of 4-amino-antipyrine on the corrosion of copper in 3 wt.% NaCl solution. Corros. Sci. 2012, 57, 270–278. [Google Scholar] [CrossRef]
- Coleman, W.F.; Arumainayagam, C.R. HyperChem 5 (by Hypercube, Inc.). J. Chem. Educ. 1998, 75, 416. [Google Scholar] [CrossRef][Green Version]
- Belghiti, M.; El Ouadi, Y.; Echihi, S.; Elmelouky, A.; Outada, H.; Karzazi, Y.; Bakasse, M.; Jama, C.; Bentiss, F.; Dafali, A. Anticorrosive properties of two 3, 5-disubstituted-4-amino-1, 2, 4-triazole derivatives on copper in hydrochloric acid environment: Ac impedance, thermodynamic and computational investigations. Surf. Interfaces 2020, 21, 100692. [Google Scholar] [CrossRef]
- Obot, I.; Macdonald, D.; Gasem, Z. Density functional theory (DFT) as a powerful tool for designing new organic corrosion inhibitors. Part 1: An overview. Corros. Sci. 2015, 99, 1–30. [Google Scholar] [CrossRef]
- Feng, L.; Yin, C.; Zhang, H.; Li, Y.; Song, X.; Chen, Q.; Liu, H. Cationic Gemini surfactants with a bipyridyl spacer as corrosion inhibitors for carbon steel. ACS Omega 2018, 3, 18990–18999. [Google Scholar] [CrossRef]
- El-Lateef, H.M.A.; Shalabi, K.; Tantawy, A.H. Corrosion inhibition and adsorption features of novel bioactive cationic surfactants bearing benzenesulphonamide on C1018-steel under sweet conditions: Combined modeling and experimental approaches. J. Mol. Liq. 2020, 320, 114564. [Google Scholar] [CrossRef]
- Nagarajan, R. Molecular packing parameter and surfactant self-assembly: The neglected role of the surfactant tail. Langmuir 2002, 18, 31–38. [Google Scholar] [CrossRef]
- Welbourn, R.J.; Truscott, C.; Skoda, M.; Zarbakhsh, A.; Clarke, S. Corrosion and inhibition of copper in hydrocarbon solution on a molecular level investigated using neutron reflectometry and XPS. Corros. Sci. 2017, 115, 68–77. [Google Scholar] [CrossRef]
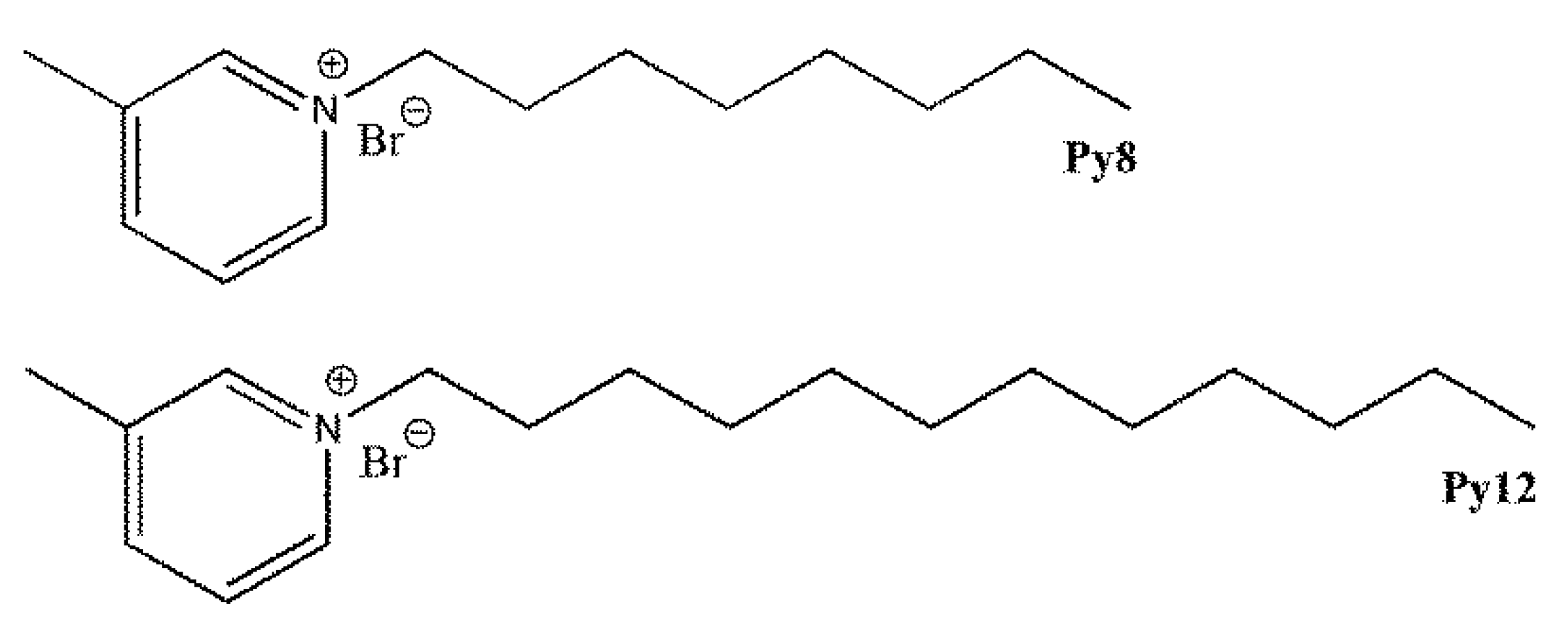






| Chemical Composition (Weight %) | C | Mn | Si | P | S | Fe |
|---|---|---|---|---|---|---|
| Minimum | 0.13 | 0.70 | 0.10 | 0 | 0 | Balance |
| Maximum | 0.18 | 0.90 | 0.40 | 0.05 | 0.05 | Balance |
| Concentration (m mol dm−3) | βa (mV/Decade) | −βc (mV/Decade) | Ecorr (mV)/(Ag/AgCl) | −icorr (µA/cm2) | IE | |
|---|---|---|---|---|---|---|
| Blank | 0 | 15.59 | 5.82 | −518 | 65.0 | - |
| Py8 | 0.5 | 11.59 | 8.81 | −507 | 29.0 | 55.38 |
| 1 | 10.00 | 7.93 | −497 | 14.0 | 78.46 | |
| 5 | 8.23 | 7.44 | −495 | 11.0 | 83.08 | |
| 10 | 8.40 | 7.00 | −503 | 9.9 | 84.74 | |
| 15 | 7.50 | 6.08 | −520 | 11.4 | 82.46 | |
| Py12 | 0.5 | 6.90 | 5.84 | −515 | 18.9 | 70.92 |
| 1 | 8.07 | 6.03 | −511 | 15.0 | 76.92 | |
| 5 | 7.57 | 6.75 | −507 | 18.7 | 71.23 | |
| 10 | 7.26 | 6.25 | −501 | 20.2 | 68.92 | |
| 15 | 8.84 | 7.47 | −458 | 17.2 | 73.54 |
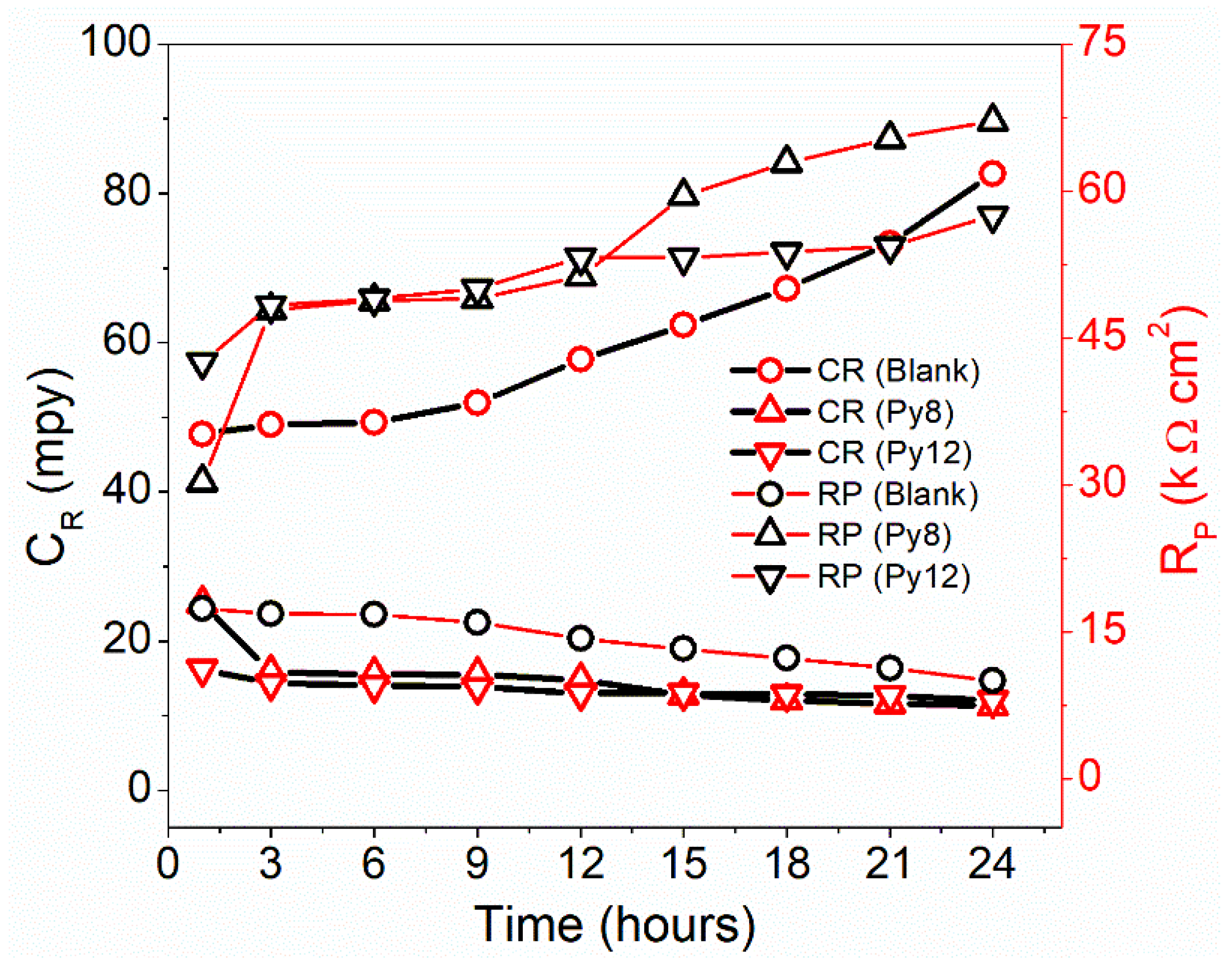
| Time (h) | Blank | Py8 | Py12 | |||||
|---|---|---|---|---|---|---|---|---|
| CR (mpy) | RP (Ω cm2) | CR (mpy) | RP (Ω cm2) | IE | CR (mpy) | RP (Ω cm2) | IE | |
| 1 | 47.70 | 17,331 | 25.05 | 30,303 | 42.81 | 16.26 | 42,553 | 59.27 |
| 3 | 49.02 | 16,863 | 15.87 | 47,847 | 64.76 | 14.32 | 48,309 | 65.09 |
| 6 | 49.27 | 16,779 | 15.56 | 48,781 | 65.60 | 14.11 | 49,019 | 65.77 |
| 9 | 51.91 | 15,924 | 15.49 | 49,020 | 67.52 | 13.84 | 50,000 | 68.15 |
| 12 | 57.78 | 14,306 | 14.80 | 51,282 | 72.10 | 13.15 | 52,632 | 72.82 |
| 15 | 62.33 | 13,263 | 12.76 | 59,524 | 77.72 | 13.01 | 53,192 | 75.07 |
| 18 | 67.20 | 12,300 | 12.07 | 62,893 | 80.44 | 12.87 | 53,763 | 77.12 |
| 21 | 73.24 | 11,287 | 11.62 | 65,360 | 82.73 | 12.73 | 54,348 | 79.23 |
| 24 | 82.66 | 10,000 | 11.31 | 67,114 | 85.10 | 12.04 | 57,471 | 82.60 |
| Compound | Conc. (mM) | Rp × 103 (Ω cm2) | Rct × 103 (Ω cm2) | Yodl × 10−6 (S sn cm−2) | Yoc × 10−6 (S sm cm−2) | n | M | χ2 × 10−6 | IE |
|---|---|---|---|---|---|---|---|---|---|
| Blank | - | - | 1.69 | 76.26 | - | 0.76 | - | 91.30 | - |
| Py8 | 0.5 | 2.65 | 5.29 | 13.85 | 5.51 | 0.62 | 0.89 | 22.91 | 68.01 |
| 1 | 2.36 | 8.16 | 14.88 | 5.04 | 0.60 | 0.86 | 16.44 | 79.24 | |
| 5 | 6.34 | 5.14 | 10.10 | 12.73 | 0.59 | 0.74 | 199.8 | 67.02 | |
| 10 | 3.52 | 9.77 | 21.26 | 3.49 | 0.57 | 0.84 | 80.32 | 82.65 | |
| 15 | 3.17 | 11.15 | 20.39 | 3.53 | 0.53 | 0.88 | 23.91 | 84.80 | |
| 0.5 | 3.50 | 9.89 | 19.24 | 3.90 | 0.56 | 0.79 | 9.80 | 82.86 | |
| 1 | 3.96 | 9.09 | 17.21 | 3.50 | 0.54 | 0.79 | 15.51 | 81.35 | |
| Py12 | 5 | 3.75 | 7.60 | 19.07 | 6.74 | 0.47 | 0.83 | 12.22 | 77.70 |
| 10 | 1.69 | 7.87 | 16.96 | 7.87 | 0.51 | 0.82 | 23.38 | 78.46 | |
| 15 | 1.06 | 7.39 | 21.05 | 8.85 | 0.55 | 0.79 | 41.92 | 77.06 |
| Compound | Time (h) | Rp × 103 (Ω cm2) | Rct × 103 (Ω cm2) | Yodl × 10−6 (S sn cm−2) | Yoc × 10−6 (S sm cm−2) | n | M | χ2 × 10−6 | IE |
|---|---|---|---|---|---|---|---|---|---|
| Py8 | 1 | 3.29 | 8.78 | 37.30 | 7.53 | 0.42 | 0.78 | 92.40 | 80.69 |
| 6 | 4.04 | 9.89 | 35.53 | 6.46 | 0.52 | 0.86 | 130.50 | 82.86 | |
| 12 | 4.52 | 11.30 | 31.70 | 6.41 | 0.39 | 0.77 | 127.70 | 85.00 | |
| 24 | 5.02 | 13.77 | 27.91 | 5.58 | 0.59 | 0.78 | 124.20 | 87.69 |
| Medium | Compound | Isotherm | R2 | Kads (L mol−1) | −ΔG°ads (kJ mol−1) |
|---|---|---|---|---|---|
| 3.5% NaCl pH = 1.5 ± 0.01 | Py8 | Langmuir | 0.999 | 7092 | 32 |
| Py12 | Langmuir | 0.998 | 11738 | 33 |
| Compound | HOMO | LUMO |
|---|---|---|
| Py8 | 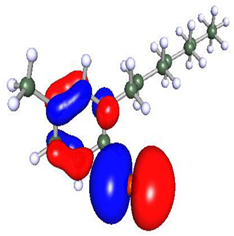 | 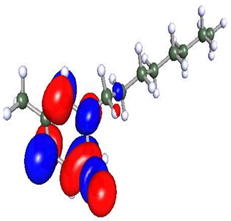 |
| Py12 |  |  |
| Compound | EHOMO (eV) | ELUMO (eV) | ΔE (eV) | I (eV) | A (eV) | η (eV) | χ (eV) | (eV−1) | ΔN |
|---|---|---|---|---|---|---|---|---|---|
| Py8 | −2.377 | −0.525 | 1.852 | 2.377 | 0.525 | 0.926 | 1.451 | 1.079 | 1.81 |
| Py12 | −4.701 | −2.562 | 2.138 | 4.701 | 2.562 | 1.069 | 3.631 | 0.935 | 0.55 |
| Atomic Charges (e) | Compounds | Atomic Charges (e) | Compounds | ||
|---|---|---|---|---|---|
| Py8 | Py12 | Py8 | Py12 | ||
| q1c | −0.173 | 0.171 | q12H | 0.078 | 0.078 |
| q2c | −0.053 | −0.056 | q15c | −0.108 | −0.112 |
| q3c | −0.080 | −0.073 | q16c | −0.124 | −0.128 |
| q4N | 0.110 | 0.106 | q20H | 0.064 | 0.067 |
| q5c | −0.049 | −0.046 | q24c | −0.099 | −0.106 |
| q6c | 0.054 | 0.051 | q27H | 0.052 | 0.054 |
| q7H | 0.115 | 0.115 | q36H | 0.072 | 0.051 |
| q8H | 0.129 | 0.131 | q39Br | −0.564 | −0.560 |
| q11c | −0.241 | −0.242 | q40C | - | −0.099 |
| Compound | EI (kJ/mol) | Esolvated surface (kJ/mol) | Eadsorbed (kJ/mol) | Eint (kJ/mol) | Ebind (kJ/mol) |
|---|---|---|---|---|---|
| Py8 | 70.27 | −2395.88 | −2534.88 | −209.27 | 209.27 |
| Py12 | 63.24 | −2395.88 | −2460.77 | −128.13 | 128.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Talat, R.; Asghar, M.A.; Tariq, I.; Akhter, Z.; Liaqat, F.; Nadeem, L.; Haider, A.; Ali, S. Evaluating the Corrosion Inhibition Efficiency of Pyridinium-Based Cationic Surfactants for EN3B Mild Steel in Acidic-Chloride Media. Coatings 2022, 12, 1701. https://doi.org/10.3390/coatings12111701
Talat R, Asghar MA, Tariq I, Akhter Z, Liaqat F, Nadeem L, Haider A, Ali S. Evaluating the Corrosion Inhibition Efficiency of Pyridinium-Based Cationic Surfactants for EN3B Mild Steel in Acidic-Chloride Media. Coatings. 2022; 12(11):1701. https://doi.org/10.3390/coatings12111701
Chicago/Turabian StyleTalat, Rabia, Muhammad Adeel Asghar, Irsa Tariq, Zareen Akhter, Faroha Liaqat, Laiba Nadeem, Ali Haider, and Saqib Ali. 2022. "Evaluating the Corrosion Inhibition Efficiency of Pyridinium-Based Cationic Surfactants for EN3B Mild Steel in Acidic-Chloride Media" Coatings 12, no. 11: 1701. https://doi.org/10.3390/coatings12111701
APA StyleTalat, R., Asghar, M. A., Tariq, I., Akhter, Z., Liaqat, F., Nadeem, L., Haider, A., & Ali, S. (2022). Evaluating the Corrosion Inhibition Efficiency of Pyridinium-Based Cationic Surfactants for EN3B Mild Steel in Acidic-Chloride Media. Coatings, 12(11), 1701. https://doi.org/10.3390/coatings12111701







