Carbon/Graphite Sheets/PTFE-Coated Porous Titanium as the Bipolar Plate by Hydrothermal Treatment
Abstract
:1. Introduction
2. Experimental
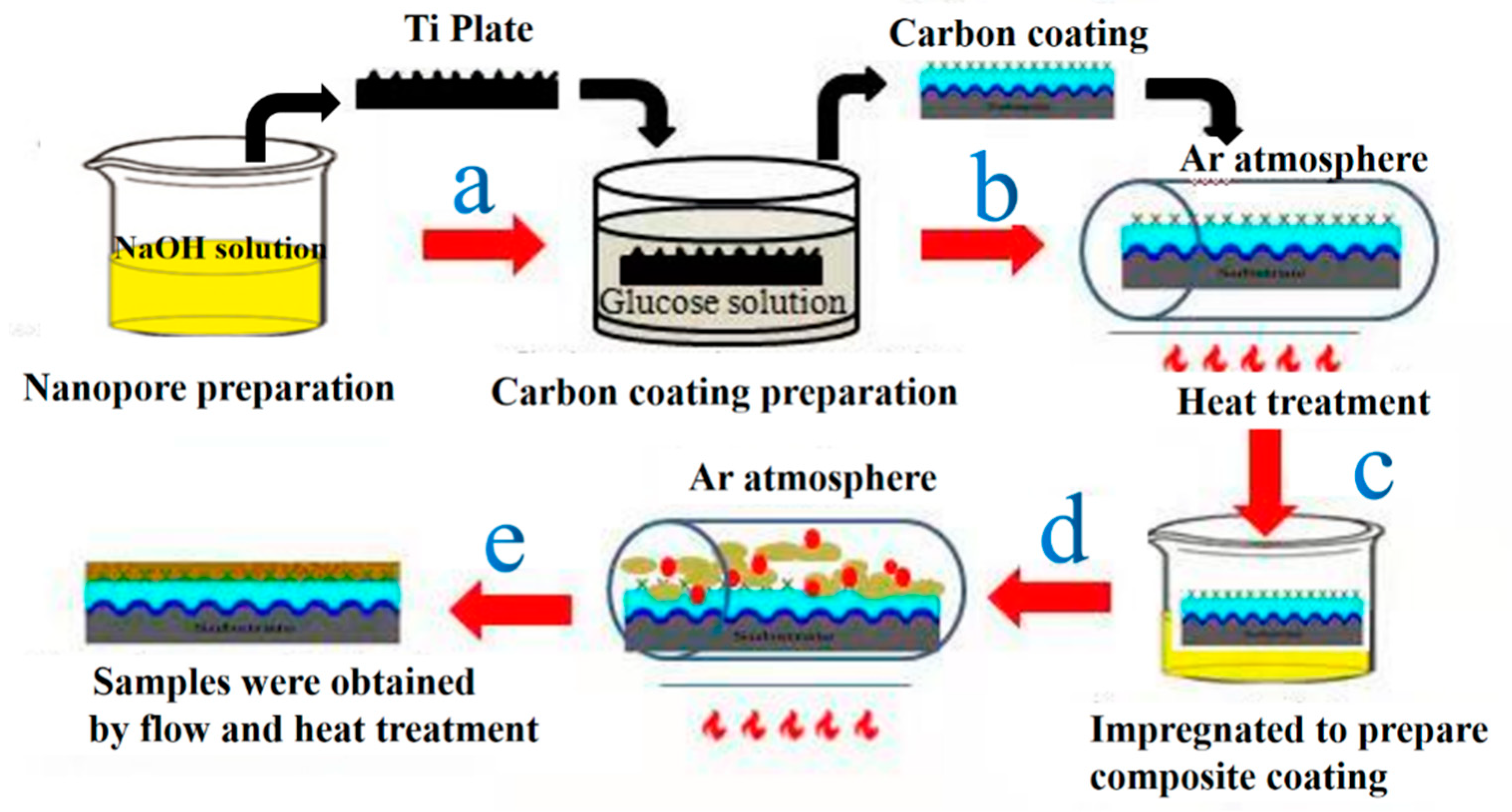
2.1. The Preparation of Bipolar Plates
2.2. The Preparation of Composite Coating
2.3. Surface Characterization
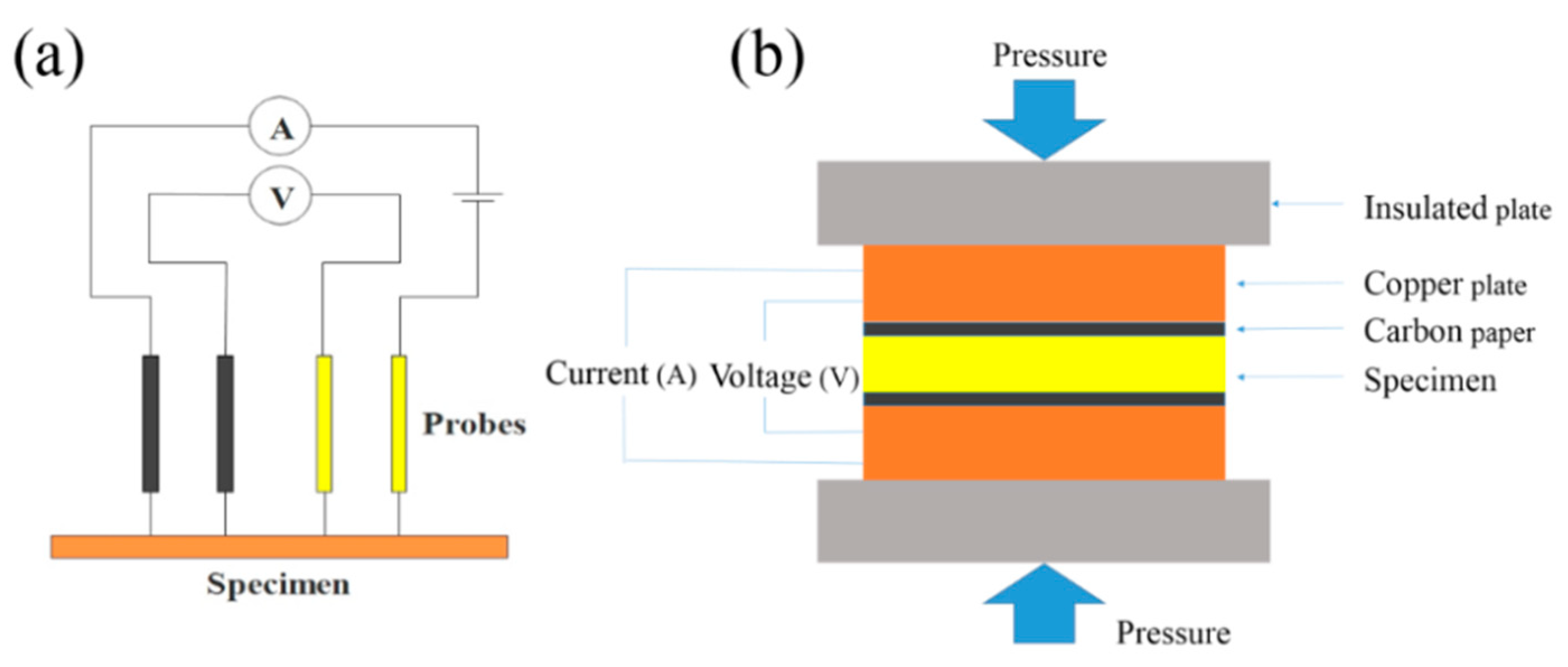
2.4. Conductivity
2.5. The Properties of Electrochemistry
3. Results and Discussion
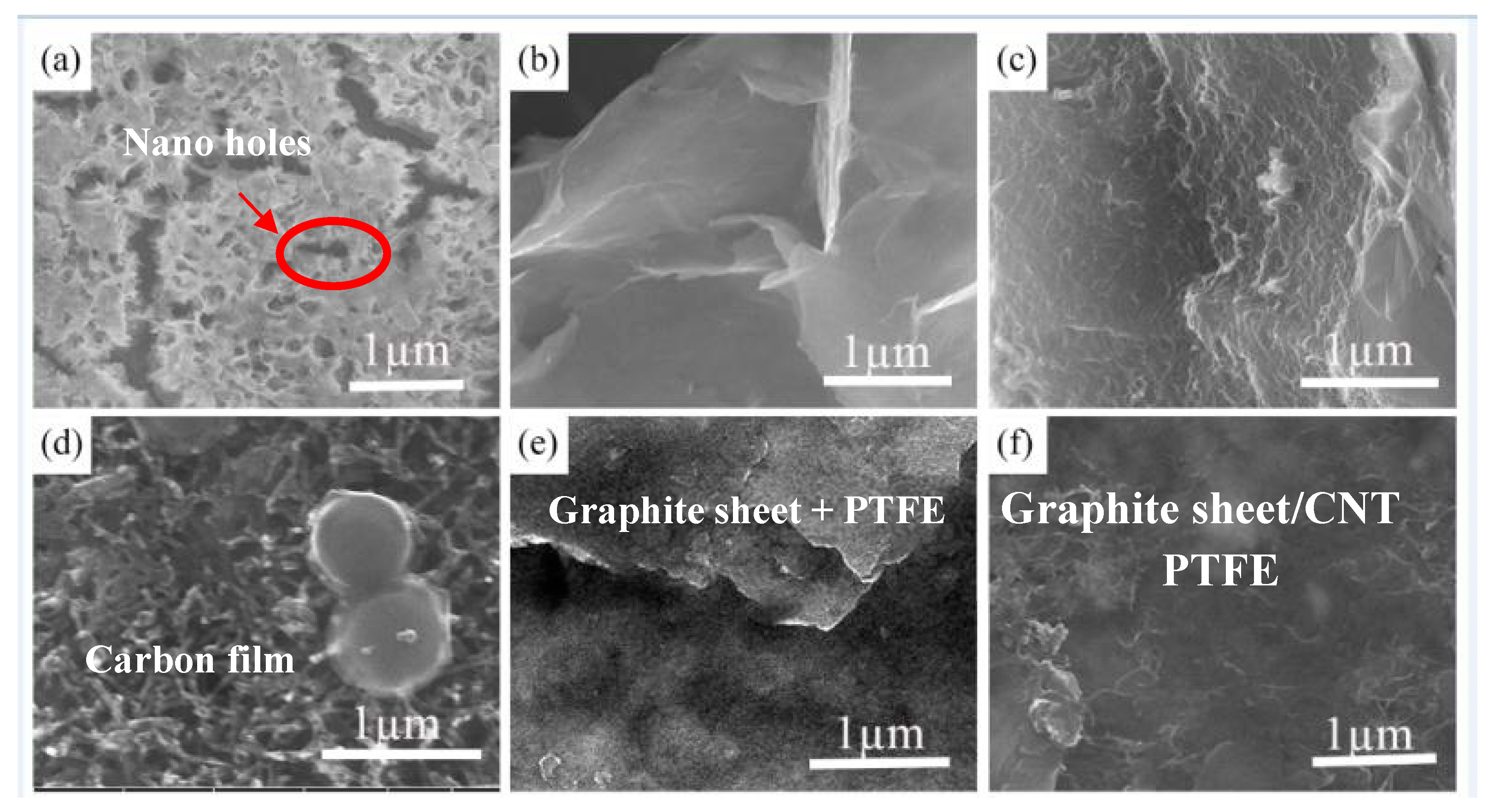
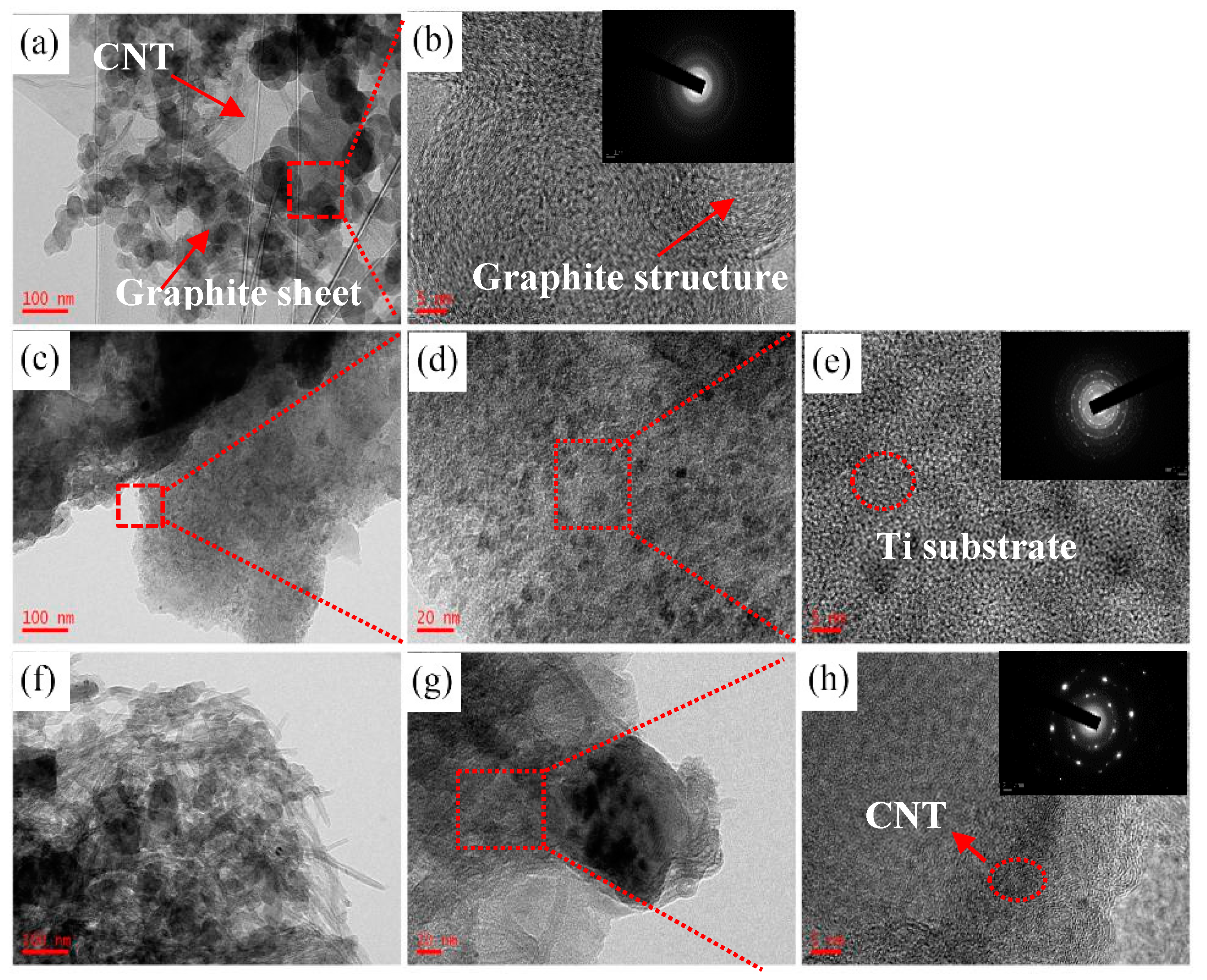
3.1. Microstructural Characterizations of the Coatings
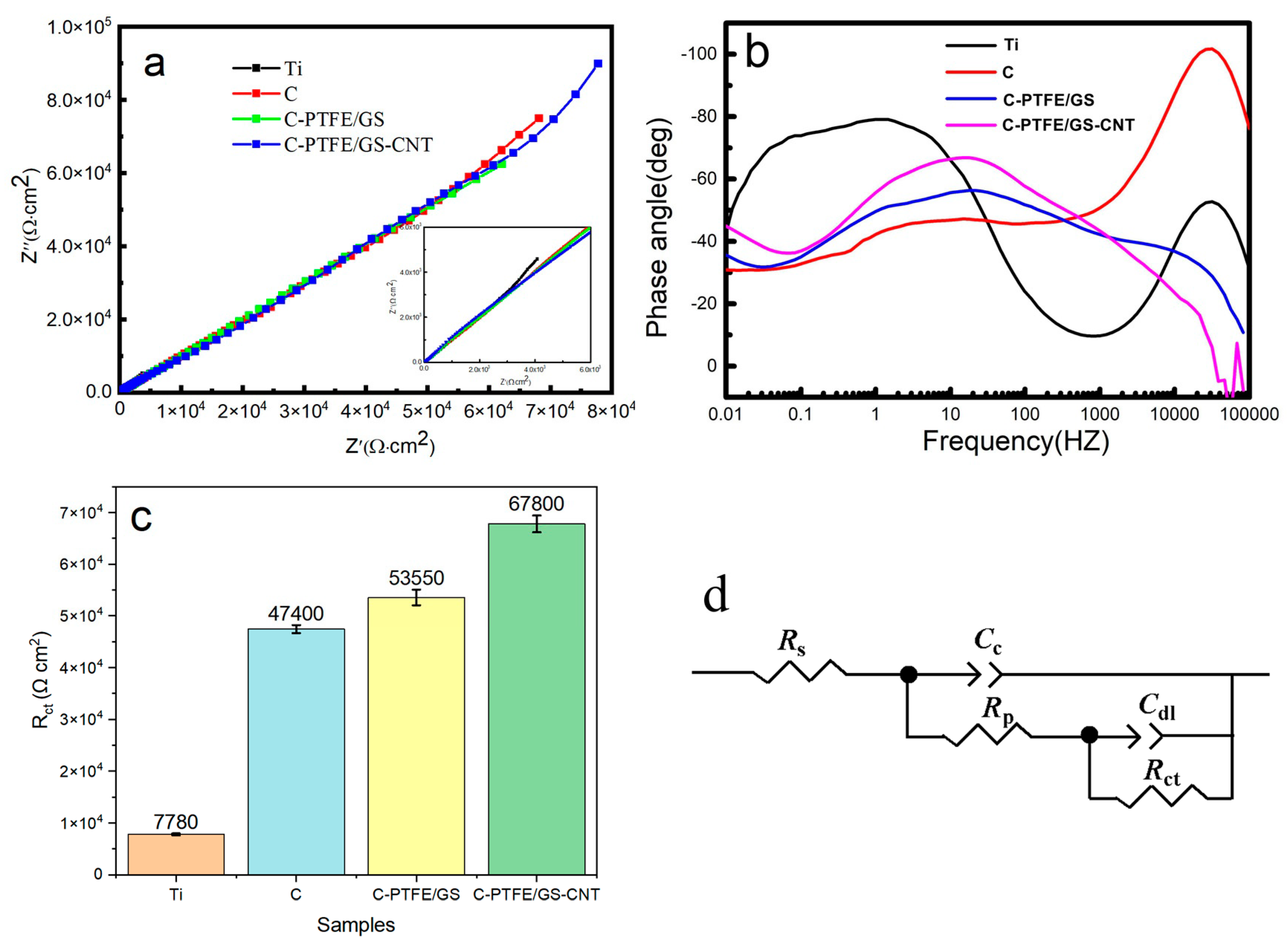
3.2. Evaluations of Electrochemical Corrosion
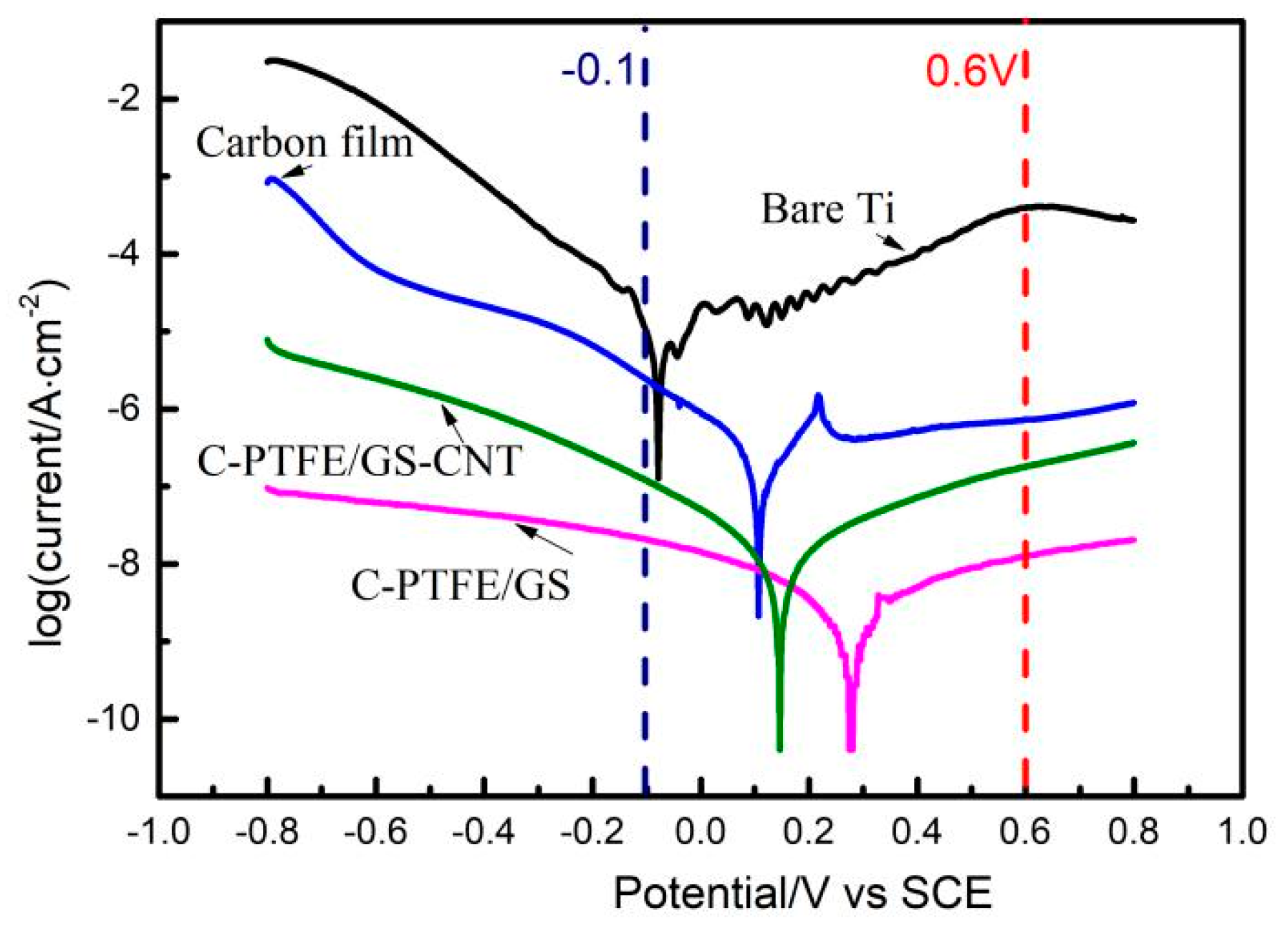
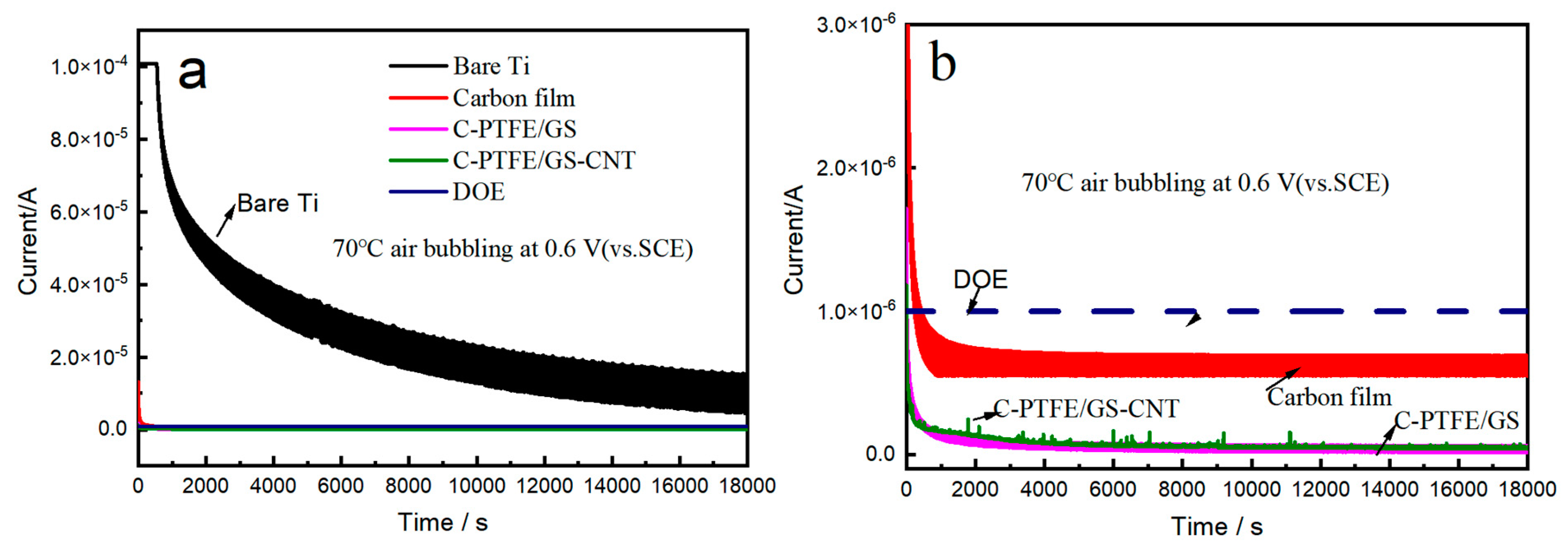
3.3. Potentiostatic Polarization
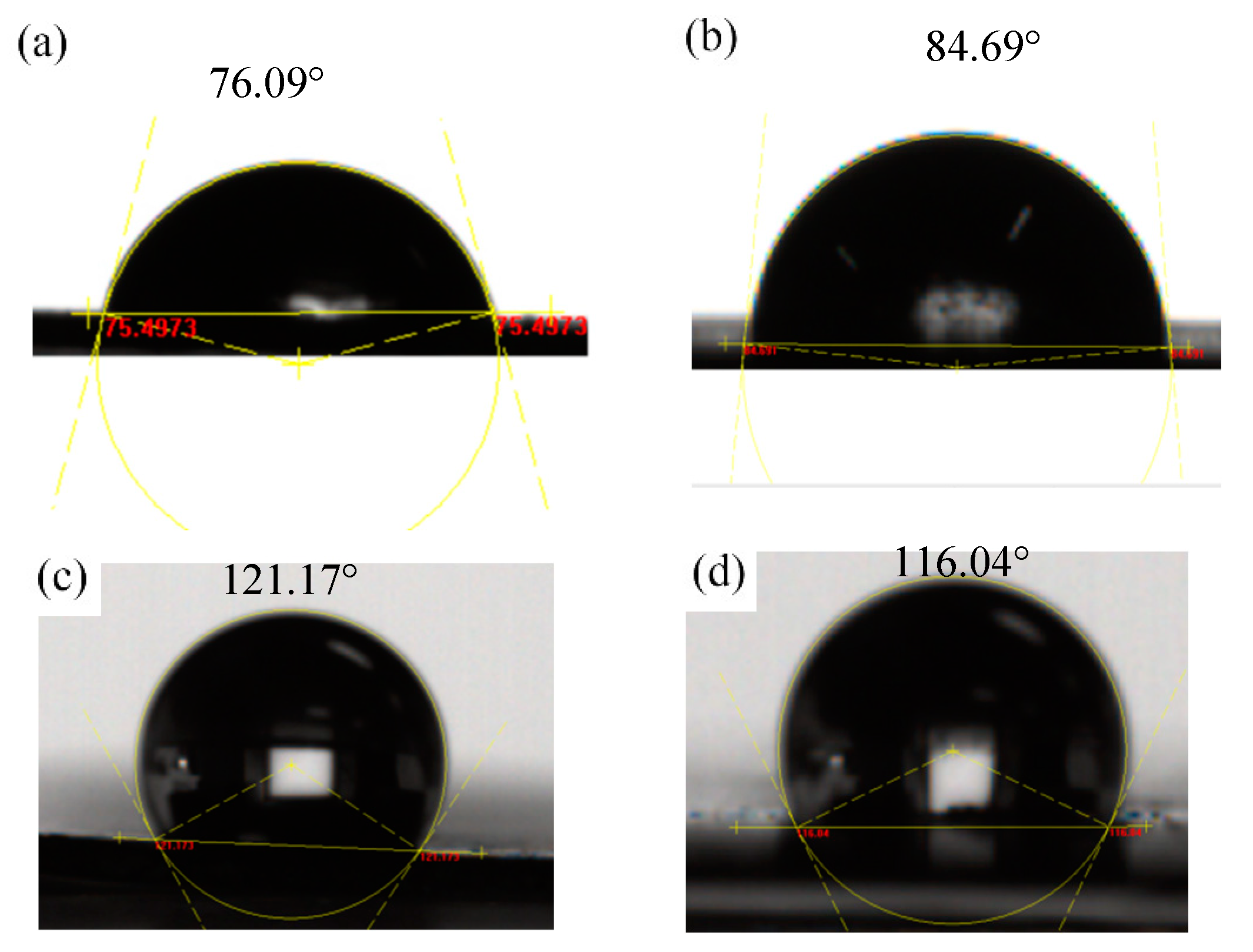
3.4. Contact Angle
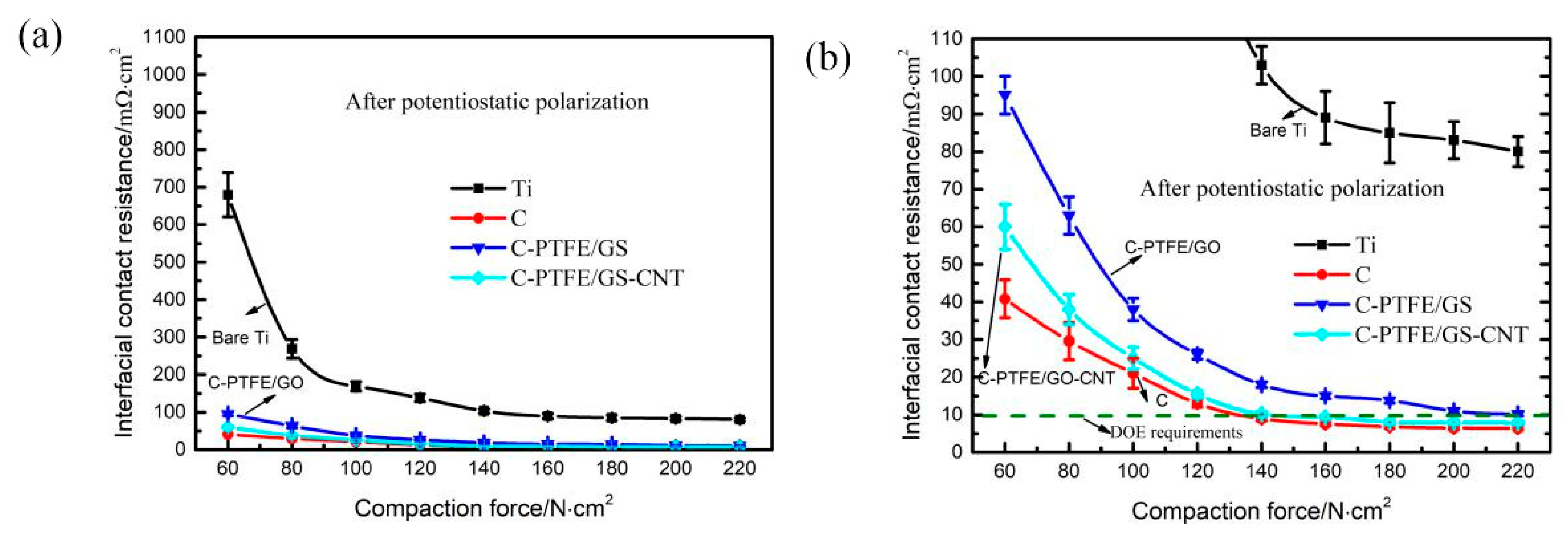
3.5. The Measurements of Conductivity
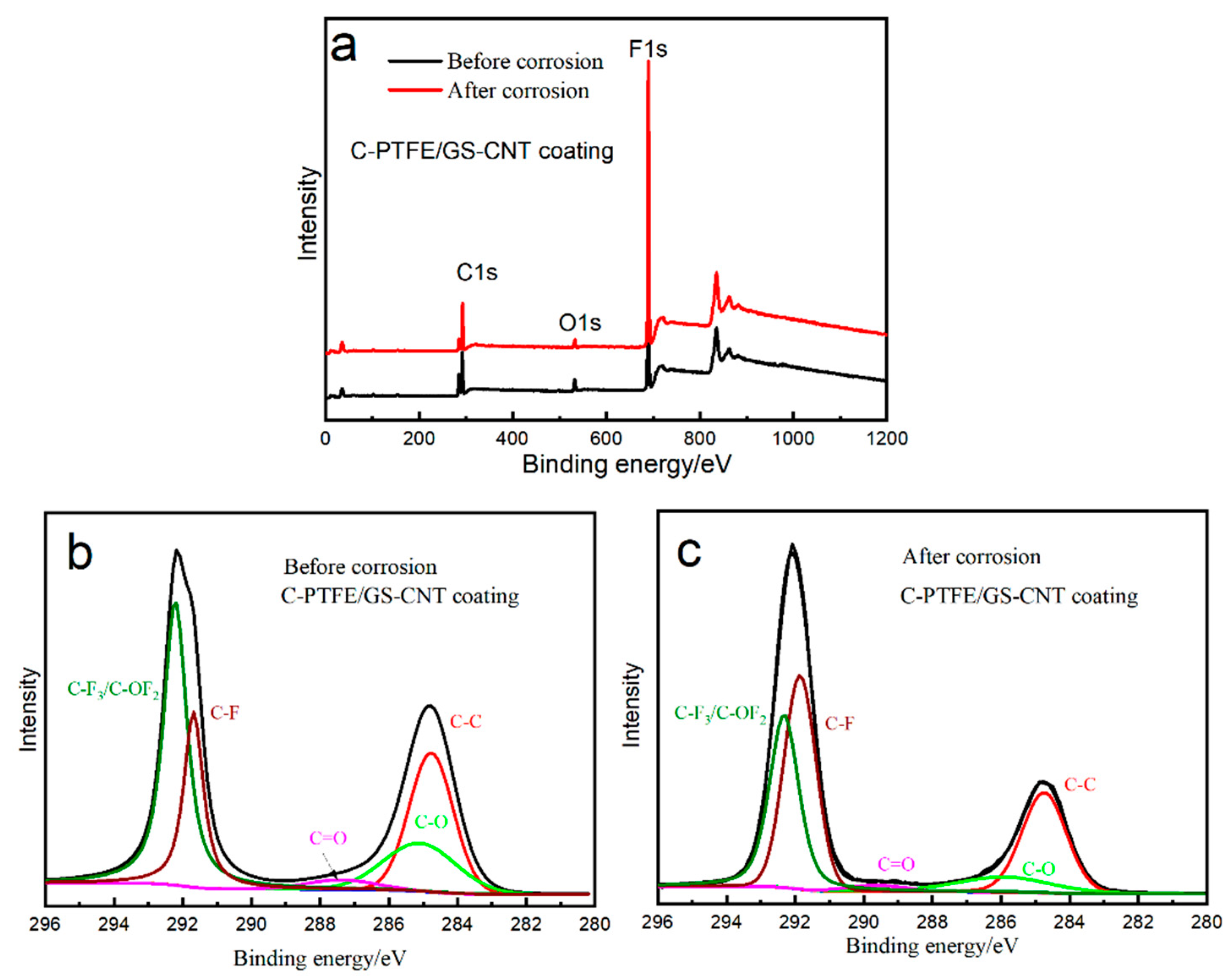
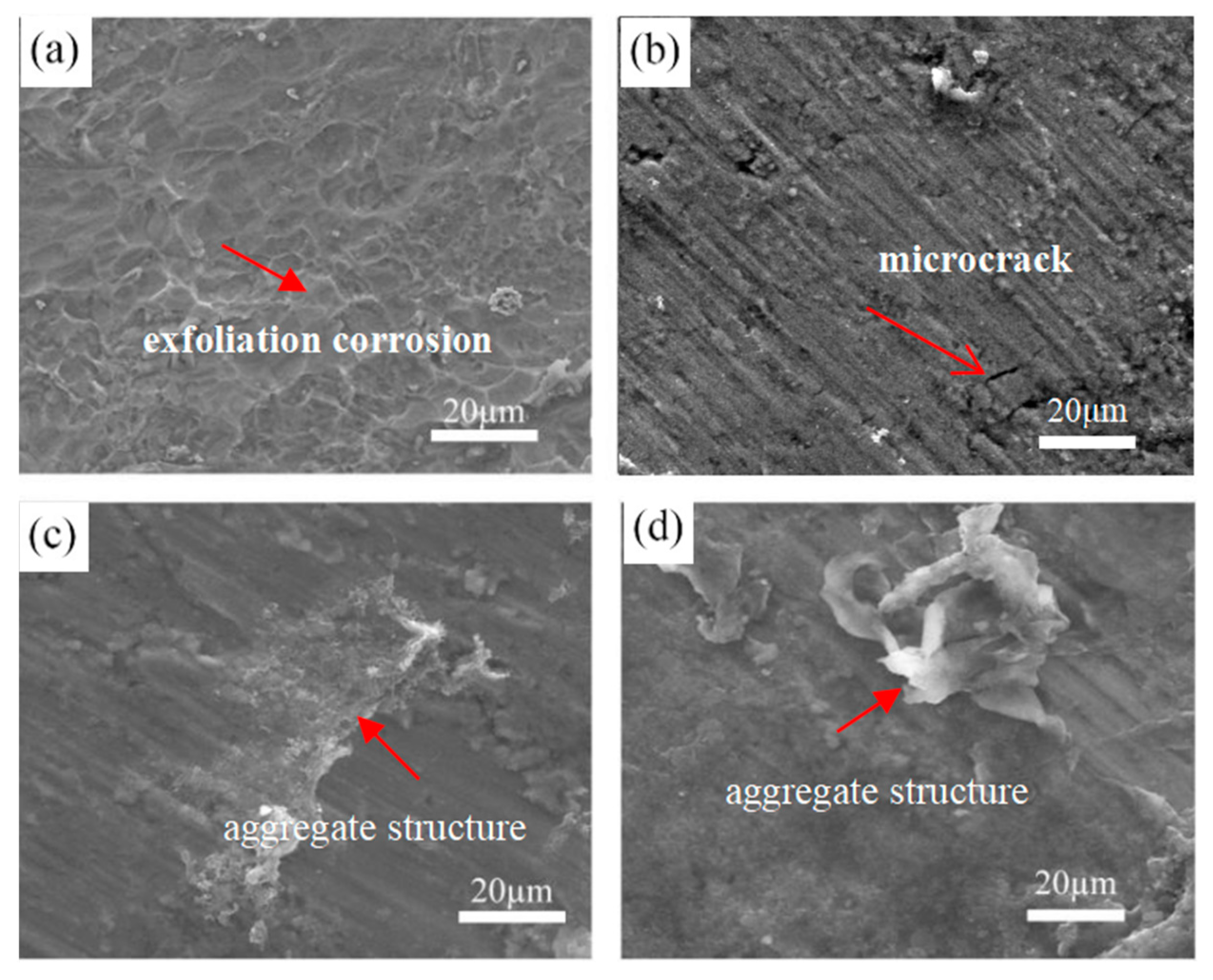
3.6. Corrosive Morphology after Potentiostatic Polarization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ouyang, C.; Xun, D.; Jian, G. N-doped and sulfonated reduced graphene oxide supported PtNi nanoparticles as highly efficient electrocatalysts for oxygen reduction reaction. Coatings 2022, 12, 1049. [Google Scholar] [CrossRef]
- Sinniah, J.D.; Wong, W.Y.; Loh, K.S.; Yunus, R.M.; Timmiati, S.N. Perspectives on carbon-alternative materials as Pt catalyst supports for a durable oxygen reduction reaction in proton exchange membrane fuel cells. J. Power Sources 2022, 534, 231422. [Google Scholar] [CrossRef]
- Barnoon, P.; Toghraie, D.; Mehmandoust, B.; Fazilati, M.A.; Eftekhari, S.A. Natural-forced cooling and Monte-Carlo multi-objective optimization of mechanical and thermal characteristics of a bipolar plate for use in a proton exchange membrane fuel cell. Energy Rep. 2022, 8, 2747–2761. [Google Scholar] [CrossRef]
- Chen, G.; Singh, S.K.; Takeyasu, K.; Hill, J.P.; Nakamura, J.; Ariga, K. Versatile nanoarchitectonics of Pt with morphology control of oxygen reduction reaction catalysts. Sci. Technol. Adv. Mater. 2022, 23, 413–423. [Google Scholar] [CrossRef]
- Park, Y.; Shin, K.; Lee, C.; Lee, S.-Y.; Lee, Y.-K.; Kim, C.-H.; Cho, H.-S.; Henkelman, G.; Lee, H.M. Iterative redox activation promotes interfacial synergy in an Ag/CuxO catalyst for oxygen reduction. Chem. Eng. J. 2022, 446, 136966. [Google Scholar] [CrossRef]
- Fortunato, G.V.; Cardoso, E.S.F.; Martini, B.K.; Maia, G. Ti/Pt−Pd-based nanocomposite: Effects of metal oxides on the oxygen reduction reaction. ChemElectroChem 2020, 7, 1610–1618. [Google Scholar] [CrossRef]
- Ouyang, C.; Zhang, X.; Wu, M.; Xun, D.; Gao, P. Physical and electrochemical properties of Ni-P/TiN coated Ti for bipolar plates in PEMFCs. Int. J. Electrochem. Sci. 2020, 15, 80–93. [Google Scholar] [CrossRef]
- Yang, L.; Chen, M.; Wang, J.; Qiao, Y.; Guo, P.; Zhu, S.; Wang, F. Microstructure and composition evolution of a single-crystal superalloy caused by elements interdiffusion with an overlay NiCrAlY coating on oxidation. J. Mater. Sci. Technol. 2020, 45, 49–58. [Google Scholar] [CrossRef]
- Wang, L.; Northwood, D.; Nie, X.; Housden, J.; Spain, E.; Leyland, A.; Matthews, A. Corrosion properties and contact resistance of TiN, TiAlN and CrN coatings in simulated proton exchange membrane fuel cell environments. J. Power Sources 2010, 195, 3814–3821. [Google Scholar] [CrossRef]
- Wang, H.-C.; Hou, K.-H.; Lu, C.-E.; Ger, M.-D. The study of electroplating trivalent CrC alloy coatings with different current densities on stainless steel 304 as bipolar plate of proton exchange membrane fuel cells. Thin Solid Film. 2014, 570, 209–214. [Google Scholar] [CrossRef]
- Zhang, H.; Lin, G.; Hou, M.; Hu, L.; Han, Z.; Fu, Y.; Shao, Z.; Yi, B. CrN/Cr multilayer coating on 316L stainless steel as bipolar plates for proton exchange membrane fuel cells. J. Power Sources 2012, 198, 176–181. [Google Scholar] [CrossRef]
- Yi, P.; Peng, L.; Zhou, T.; Wu, H.; Lai, X. Development and characterization of multilayered Cr–C/a-C:Cr film on 316L stainless steel as bipolar plates for proton exchange membrane fuel cells. J. Power Sources 2013, 230, 25–31. [Google Scholar] [CrossRef]
- Yi, P.; Peng, L.; Zhou, T.; Huang, J.; Lai, X. Composition optimization of multilayered chromium-nitride–carbon film on 316L stainless steel as bipolar plates for proton exchange membrane fuel cells. J. Power Sources 2013, 236, 47–53. [Google Scholar] [CrossRef]
- Feng, K.; Li, Z.; Lu, F.; Huang, J.; Cai, X.; Wu, Y. Corrosion resistance and electrical properties of carbon/chromium–titanium–nitride multilayer coatings on stainless steel. J. Power Sources 2014, 249, 299–305. [Google Scholar] [CrossRef]
- Zada, A.; Ali, N.; Subhan, F.; Anwar, N.; Ali Shah, M.I.; Ateeq, M.; Hussain, Z.; Zaman, K.; Khan, M. Suitable energy platform significantly improves charge separation of g-C3N4 for CO2 reduction and pollutant oxidation under visible-light. Prog. Nat. Sci. 2019, 29, 138–144. [Google Scholar] [CrossRef]
- Gao, P.; Xie, Z.; Wu, X.; Ouyang, C.; Lei, T.; Yang, P.; Liu, C.; Wang, J.; Ouyang, T.; Huang, Q. Development of Ti bipolar plates with carbon/PTFE/TiN composites coating for PEMFCs. Int. J. Hydrog. Energy 2018, 43, 20947–20958. [Google Scholar] [CrossRef]
- Wang, W.-L.; He, S.-M.; Lan, C.-H. Protective graphite coating on metallic bipolar plates for PEMFC applications. Electrochim. Acta 2012, 62, 30–35. [Google Scholar] [CrossRef]
- Larijani, M.; Yari, M.; Afshar, A.; Jafarian, M.; Eshghabadi, M. A comparison of carbon coated and uncoated 316L stainless steel for using as bipolar plates in PEMFCs. J. Alloys Compd. 2011, 509, 7400–7404. [Google Scholar] [CrossRef]
- Li, W.; Liu, L.-T.; Li, Z.-X.; Wang, Y.-F.; Li, H.-Z.; Lei, J.-J. Corrosion resistance and conductivity of amorphous carbon coated SS316L and TA2 bipolar plates in proton-exchange membrane fuel cells. Diam. Relat. Mater. 2021, 118, 108503. [Google Scholar] [CrossRef]
- Bi, F.; Peng, L.; Yi, P.; Lai, X. Multilayered Zr–C/a-C film on stainless steel 316L as bipolar plates for proton exchange membrane fuel cells. J. Power Sources 2016, 314, 58–65. [Google Scholar] [CrossRef]
- Lin, M.-T.; Wan, C.-H.; Wu, W. Comparison of corrosion behaviors between SS304 and Ti substrate coated with (Ti,Zr)N thin films as Metal bipolar plate for unitized regenerative fuel cell. Thin Solid Films 2013, 544, 162–169. [Google Scholar] [CrossRef]
- Pingping, G.; Chun, O.; Zhiyong, X.; Tao, T. Corrosion protection of electroless plating Ni-P including tin nanoparticles for bipolar plates of PEMFCs. Surf. Rev. Lett. 2018, 25, 1850052. [Google Scholar] [CrossRef]
- Gao, M.L.; Wu, X.B.; Gao, P.P.; Ting, L.E.I.; Liu, C.X.; Xie, Z.Y. Properties of hydrophobic carbon–PTFE composite coating with high corrosion resistance by facile preparation on pure Ti. Trans. Nonferrous Met. Soc. China 2019, 29, 2321–2330. [Google Scholar] [CrossRef]
- Zhou, H.; Gao, P.; Wang, P.; Xie, Z.; Wu, X. Titanium nitride (TiN)–polytetrafluoroethylene (PTFE)-modified carbon paper used in PEM fuel cells: Characterization and corrosion-resistant mechanism. Appl. Phys. A 2019, 126, 37. [Google Scholar] [CrossRef]
- Liu, R.; Jia, Q.; Zhang, B.; Lai, Z.; Chen, L. Protective coatings for metal bipolar plates of fuel cells: A review. Int. J. Hydrog. Energy 2022, 47, 22915–22937. [Google Scholar] [CrossRef]
- Tokutake, K.; Nishi, H.; Ito, D.; Okazaki, S.; Serizawa, Y. Relationship between degradation characteristics of organic coating on internal bottom plate of oil storage tank and constant-phase element parameter values. Prog. Org. Coatings 2015, 87, 69–74. [Google Scholar] [CrossRef]
- Yin, Y.; Yang, Y.; Liu, G.; Chen, H.; Gong, D.; Ying, Y.; Fan, J.; Liu, S.; Li, Z.; Wang, C.; et al. Ultrafast solid-phase synthesis of 2D pyrene-alkadiyne frameworks towards efficient capture of radioactive iodine. Chem. Eng. J. 2022, 441, 135996. [Google Scholar] [CrossRef]
- Dang, A.; Sun, Y.; Fang, C.; Li, T.; Liu, X.; Xia, Y.; Ye, F.; Zada, A.; Khan, M. Rational design of Ti3C2/carbon nanotubes/MnCo2S4 electrodes for symmetric supercapacitors with high energy storage. Appl. Surf. Sci. 2022, 581, 152432. [Google Scholar] [CrossRef]
- Dang, A.; Sun, Y.; Liu, Y.; Xia, Y.; Liu, X.; Gao, Y.; Wu, S.; Li, T.; Zada, A.; Ye, F. Flexible Ti3C2Tx/carbon nanotubes/CuS film electrodes based on a dual-structural design for high-performance all-solid-state supercapacitors. ACS Appl. Energy Mater. 2022, 5, 9158–9172. [Google Scholar] [CrossRef]

| Sample Number | Suspension Solution |
|---|---|
| Ti | Bare Ti |
| C | Carbon film |
| C-PTFE/GS | 1 wt.% PTFE + graphite sheet |
| C-PTFE/GS-CNT | 1 wt.% PTFE + graphite sheet/Carbon nanotubes |
| Samples | At −0.1 V icorr.(μA/cm2) | At 0.6 V icorr.(μA/cm2) | Polarization Voltage (V) | Polarization icorr.(μA/cm2) |
|---|---|---|---|---|
| Ti | 11 | 26 | −0.08 | 7.8 |
| C | 6.3 | 0.89 | 0.1 | 0.06 |
| C-PTFE/GS | 0.32 | 0.43 | 0.15 | 0.006 |
| C-PTFE/GS-CNT | 0.08 | 0.05 | 0.28 | 0.014 |
| Chemical Bonding | Binding Energy/eV | Surface Composition/at.% | |
|---|---|---|---|
| Before | After | ||
| C–C | 284.6 | 25.44% | 23.39% |
| C–O | 286.4 | 14.31% | 8.04% |
| C=O | 288.9 | 2.74% | 1.81% |
| C–F | 291.6 | 37.96% | 35.61% |
| C–F3/C–OF2 | 293.1 | 19.55% | 31.15% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ouyang, C.; Xun, D. Carbon/Graphite Sheets/PTFE-Coated Porous Titanium as the Bipolar Plate by Hydrothermal Treatment. Coatings 2022, 12, 1649. https://doi.org/10.3390/coatings12111649
Ouyang C, Xun D. Carbon/Graphite Sheets/PTFE-Coated Porous Titanium as the Bipolar Plate by Hydrothermal Treatment. Coatings. 2022; 12(11):1649. https://doi.org/10.3390/coatings12111649
Chicago/Turabian StyleOuyang, Chun, and Damao Xun. 2022. "Carbon/Graphite Sheets/PTFE-Coated Porous Titanium as the Bipolar Plate by Hydrothermal Treatment" Coatings 12, no. 11: 1649. https://doi.org/10.3390/coatings12111649
APA StyleOuyang, C., & Xun, D. (2022). Carbon/Graphite Sheets/PTFE-Coated Porous Titanium as the Bipolar Plate by Hydrothermal Treatment. Coatings, 12(11), 1649. https://doi.org/10.3390/coatings12111649





