Advanced Methods for Hydroxylation of Vegetable Oils, Unsaturated Fatty Acids and Their Alkyl Esters
Abstract
:1. Introduction
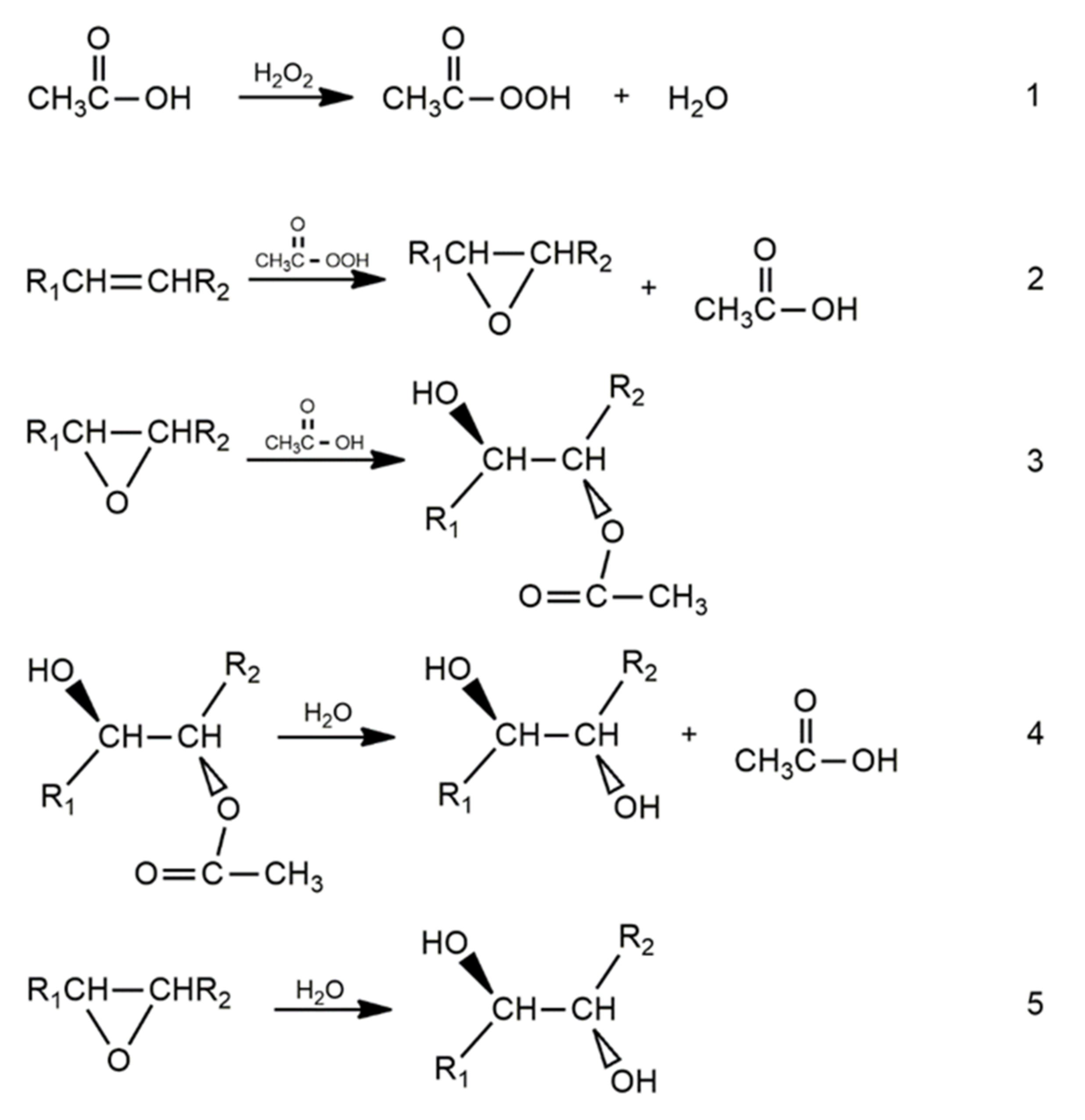
2. Hydroxylation of Epoxide Vegetable Oils Using Glycols and Other Polyols
Hydroxylation of Epoxidized Oils by Methanol and Water
3. Hydroxylation of Epoxidized Oils with Alkanolamines
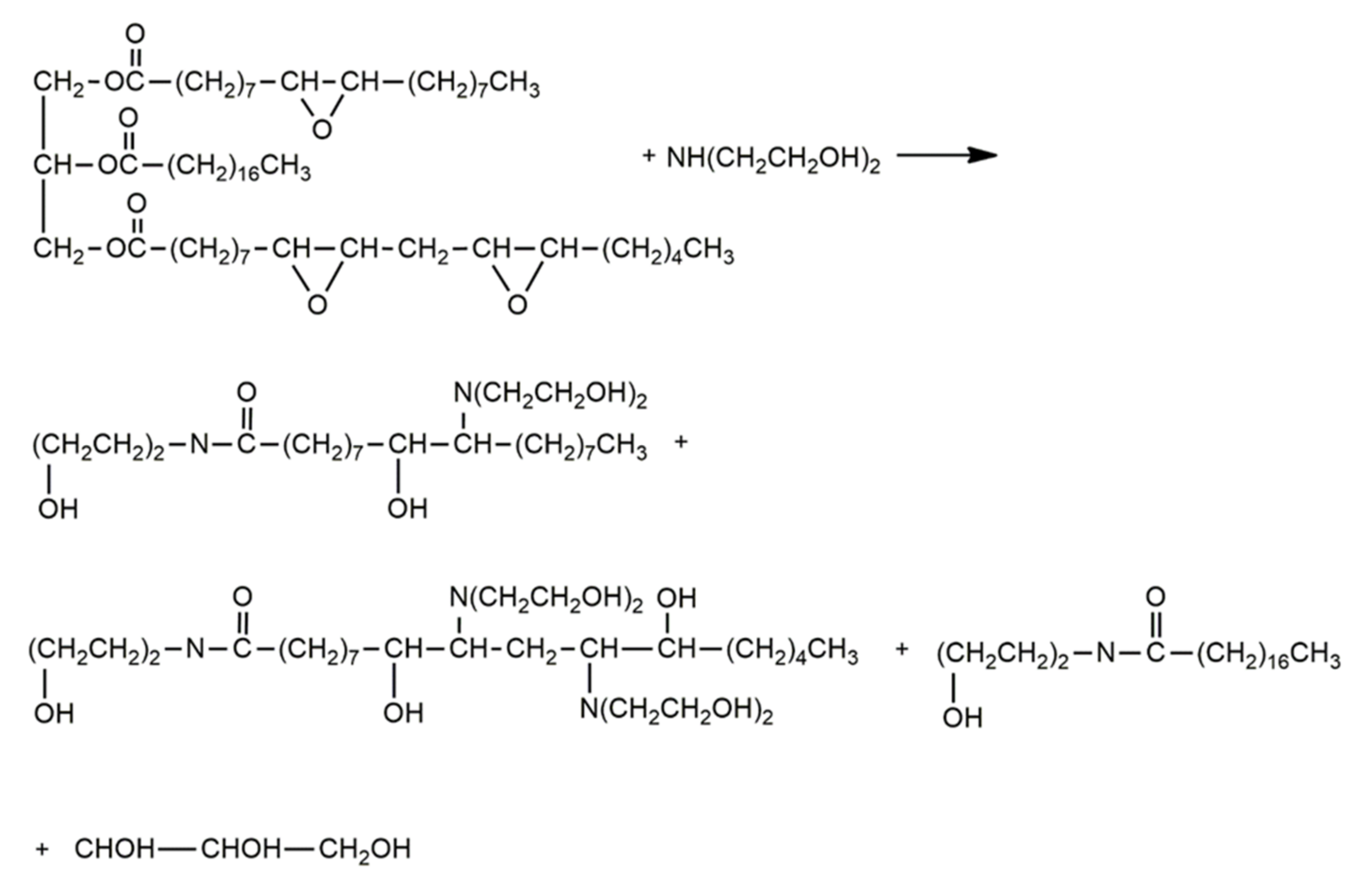
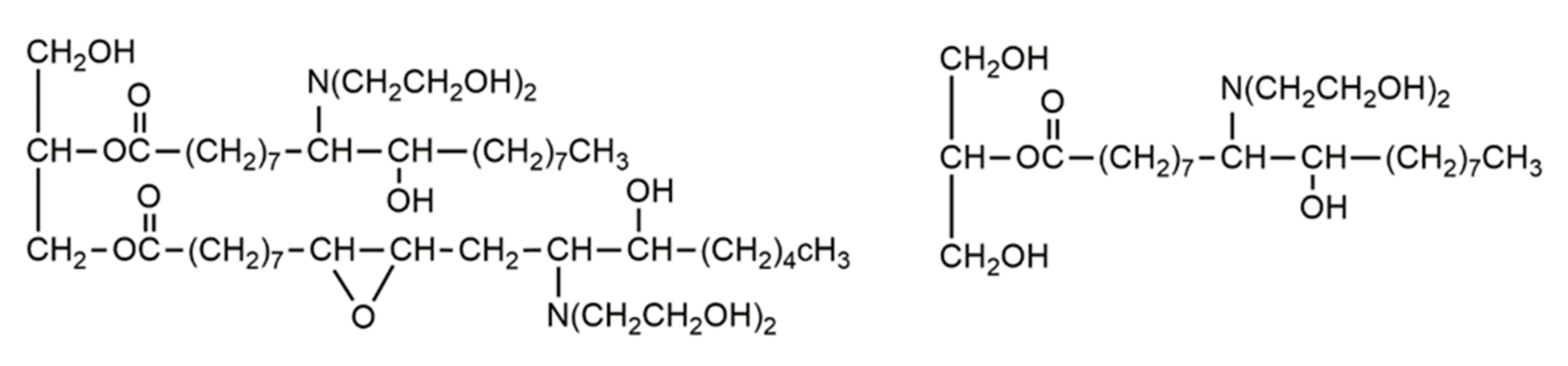
Aminolysis of Primary Polyols from Vegetable Oils with the Use of Hydroxyalkylamines
4. Alkoxylation of Secondary Hydroxyl Groups
4.1. Polyol Synthesis by Hydroformylation of Vegetable Oils
4.2. Obtaining of Polyols by Ozonolysis of Oils
5. Polyols Attractive for Production of Polyurethane Resins
6. Properties of Polyols and Their Application
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zora, N.; Rigaux, T.; Buvat, J.C.; Lefebvre, D.; Leveneur, S. Influence assessment of inlet parameters on thermal risk and productivity: Application to the epoxidation of vegetable oils. J. Loss Prev. Process Ind. 2021, 72, 104551. [Google Scholar] [CrossRef]
- Borugadda, V.B.; Goud, V.V. Hydroxylation and hexanoylation of epoxidized waste cooking oil and epoxidized waste cooking oil methyl esters: Process optimization and physico-chemical characterization. Ind. Crop. Prod. 2019, 133, 151–159. [Google Scholar] [CrossRef]
- Su, Y.; Zhang, S.; Chen, Y.; Yuan, T.; Yang, Z. One-step synthesis of novel renewable multi-functional linseed oil-based acrylate prepolymers and its application in UV-curable coatings. Prog. Org. Coat. 2020, 148, 105820. [Google Scholar] [CrossRef]
- Dominici, F.; Samper, M.D.; Carbonell-Verdu, A.; Luzi, F.; López-Martínez, J.; Torre, L.; Puglia, D. Improved toughness in lignin/natural fiber composites plasticized with epoxidized and maleinized linseed oils. Materials 2020, 13, 600. [Google Scholar] [CrossRef] [Green Version]
- Laverdura, U.P.; Rossi, L.; Ferella, F.; Courson, C.; Zarli, A.; Alhajyoussef, R.; Gallucci, K. Selective catalytic hydrogenation of vegetable oils on lindlar catalyst. Asc Omega 2020, 5, 22901–22913. [Google Scholar] [CrossRef]
- Soares, S.; Rocha, F.R.P. Multi-energy calibration to circumvent matrix effects in the determination of biodiesel quality parameters by UV-Vis spectrophotometry. Talanta 2020, 209, 120584. [Google Scholar] [CrossRef]
- Zhou, Z.; Abbatt, J.P.D. Formation of gas-phase hydrogen peroxide via multiphase ozonolysisi of unsaturated lipids. Environ. Sci. Technol. Lett. 2021, 8, 114–120. [Google Scholar] [CrossRef]
- Paraskar, P.M.; Kulkarni, R.D. Synthesis of isostearic acid/dimer fatty acid-based polyesteramide polyol for the development of green polyurethane coatings. J. Polym. Environ. 2021, 29, 54–70. [Google Scholar] [CrossRef]
- Dubois, J.L.; Couturier, J.L.; Asadauskas, S.J.; Labanauskas, L.; Bražinskienė, D.; Blaauw, R. Conversion of fatty acid methyl esters into dibasic esters by metathesis and their lubricant properties. Rsc Adv. 2021, 11, 31030–31041. [Google Scholar] [CrossRef]
- Pėrez-Sena, W.Y.; Salmi, T.; Estel, L.; Leveneur, S. Thermal risk assessment for the epoxidation of linseed oil by classical Prisleschajew epoxidation and by direct epoxidation by H2O2 on alumina. J. Therm. Anal. Calorim. 2020, 140, 673–684. [Google Scholar] [CrossRef]
- Kurańska, M.; Niemiec, M. Cleaner production of epoxidized cooking oil using a heterogenous catalyst. Catalysts 2020, 10, 1261. [Google Scholar] [CrossRef]
- Abolis, A.; Pomilovskis, R.; Vanags, E.; Mierina, I.; Michalowski, S.; Fridrihsone, A.; Kirpluks, M. Impact of different epoxidation approaches of tall oil fatty acids on rigid polyurethane foam thermal insulation. Materials 2021, 14, 894. [Google Scholar]
- Musik, M.; Janus, E.; Pełech, R.; Sałaciński, Ł. Effective epoxidation of fatty acid methyl esters with hydrogen peroxide by the catalytic system H3PW12O40/quaternary phosphonium salts. Catalysts 2021, 11, 1058. [Google Scholar] [CrossRef]
- Vondran, J.; Pela, J.; Palczewski, D.; Skiborowski, M.; Seidensticker, T. Curse and blessing—The role of water in the homogenously Ru-catalyzed epoxidation of technical grade methyl oleate. ACS Sustain. Chem. Eng. 2021, 9, 11469–11478. [Google Scholar] [CrossRef]
- Pfister, D.P.; Xia, Y.; Larock, R.C. Recent advances in vegetable oil-based polyurethanes. Chem. Sus. Chem. 2011, 4, 703–717. [Google Scholar] [CrossRef]
- Yan, Z.; Ma, Z.; Deng, J.; Luo, G. Mechanism and kinetics of epoxide ring-opening with carboxylic acids catalyzed by the corresponding carboxylates. Chem. Eng. Sci. 2021, 242, 116746. [Google Scholar] [CrossRef]
- Guo, A.; Cho, Y.; Petrović, Z.S. Structure and properties of halogenated and nonhalogenated soy-based polyols. J. Polym. Sci. A Polym. Chem. 2000, 38, 3900–3910. [Google Scholar] [CrossRef]
- Poussard, L.; Lazko, J.; Mariage, J.; Raquez, J.-M.; Dubois, P. Biobased waterborne polyurethanes for coating applications: How fully biobased polyols may improve the coating properties. Prog. Org. Coat. 2016, 97, 175–183. [Google Scholar] [CrossRef]
- Czub, P. Kompozycje epoksydowe z wykorzystaniem modyfikowanych olejów roślinnych. Polymers 2008, 53, 182–189. [Google Scholar]
- Czub, P. Modyfikowane Oleje Roślinne Oraz Produkty Chemicznej Degradacji Odpadowego Poli(Tereftalanu Etylenu) Jako Ekologiczne Surowce Do Żywic Epoksydowych; Wydawnictwo Politechniki Krakowskiej: Kraków, Poland, 2008. [Google Scholar]
- PN-EN ISO 2554:2001; Plastics—Unsaturated Polyester Resins—Determination of Hydroxyl Value. Polish Committee for Standardization: Warszawa, Poland, 2001. Available online: https://sklep.pkn.pl/pn-en-iso-2554-2001p.html (accessed on 5 December 2001).
- Rojek, R.; Pawlik, H.; Prociak, A. Influence of chemical structure of rapeseed bio-polyols on the properties of viscoelastic polyurethane foams. Czas. Tech. Chem. Politech. Krakowaska 2010, 10, 278–284. [Google Scholar]
- Petrović, Z. Polyurethanes from vegetable oils. Polym. Rev. 2008, 48, 109–155. [Google Scholar] [CrossRef]
- Datta, J.; Głowińska, E.; Haponiuk, J. Sposób Otrzymywania Hydroksylowanego Oleju Sojowego, Politechnika Gdańska. PL Patent 218,912,B1, 27 February 2015. [Google Scholar]
- Hassan, H.A.; Ismail, T.N.M.T.; Sattar, M.N.; Hoong, S.S.; Ooi, T.L.; Ahmad, S.; Palam, K.D.P.; Cheong, M.Y. Process to Produce Polyols. U.S. Patent 7,932,409,B2, 26 April 2011. [Google Scholar]
- Harrington, R.; Malsam, J. Flexible Polyurethane Foams Prepared Using Modified Vegetable Oil Based Polyols Pat. WO Patent 033,167,A2, 14 April 2005. [Google Scholar]
- Petrovic, S.Z.; Javni, I.X.; Zlatanic, A.; Guo, A.X. Modified Vegetable Oil-Based Polyols. U.S. Patent 8,153,746,B2, 10 April 2012. [Google Scholar]
- Guo, A.; Javni, I.; Petrovic, Z. Rigid polyurethane foams based on soybean oil. J. Appl. Polym. Sci. 2000, 77, 467–473. [Google Scholar] [CrossRef]
- Dai, H.; Yang, L.; Lin, B.; Wang, C.; Shi, G. Synthesis and characterization of different soy-based polyols by ring opening of epoxidized soybean oil with methanol. J. Am. Oil Chem. Soc. 2009, 86, 261–267. [Google Scholar] [CrossRef]
- Petrovic, Z.; Guo, A.; Javni, I. Process for the Preparation of Vegetable Oil-Based Polyols and Electroninsulating Casting Compounds Created from Vegetable Oil-Based Polyols. U.S. Patent 6,107,433, 22 August 2000. [Google Scholar]
- Hellbardt, S.A.; Zech, W.; Schlandt, K. Process for the Preparation of Compounds of Hydroxylated Fatty Acid. EP Patent 0,554,590,A2, 11 August 1993. [Google Scholar]
- Zlatanic, A.; Lava, C.; Zhang, W.; Petrovic, Z.S. Effect of structure on properties of polyols and polyurethanes based on different vegetable oils. J. Polym. Sci. Part B Polym. Phys. 2004, 42, 809–819. [Google Scholar] [CrossRef]
- Metivier, P. Procédés D’époxydation ou de Dihydroxylation D’acides Gras Unsatures. WO Patent 2000/44704, 3 August 2000. [Google Scholar]
- Metivier, P. Preparation de Derives D’acides Gras Monocarboxyles Portant deux Functions Hydroxyls vicinales ou une Function Epoxyde. FR Patent 2,789,013,A1, 4 August 2000. [Google Scholar]
- Desroches, M.; Escouvois, M.; Auvergne, R.; Caillol, S.; Boutevin, B. From vegetable oils to polyurethanes: Synthetic routes to polyols and main industrial products. Polym. Rev. 2012, 52, 38–79. [Google Scholar] [CrossRef] [Green Version]
- Casper, D.M. Method of Preparing a Hydroxyl Functional Vegetable oil. U.S. Patent 0,041,155,A1, 23 February 2006. [Google Scholar]
- Casper, D.M.; Newbold, T. Method of Preparing a Hydroxyl Functional Vegetable Oil. U.S. Patent 0,041,156,A1, 23 February 2006. [Google Scholar]
- Luo, N.; Newbold, T. Process for the Manufacture of Natural Oil Hydroxylates. U.S. Patent 8,097,739,B2, 17 January 2012. [Google Scholar]
- Hsiao, Y.L.; Skorpenske, R.; Kaushiva, B.; McDaniel, K.; Pazos, J.; Hager, S.; Haider, K.; Covestro LLC. Polyurethane Foams Made with Alkoxylated Vegetable Oils. U.S. Patent 0,229,375,A1, 10 December 2006. [Google Scholar]
- Lorenz, K. Process for the Production of Polyols Based on Natural Oils. U.S. Patent 0,123,725,A1, 31 May 2007. [Google Scholar]
- Newbold, T.; Casper David, M. Method of preparing enhanced reactive vegetable oils. U.S. Patent 0,306,435,A1, 12 October 2009. [Google Scholar]
- Li, Y.; Luo, X.; Hu, S. Bio-Based Polyols and Polyurethanes, 1st ed.; Springer: Cham, Switzerland; Heidelberg, Germany; New York, NY, USA; Dordrecht, The Netherlands; London, UK, 2015. [Google Scholar]
- Lysenko, Z.; Morrison, D.L.; Babb, D.A.; Bunning, D.L.; Derstine, C.W.; Gilchrist, J.H.; Jouett, R.H.; Kanel, J.S.; Olson, K.D.; Peng, W.-J.; et al. Aldehyde and Alcohol Compositions Derived from Seed Oils. WO Patent 096,744,A2, 11 November 2004. [Google Scholar]
- Petrović, Z.S.; Guo, A.; Javni, I.; Cvetković, I.; Hong, D.P. Polyurethane networks from polyols obtained by hydroformylation of soybean oil. Polym. Int. 2008, 57, 275–281. [Google Scholar] [CrossRef]
- Sałaciński, Ł.; Lewandowski, G.; Milchert, E. Review of technological methods for the production of biodegradable polyols (oligomerols). Przem. Chem. 2017, 96, 2291–2295. [Google Scholar]
- Akram, D.; Sharmin, E.; Ahmad, S. Synthesis and characterization of boron incorporated polyester polyol from linseed oil: A sustainable material. Macromol. Symp. 2009, 277, 130–137. [Google Scholar] [CrossRef]
- Rogier, E.R. High Molecular Weight Products. U.S. Patent 4,304,945, 12 August 1981. [Google Scholar]
- Fitchett, C.S.; Laughton, N.G.; Chappel, C.G.; Khan, M.L.; Tverezovskiy, V.; Tomkinson, J.; Fowler, P. Oil Ozonolysis. EP Patent 1,453,799,A, 9 August 2004. [Google Scholar]
- Omonov, T.S.; Kharraz, F.; Curtis, J.M. Ozonolysis of canola oil: A study of product yields and ozonolysis kinetics in differet solvent systems. J. Am. Oil Chem. Soc. 2011, 88, 689–705. [Google Scholar] [CrossRef]
- Lysenko, Z.; Schrock, A.K.; Babb, D.A.; Sanders, A. Vegetable Oil Based Polyols and Polyurethanes Made Therefrom. U.S. Patent 0,276,609,A1, 12 July 2006. [Google Scholar]
- Guo, Z.; Demydov, D.; Zhang, W.; Petrovic, Z.S. Polyols and polyurethanes from hydroformylation of soybean oil. J. Polym. Environ. 2002, 10, 49–52. [Google Scholar] [CrossRef]
- Frankel, E.N. Selective Hydroformylation of Unsaturated Fatty Compounds. U.S. Patent 3,787,459, 22 January 1974. [Google Scholar]
- Frankel, E.N.; Pryde, E.H. Acetoxymethyl Derivatives of Polyunsaturated Fatty Triglycerides as Primary Plasticizers for Polyvinylchloride. U.S. Patent 4,083,816, 4 November 1978. [Google Scholar]
- Petrović, Z.S.; Cvetković, I.; Hong, D.P.; Wan, X.; Zhang, W.; Abraham, T.W.; Malsam, J. Vegetable oil-based triols from hydroformylated fatty acids and polyurethane elastomers. Eur. J. Lipid Sci. Technol. 2010, 112, 97–102. [Google Scholar] [CrossRef]
- Vanbesien, T.; Hapiot, F.; Monflier, E. Hydroformylation of vegetable oils and the potential use of hydroformylated fatty acids. Lipid Technol. 2013, 25, 175–178. [Google Scholar] [CrossRef]
- Akram, D.; Sharmin, D.; Ahmad, S. Synthesis, characterization and corrosion protective properties of boron-modified polyurethane from natural polyols. Prog. Org. Coat. 2008, 63, 25–32. [Google Scholar] [CrossRef]
- Akram, D.; Sharmin, D.; Ahmad, S. Development and characterization of boron incorporated linseed oil polyurethanes. J. Appl. Polym. Sci. 2010, 116, 499–508. [Google Scholar] [CrossRef]
- Hu, Y.H.; Gao, Y.; Wang, D.N.; Hu, C.P.; Zu, S.; Vanoverloop, S.; Randal, D. Rigid polyurethane foam prepared from a rape seed oil based polyol. J. Appl. Polym. Sci. 2002, 84, 591–597. [Google Scholar] [CrossRef]
- Yakushin, V.; Abolins, A.; Vilsone, D.; Sevastyanova, I. Polyurethane coatings based on linseed oil phosphate ester polyols with intumescent flame retardants. Fire Mater. 2018, 43, 92–100. [Google Scholar] [CrossRef]
- Zhong, B.; Shaw, C.; Rahim, M.; Massingill, J. Novel coatings from soybean oil phosphate ester polyols. J. Coat. Technol. 2001, 73, 83–90. [Google Scholar] [CrossRef]
- Brasil, M.C.; Gerbase, A.E.; De Luca, M.A.; Gregario, J.R. Organic-inorganic hybrid film based on hydroxylated soybean oils. J. Am. Oil Chem. Soc. 2007, 84, 289–295. [Google Scholar] [CrossRef]
- Ferrer, M.C.C.; Babb, D.; Ryan, A.J. Characterization of polyurethane networks based on vegetable derived polyol. Polymer 2008, 49, 3279–3287. [Google Scholar] [CrossRef]
- Xu, Y.; Petrovic, Z.; Das, S.; Wilkes, G.L. Polyols and polyurethanes from hydroformylation of soybean oil. Polymer 2008, 49, 4248–4258. [Google Scholar] [CrossRef]
- Acik, G. Preparation of antimicrobial and biodegradable hybrid soybean oil and poly (L-lactide) based polymer with quaternized ammonium salt. Polym. Degrad. Stab. 2020, 181, 109317. [Google Scholar] [CrossRef]
- Campanella, A.; Bonnaillie, L.M.; Wool, R.P. Polurethane foams from soyoi-based plyols. J. Appl Polym. Sci. 2009, 112, 2567–2578. [Google Scholar] [CrossRef]
- Lligadas, G.; Ronda, J.C.; Galia, M.; Cadiz, V. Plant oils as platform chemicals for polyurethane synthesis: Current state of the art. Biomacromolecules 2010, 11, 2825–2835. [Google Scholar] [CrossRef]
- Kaujalgikar, S.; Rao, N.; Chaudhary, B.I.; Ghosh-Dastidar, A. Methods for Making Epoxidized Fatty Acid Alkyl Esters. U.S. Patent 9,593,091,B2, 14 March 2017. [Google Scholar]
- Monteavaro, L.L.; Da Silva, E.O.; Costa, A.P.O.; Samios, D.; Garbase, A.E.; Petzhold, C. Polyurethane network from formiated soy polyols: Synthesis and mechanical characterization. J. Am. Oil Chem. Soc. 2005, 82, 365–371. [Google Scholar] [CrossRef]
- Zhao, H.; Herrington, R.; Rodriguez, F.; Hunstman Petrochemical LLC. Natural Oil Based AUTOCATALYTIC Polyols. U.S. Patent 0,118,432,A1, 19 May 2011. [Google Scholar]
- Ionescu, M. Polyols for Polyurethanes, Chemistry and Technology, 3rd ed.; De Gruyter: Berlin, Germany; Boston, MA, USA, 2019; Volume 2. [Google Scholar]
- Sharmin, E.; Ashraf, S.M.; Ahmad, S. Epoxidation, hydroxylation, acrylation and urethanation of linum usitatissimum seed oil and its derivatives. J. Lipid Sci. Technol. 2007, 109, 134–146. [Google Scholar] [CrossRef]
- Cifarelli, A.; Boggioni, L.; Vignali, A.; Tritto, I.; Bertini, F.; Losio, S. Flexible polyurethane foams from epoxidized vegetable oils and a bio-based diisocyyanate. Polymers 2021, 13, 612. [Google Scholar] [CrossRef]
- Polaczek, K.; Kurańska, M.; Auguścik-Królikowska, M.; Prociak, A.; Ryszkowska, J. Open-cell polyurethanes foams of very low density modified with various palm oil-based bio-polyols in accordance with cleaner production. J. Clean. Prod. 2021, 290, 125875. [Google Scholar] [CrossRef]
- SaifulAzry, S.O.A.; Chuah, T.G.; Paridah, M.T.; Aung, M.M.; Ridzuan, M.A.; Lee, C.H.; Sariah, S.; Lee, S.H.; Juliana, A.H. Influence of cellulose II polymorph nanowhiskers on bio-based nanocomposite film from Jatropha oil polyurethane. Mater. Res. Express 2021, 8, 015003. [Google Scholar] [CrossRef]
- Majdoub, M.; Essamlali, Y.; Amadine, O.; Ganetri, I.; Zahouily, M. Organophilic graphene nanosheets as a promising nanofiller for bio-based polyurethane nanocomposites: Investigation of thermal, barrier and mechanical properties. New J. Chem. 2019, 43, 15659. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, P.; Fan, M.; Jiang, P.; Bao, Y.; Gao, X.; Xia, J. Dual bond synergy enhancement to mechanical and thermal properties of castor oil-based waterborne polyurethane composites. Polymer 2019, 182, 121832. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Liang, H.; Zhou, X.; Fang, C.; Zhang, C.; Luo, Y. Synthesis and properties of castor oil-based waterborne polyurethane/sodium alginate composites with tunable properties. Carbohydr. Polym. 2019, 208, 391–397. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, J.; Wang, H.; He, M.; Ding, L. Flame retardant modified bio-based waterborne polyurethane dispersions derived from castor oil and soy polyol. Eur. J. Lipid Sci. Technol. 2021, 123, 2000248. [Google Scholar] [CrossRef]
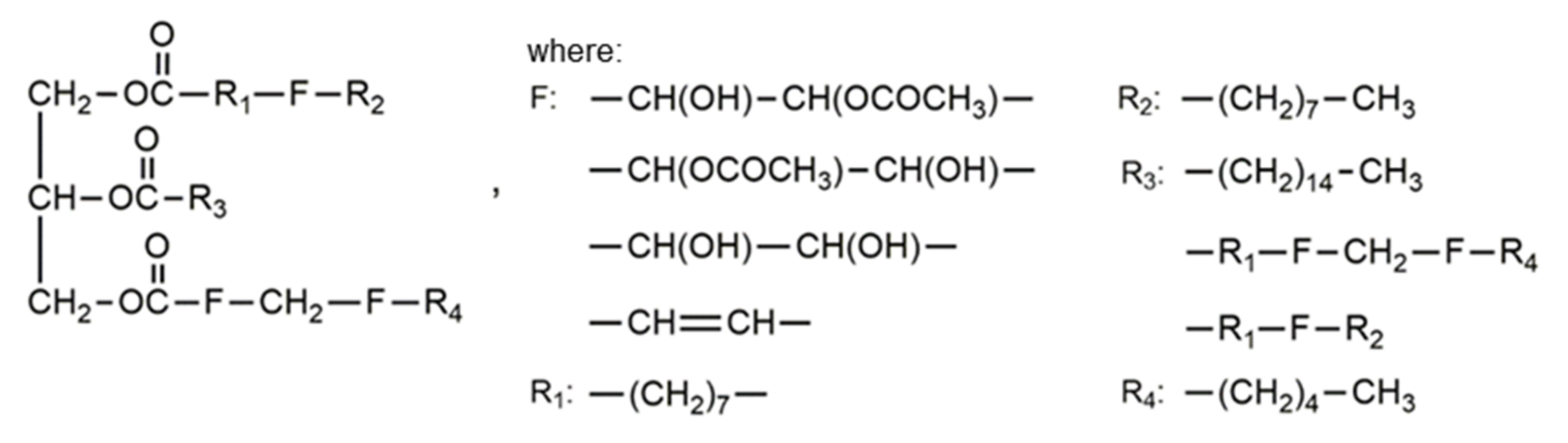
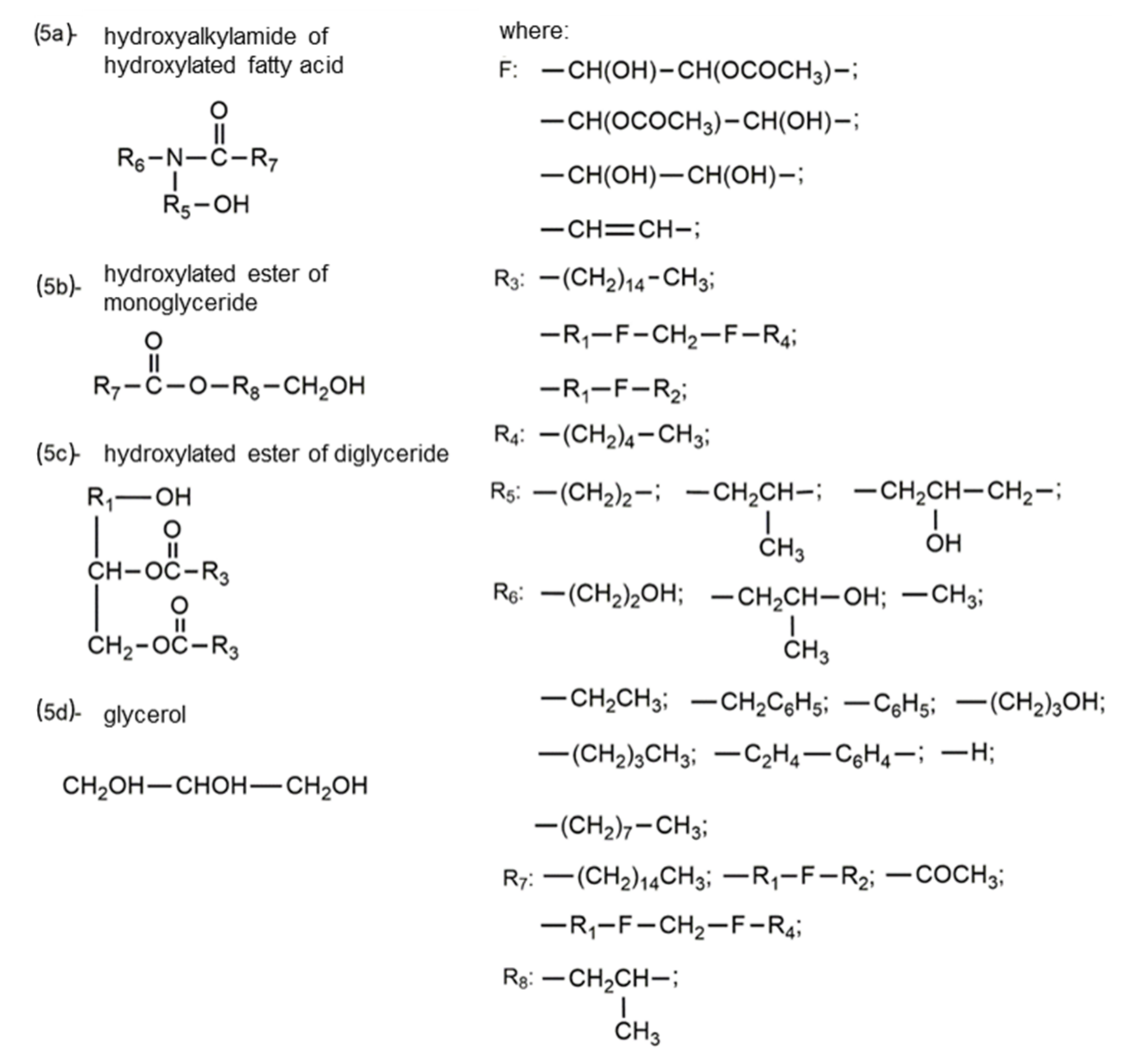
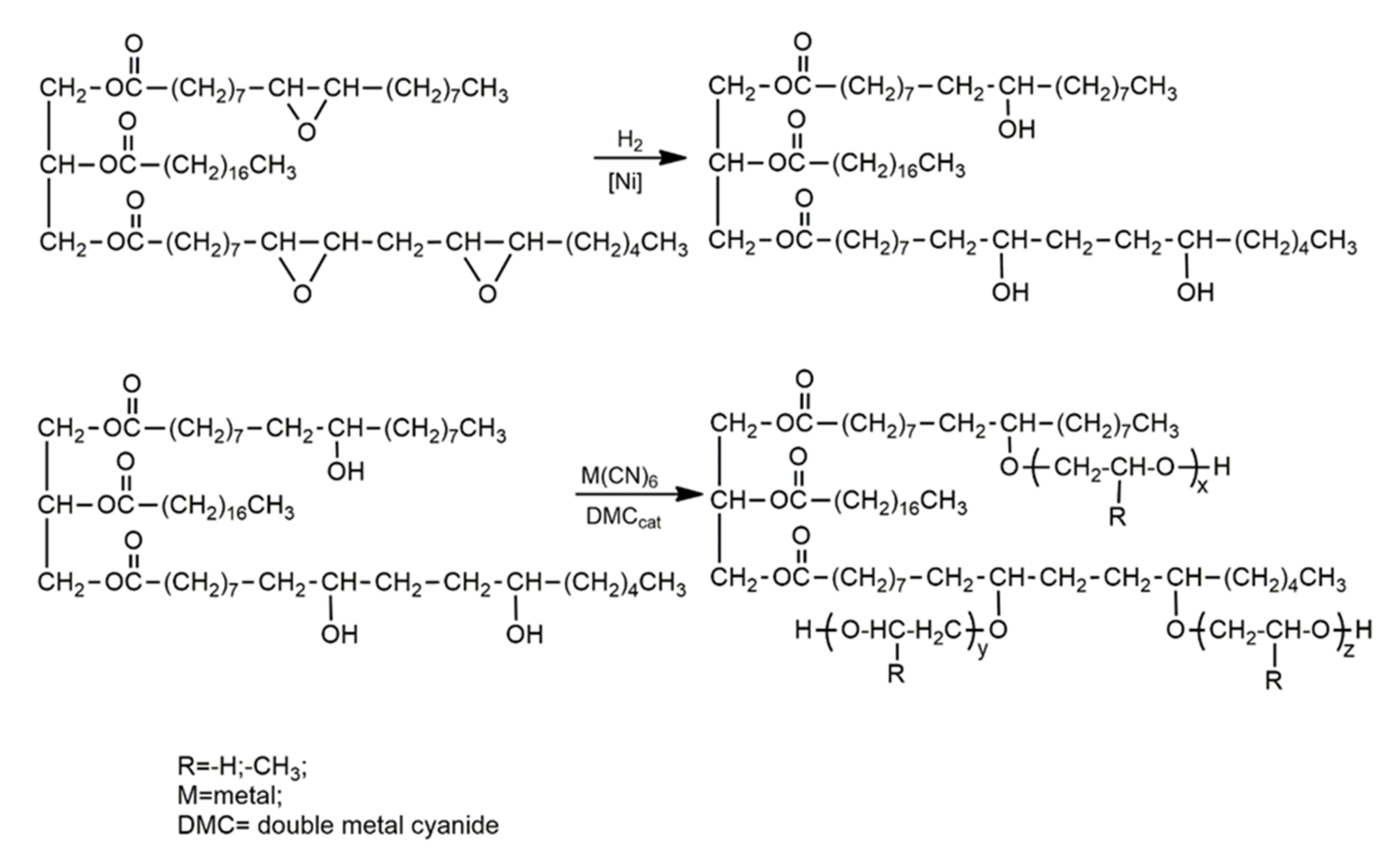
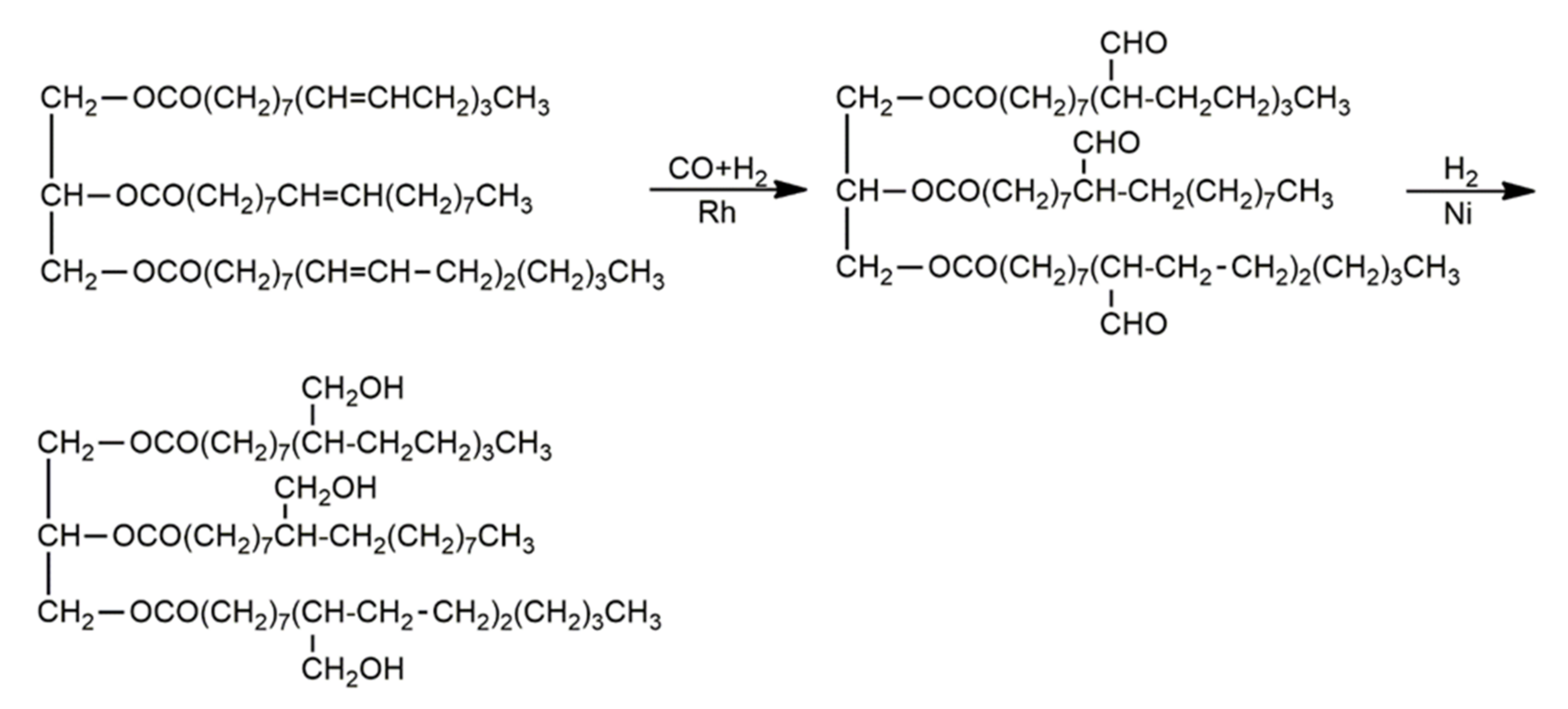
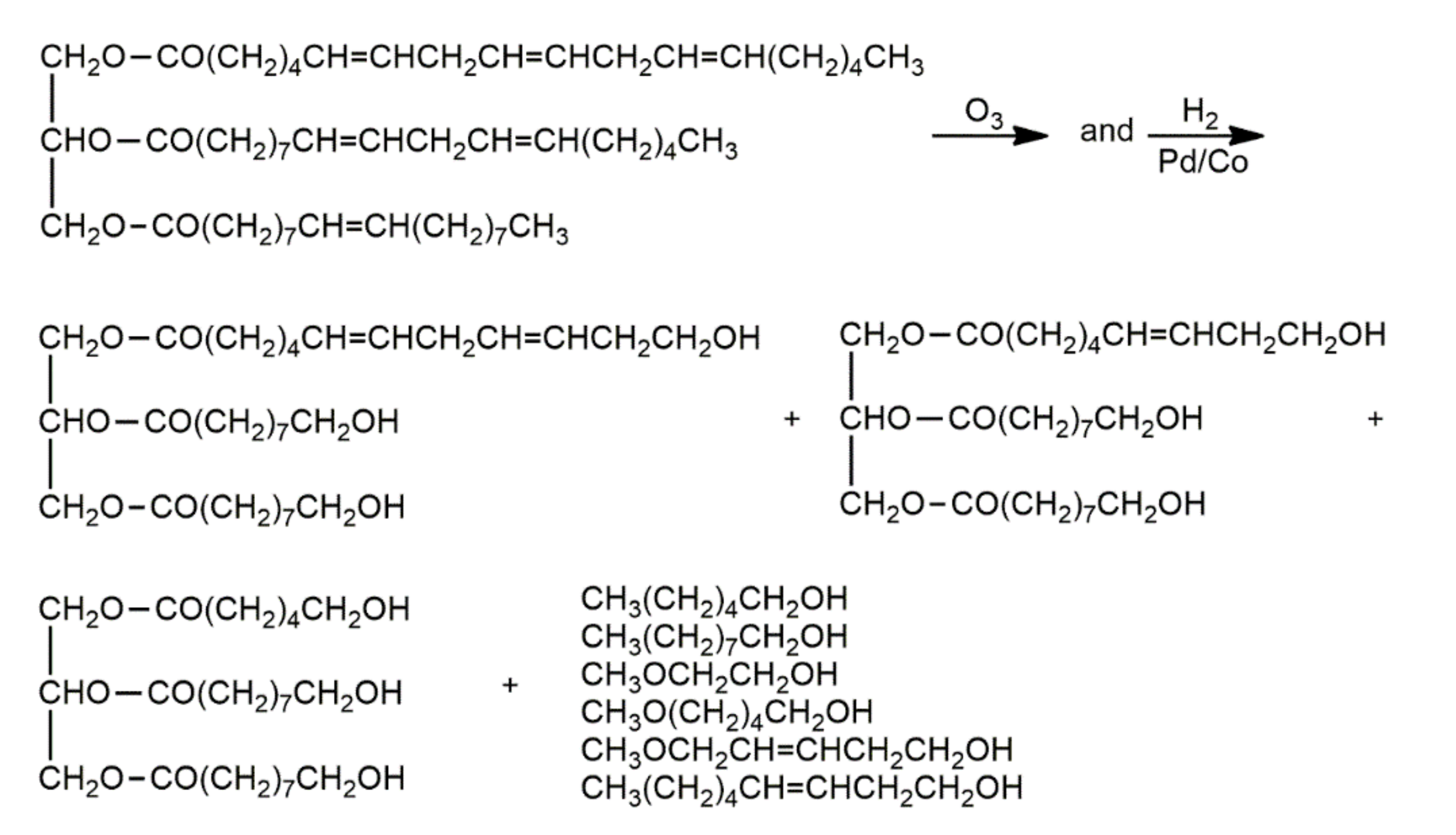
| Oils | Epoxy Number [mol/100 g] or Epoxy Oxygen Content [%] | Used Catalyst | Epoxy Ring Opening Reagents | OH Values [mg KOH/g] | References |
|---|---|---|---|---|---|
| Soybean oil | 0.353 a | H2SO4 | Ethylene glycol | 209.2 | [19] |
| Soybean oil | 0.379 a | H3PO4 | 1,2-propanodiol | 153 | [23] |
| 0.366 a | H3PO4 | 1,3-propanodiol | 199 | ||
| Soybean and palm oil | 2.02 b | Complex of BF3–diethyl ether | Ethylene glycol | 70–90 | [24] |
| Soybean oil | 5.4 b | HBF4 | Methanol | 192 | [25] |
| Soybean oil | - | HBF4 | Methanol | 184–215 | [27] |
| Soybean oil | 0.385 a | HBF4 | Methanol | 180.3 | [28] |
| Soybean oil | 7.0 b | HBF4 | Methanol | 110–213 | [29] |
| Beet oil | 5.95 b | H2SO4 | Ethanol | 144 | [30] |
| Beet oil | 5.95 b | H2SO4 | n-Butanol | 193 | |
| Beet oil | 5.95 b | H2SO4 | Diethanolamine | 462 | |
| Canola oil | 6.69 b | HBF4 | Methanol | 173.6 | [31] |
| Midoleic sunflower oil | 6.32 b | HBF4 | Methanol | 163.5 | |
| Soybean oil | 7.60 b | HBF4 | Methanol | 179.3 | |
| Linseed oil | 10.58 b | HBF4 | Methanol | 247.8 | |
| Sunflower oil | 7.82 b | HBF4 | Methanol | 177.8 | |
| Corn oil | 7.43 b | HBF4 | Methanol | 179.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Musik, M.; Bartkowiak, M.; Milchert, E. Advanced Methods for Hydroxylation of Vegetable Oils, Unsaturated Fatty Acids and Their Alkyl Esters. Coatings 2022, 12, 13. https://doi.org/10.3390/coatings12010013
Musik M, Bartkowiak M, Milchert E. Advanced Methods for Hydroxylation of Vegetable Oils, Unsaturated Fatty Acids and Their Alkyl Esters. Coatings. 2022; 12(1):13. https://doi.org/10.3390/coatings12010013
Chicago/Turabian StyleMusik, Marlena, Marcin Bartkowiak, and Eugeniusz Milchert. 2022. "Advanced Methods for Hydroxylation of Vegetable Oils, Unsaturated Fatty Acids and Their Alkyl Esters" Coatings 12, no. 1: 13. https://doi.org/10.3390/coatings12010013
APA StyleMusik, M., Bartkowiak, M., & Milchert, E. (2022). Advanced Methods for Hydroxylation of Vegetable Oils, Unsaturated Fatty Acids and Their Alkyl Esters. Coatings, 12(1), 13. https://doi.org/10.3390/coatings12010013






