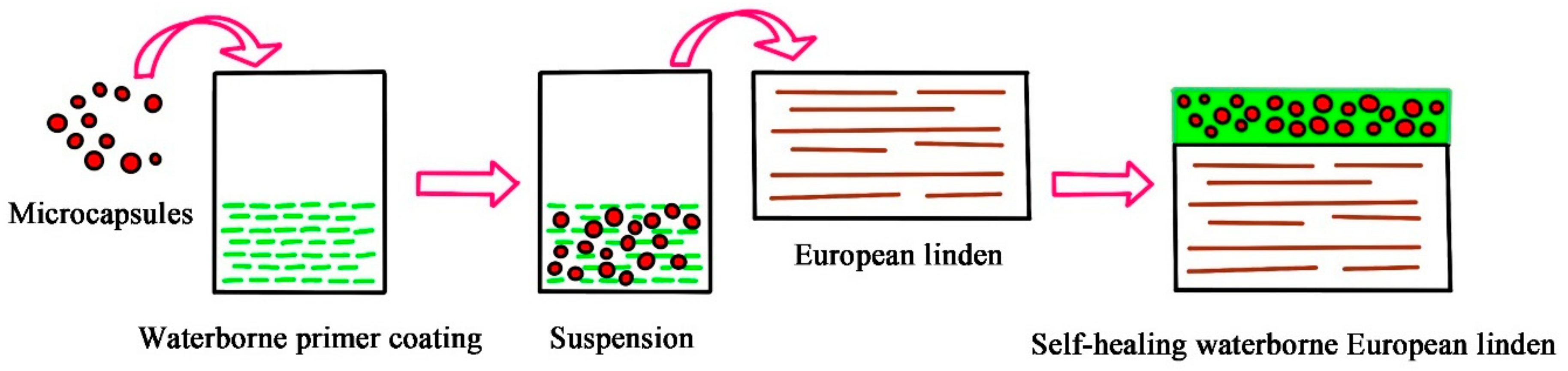
2.1. Experimental Materials
Waterborne acrylic resin (CAS No.: 9003-01-4) was offered by Dulux Paint Co., Ltd., Shanghai, China. Urea (Mw: 60.06 g/mol, CAS No.: 57-13-6), 37.0% formaldehyde solution (Mw: 30.03 g/mol, CAS No.: 50-00-0), triethanolamine (Mw: 149.19 g/mol, CAS No.: 102-71-6), citric acid monohydrate (CAM, Mw: 210.14 g/mol, CAS No.: 5949-29-1), sodium dodecyl benzene sulfonate (SDBS, MW: 348.48 g/mol, CAS No. 25155-30-0), anhydrous ethanol (Mw: 46.07 g/mol, CAS No.: 64-17-5) were supplied by Chemical Reagent Co., Ltd., Nanjing, China. Octyl alcohol (MW: 130.23 g/mol, CAS No. 117-87-5) was offered by Yatai United Chemical Co., Ltd., Wuxi, China. European linden boards (100 mm × 65 mm × 4 mm, uniform material chroma) were offered by Yihua Lifestyle Technology Co., Ltd., Shantou, China. Waterborne primer was supplied by Dulux Paint Co., Ltd., Shanghai, China. The main components of waterborne primer were aqueous acrylic copolymer dispersion, matting agent, additives and water. Solid concentration of the waterborne primer was higher than 30.0%.
2.4. Testing and Characterization
The chroma values of coating were tested by HP-2136 portable chromatic aberration instrument (Hanpu Testing Instrument Co., Ltd., Shenzhen, China). The L-sign represents the brightness of the coating surface. The a-sign represents the chroma value from red to green, the b-sign represents the chroma value from yellow to blue, while the c-sign represents the saturation. The H-sign represents hue. L
1, a
1 and b
1 are the chroma values of one point on the coating; meanwhile, L
2, a
2 and b
2 are the chroma values of another point. ΔL (brightness difference) = L
1−L
2, Δa (red–green difference) = a
1−a
2, Δb (yellow–blue difference) = b
1−b
2. The chroma difference (ΔE) was computed in the light of Equation (1):
The 60° gloss of coatings was determined using the HG268-gloss meter (3NH Technology Co., Ltd., Shenzhen, China). Grades of 6H–6B pencils were used to determine the coating hardness. The angle between the pencil and the coating was about 45°. The pencil was pushed forward on the coating surface at the speed of 1 cm/s under the 1.0 kg load. As scratches appeared on the coatings, the coating hardness was measured. The adhesion was determined by a QFH-HG600 coating grader (Yueqing Liushi Li Chuang Measurement Equipment Firm, Zhejiang, China). The handle was held, and the multi-edge cutter was perpendicular to the board; the coating was cut at a speed of 20-50 mm/s under uniform pressure. Afterward, this operation was repeated by rotating the setup 90° to form a grid pattern. Tape was applied to the entire grid and then removed at a minimum angle. A magnifying glass was used to observe the coating damage. According to the damage degree, the adhesion level was determined. The impact resistance was measured by the QCJ impactor (Maike Instrument Equipment Co., Ltd., Dongguan, China). The sample coating was placed on the iron plate horizontally upward, and the heavy hammer was fixed at a certain height of the sliding cylinder by the control device. After the control button was pressed, the heavy hammer would fall on the punch freely. When the heavy hammer was lifted and the test panel was taken out, the deformation degree of coating surface shall be observed by a magnifying glass and the height of the heavy hammer falling on the test panel shall be recorded. Impact resistance was represented by the maximum height of the fixed weight falling on the test plate without causing damage to the coating. The 680 tests were performed for mechanical testing.
Elongations at break were gauged with the Model AG-IC100KN precision electronic universal capability experiment machine and TRview X optical displacement meter (Shimadzu Co., Ltd., Kyoto, Japan). The coating was applied to the glass substrate and then soaked in water to remove it with a knife. Both ends of the coating were clamped by clamps to guarantee that the coating did not slid. The coating was out of shape at the tensile rate of 0.12 mm/min and was destroyed under a certain longitudinal load. Elongation at break was computed based on the displacement length of the coating at fracture and initial length of the coating before drawing.
Liquid resistance was gauged by a 15.0% NaCl solution, 70.0% medical ethanol, Whitecat Detergent containing 25.0% fatty alcohol ethylene oxide and 75.0% water (Hutchison Whitecat Co., Ltd., Shanghai, China) and red ink (Fine Stationery Co., Ltd., Shanghai, China). The first two liquids were supplied by Qingdao Haishi Hainuo Co., Ltd., Qingdao, China. After soaking in the test solutions, the filter paper was removed with a tweezer and placed in the coating test area. Samples were covered with glass cover. After 24 h, the glass cover and filter paper were taken off. The residual liquid on the coating surface was absorbed, and the imprint and discoloration were examined. The precision contact angle measuring instrument (Swedish Attention Theta, provided by Baioulin Technology Co., Ltd., Finland, Sweden) was used to test the contact angle of the coating containing microcapsule. The test liquid was distilled water.
A ZN ultraviolet weather resistance tester (Environmental Test Equipment Co., Ltd., Nanjing, China) was used to test the coating aging and stability. The aging process under the action of natural climate is simulated in the laboratory, and the UV light with the wavelength of 280–340 nm is used. The light source was adopted 8 UV fluorescent tubes with rated power of 40 W, which were distributed on both sides of the machine, 4 on each side. The center distance of the lamp was 79 mm. The distance between the sample surface and the UV-lamp plane was 50 mm and parallel. The test sample was fixed on the sample rack, facing the fluorescent lamp. When the sample holder was not filled completely, the blackboard was used to fill the sample holder and to keep the inner wall of the test chamber closed. The illumination temperature was 60 °C; the condensation temperature was 50 °C; the cycle time was 4 h illumination with 4 h condensation. The chroma value and gloss of the coating before and after aging and stability test were tested and the total testing time was 200 h. In the aging and stability measurement, L1, a1, b1 c1 and H1 were the coating chroma values before aging and stability test and L2, a2, b2, c2 and H2 were the chroma after aging and stability test.
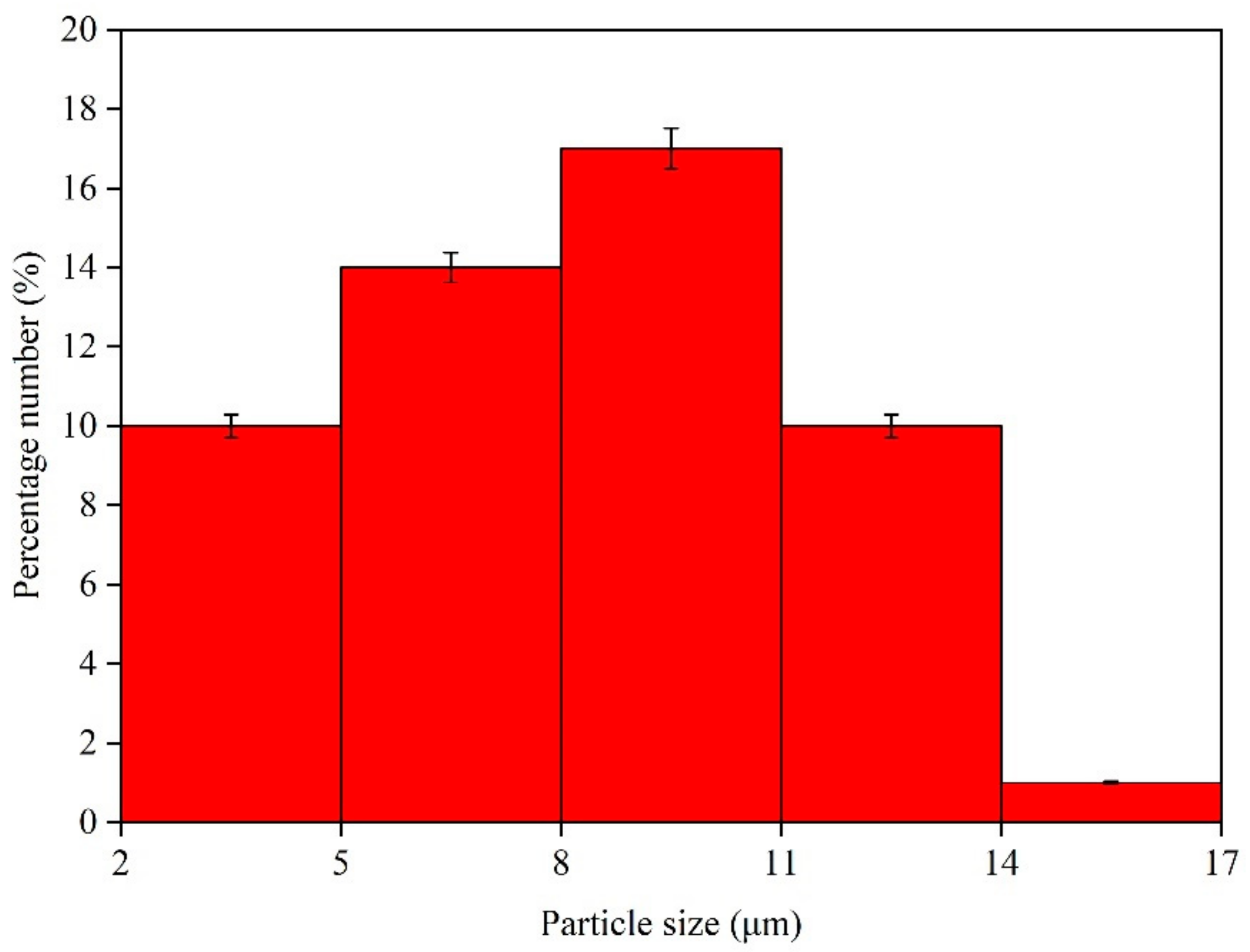
The morphology of the microcapsules and the waterborne primer coating for aging resistance test measurement was analyzed by Quanta 200 environment scanning electron microscope (SEM) from FEI Company (Hillsboro, OR, USA). The optical microscope (OM) image of the microcapsule and coating was observed with a ZEISS light microscope AX10 (Carl Zeiss AG, Aalen, Germany). The coating composition was analyzed by Vertex 80 V infrared spectrum analyzer (Germany Bruker Co., Ltd., Karlsruhe, Germany). The test range was 4000–500 cm−1, the scanning sample was 16 s, and the resolution was 4 cm−1. The infrared spectrum of the microcapsules were measured by potassium bromide tablet method. The specific operation steps were as follows: 200 mg KBr and 1–2 mg microcapsules were taken out and ground in an agate mortar for about 1–2 min. During grinding, the samples were scraped to the center of the mortar with a small stainless-steel shovel to grind them more finely and avoid uneven generation and scattering of particles, resulting in uneven baseline. The mold was taken out and wiped clean, and the sample was put into the medicine spoon evenly. The whole process was finished by baking with infrared lamp. The mold was placed on tablet press, screwed tightly to close the vent valve, pressurized to 20 MPa and stayed for 1–2 min. The bleeder valve was opened slowly to reduce the pressure to 0. The screw was unscrewed, and the mold was taken out. The base was open, the inner module was pressed out in the opposite direction, carefully the pressed tablet was taken out with a flat chemical shovel and put into tablet holder. The infrared spectrum of coating was measured by ATR method. The coating was to place the sample on the test bench, and the sample was fixed on the top surface of ATR crystal by pressure bar. All the experiments were repeated four times with an error of less than 5.0%.