Investigation of the Properties of Color-Changing Powder Water-Based Coating
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
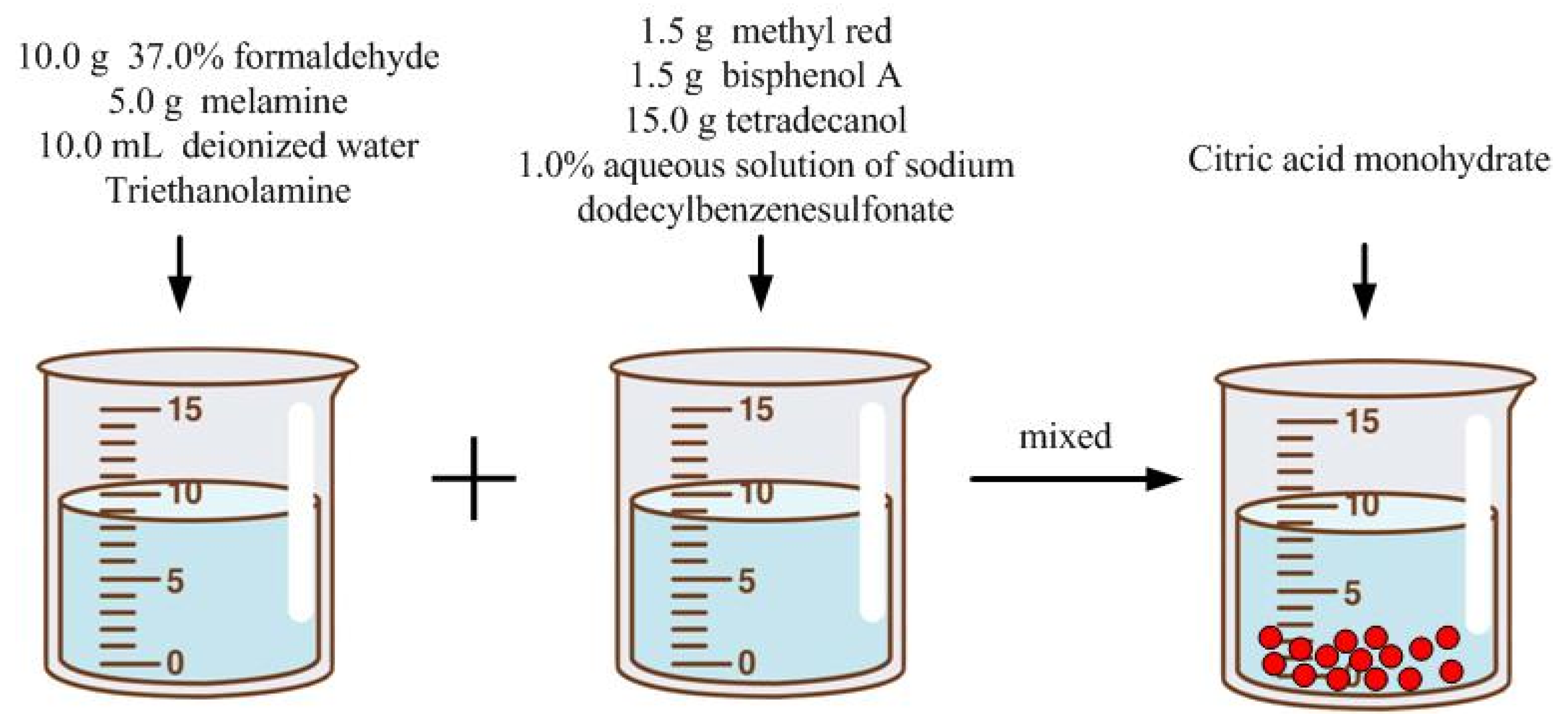
2.2. Preparation of Microcapsules with Color-Changing Powder
2.3. Preparation of Coating
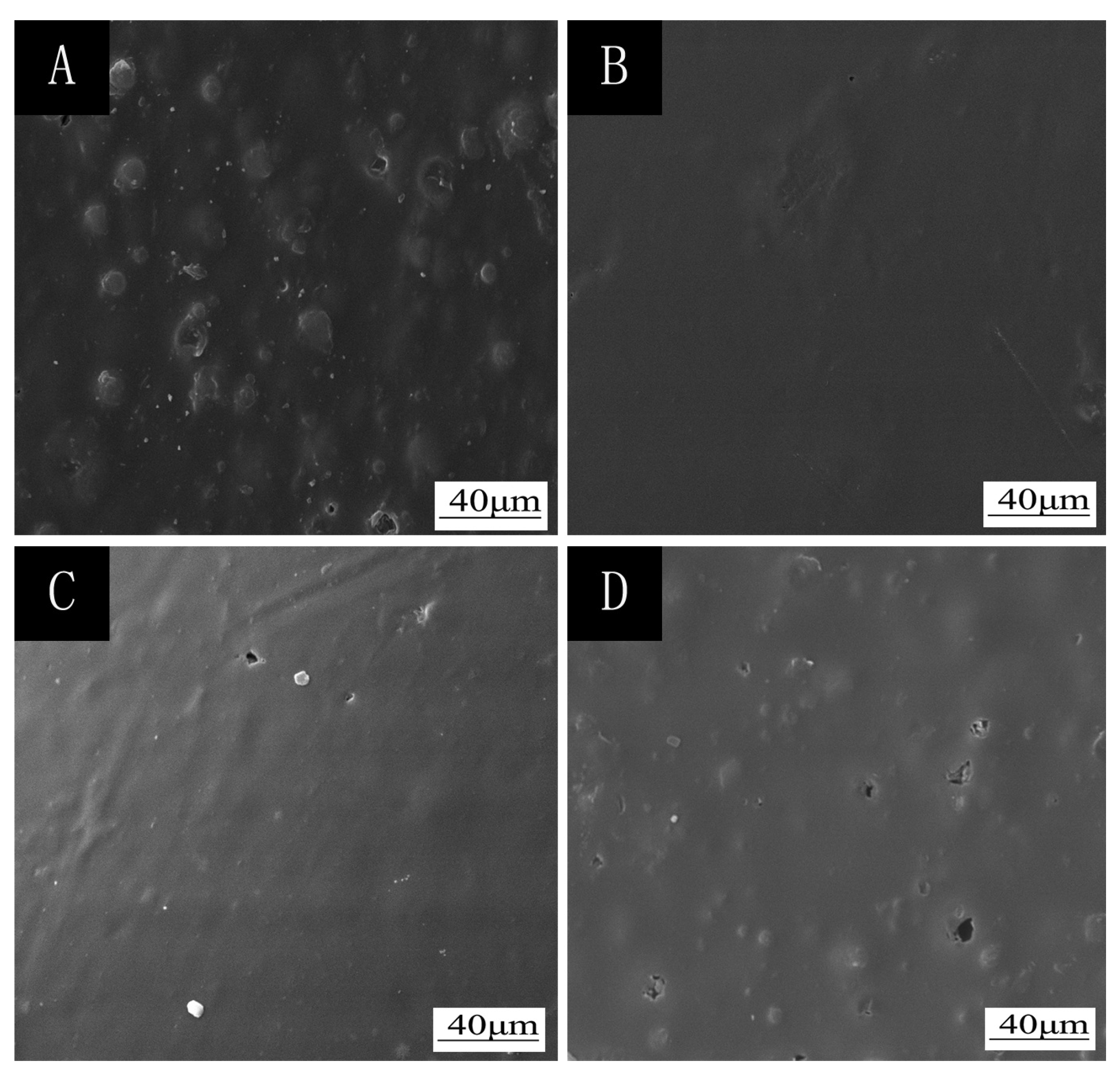
2.4. Testing and Characterization
3. Results and Discussion
3.1. Orthogonal Experiment Analysis
3.2. Performance Optimization of Reversible Color Changing Waterborne Coatings
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wu, Y.; Wu, X.; Yang, F.; Ye, J. Preparation and characterization of waterborne UV lacquer product modified by zinc oxide with flower shape. Polymers 2020, 12, 668. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Fang, X.Y.; Han, J.T.; Wu, Z.H.; Zhang, J.L. Effect of coating thickness on sound absorption property of four wood species commonly used for piano soundboards. Wood Fiber Sci. 2020, 52, 28–43. [Google Scholar] [CrossRef]
- Xiong, X.Q.; Niu, Y.T.; Yuan, Y.Y.; Zhang, L.T. Study on dimensional stability of veneer rice straw particleboard. Coatings 2020, 10, 558. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, J.C.; Huang, Q.T.; Yang, F.; Wang, Y.J.; Liang, X.M.; Li, J.Z. Study on the colorimetry properties of transparent wood prepared from six wood species. ACS Omega 2020, 5, 1782–1788. [Google Scholar] [CrossRef]
- Houska, J.; Kolenaty, D.; Vlcek, J.; Barta, T.; Rezek, J.; Cerstvy, R. Significant improvement of the performance of ZrO2/V1−xWxO2/ZrO2 thermochromic coatings by utilizing a second-order interference. Sol. Energy Mater. Sol. C 2019, 191, 365–371. [Google Scholar] [CrossRef]
- Ahangari, M.G.; Fereidoon, A.; Jahanshahi, M.; Sharifi, N. Effect of nanoparticles on the micromechanical and surface properties of poly(urea-formaldehyde) composite microcapsules. Compos. Part B Eng. 2014, 56, 450–455. [Google Scholar] [CrossRef]
- Sun, K.; Liu, H.; Wang, X.D.; Wu, D.Z. Innovative design of superhydrophobic thermal energy-storage materials by microencapsulation of n-docosane with nanostructured ZnO/SiO2 shell. Appl. Energy 2019, 237, 549–565. [Google Scholar] [CrossRef]
- Geng, X.Y.; Li, W.; Wang, Y.; Lu, J.W.; Wang, J.P.; Wang, N.; Li, J.J.; Zhang, X.X. Reversible thermochromic microencapsulated phase change materials for thermal energy storage application in thermal protective clothing. Appl. Energy 2018, 217, 281–294. [Google Scholar] [CrossRef]
- Dai, J.Y.; Ma, S.Q.; Liu, X.Q.; Han, L.J.; Wu, Y.G.; Dai, X.Y.; Zhu, J. Synthesis of bio-based unsaturated polyester resins and their application in waterborne UV-curable coatings. Prog. Org. Coat. 2015, 78, 49–54. [Google Scholar] [CrossRef]
- Herrera, R.; Muszynska, M.; Krystofiak, T.; Labidi, J. Comparative evaluation of different thermally modified wood samples finishing with UV curable and waterborne coatings. Appl. Surf. Sci. 2015, 357, 1444–1453. [Google Scholar] [CrossRef]
- Zhang, X.P.; Wen, J.; Hu, B.S.; Yuan, J.F.; Wang, J.; Zhu, L.; Pan, M.W. Dispersity control and anti-corrosive performance of graphene oxide modified by functionalized nanosilica in waterborne polyurethane. Nanotechnology 2020, 31, 205708. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Y.; Cui, Y.X.; Li, Z.K.; Zhu, Y.J.; Wang, H.Y. Fabrication of microcapsules containing dual-functional tung oil and properties suitable for self-healing and self-lubricating coatings. Prog. Org. Coat. 2018, 115, 164–171. [Google Scholar] [CrossRef]
- Lei, Y.; Liu, Z.M.; Wu, B.; Jiang, L.; Lei, J.X. Preparation and properties of cross-linked waterborne polyurethane based on solvent-free route. Polym. Bull. 2020, 77, 3263–3275. [Google Scholar] [CrossRef]
- Han, Y.T.; Jiang, Y.Z.; Hu, J.L. Collagen incorporation into waterborne polyurethane improves breathability, mechanical property, and self-healing ability. Compos. Part A Appl. Sci. Manuf. 2020, 133, 105854. [Google Scholar] [CrossRef]
- Tounici, A.; Martin, M.; Jose, M. Addition of graphene oxide in different stages of the synthesis of waterborne polyurethane-urea adhesives and its influence on their structure, thermal, viscoelastic and adhesion properties. Materials 2020, 13, 2899. [Google Scholar] [CrossRef]
- Yan, X.X.; Chang, Y.J.; Qian, X.Y. Effect of concentration of thermochromic ink on performance of waterborne finish films for the surface of Cunninghamia lanceolata. Polymers 2020, 12, 552. [Google Scholar] [CrossRef]
- Yan, X.X.; Chang, Y.J. Investigation of waterborne thermochromic topcoat film with color changing microcapsules on Chinese fir surface. Prog. Org. Coat. 2019, 136, 105262. [Google Scholar] [CrossRef]
- Yan, X.X.; Wang, L.; Qian, X.Y. Influence of thermochromic pigment powder on properties of waterborne primer film for Chinese fir. Coatings 2019, 9, 742. [Google Scholar] [CrossRef]
- GB/T 4893.1-2005 Furniture-Assessment of Surface Resistance to Cold Liquids; Standardization Administration of the People’s Republic of China: Beijing, China, 2005; pp. 1–5. (In Chinese)
- GB/T 9754-2007 Paints and Varnishes Determination of Specular Gloss of Non Metallic Paint Films at 20°, 60° and 85°; Standardization Administration of the People’s Republic of China: Beijing, China, 2007; pp. 1–4. (In Chinese)
- ISO 2813-2004 Paints and Varnishes-Determination of Gloss Value at 20°, 60° and 85°; International Organization for Standardization: Geneva, Switzerland, 2014.
- GB/T 3181-2008 Colour Standard for Paint Film; Standardization Administration of the People’s Republic of China: Beijing, China, 2008; pp. 4–10. (In Chinese)
- ISO 2409-2007 Paints and Varnishes—Cross-Cut Test; International Organization for Standardization: Geneva, Switzerland, 2007.
- GB/T 1732-1993 Determination of Impact Resistance of Film; Standardization Administration of the People’s Republic of China: Beijing, China, 1993; pp. 418–419. (In Chinese)
- Ishizaka, K.; White, B.; Watson, M.; Lewis, S.R.; Lewis, R. Influence of temperature on adhesion coefficient and bonding strength of leaf films: A twin disc study. Wear 2020, 454, 203330. [Google Scholar] [CrossRef]
- Peng, X.R.; Zhang, Z.K.; Zhang, R. Effects of sanding process on adhesion of waterborne paint film on a polypropylene membrane surface. Forest Prod. J. 2020, 70, 232–240. [Google Scholar]
- Yan, X.X.; Wang, L.; Qian, X.Y. Effect of coating process on performance of reversible thermochromic waterborne coatings for Chinese fir. Coatings 2020, 10, 223. [Google Scholar] [CrossRef]





| Sample | Primer Application Method (Time) | Topcoat Application Method (Time) | Adding Method of Color-Changing Powder |
|---|---|---|---|
| #1 | 2 | 2 | Topcoat Addition |
| #2 | 2 | 3 | Primer Addition |
| #3 | 3 | 2 | Primer Addition |
| #4 | 3 | 3 | Topcoat Addition |
| Sample | Number of Primers | Color-Changing Powder Weight (g) | Primer Weight (g) | Topcoat Weight (g) |
|---|---|---|---|---|
| #1, #4 | 2, 3 | 0.1 | 2.0 | 1.9 |
| #2, #3 | 2, 3 | 0.1 | 1.9 | 2.0 |
| #5 | 0 | 0.1 | 2.0 | 1.9 |
| #6 | 1 | 0.1 | 2.0 | 1.9 |
| #7 | 2 | 0.1 | 2.0 | 1.9 |
| #8 | 3 | 0.1 | 2.0 | 1.9 |
| #9 | 4 | 0.1 | 2.0 | 1.9 |
| #10 | 5 | 0.1 | 2.0 | 1.9 |
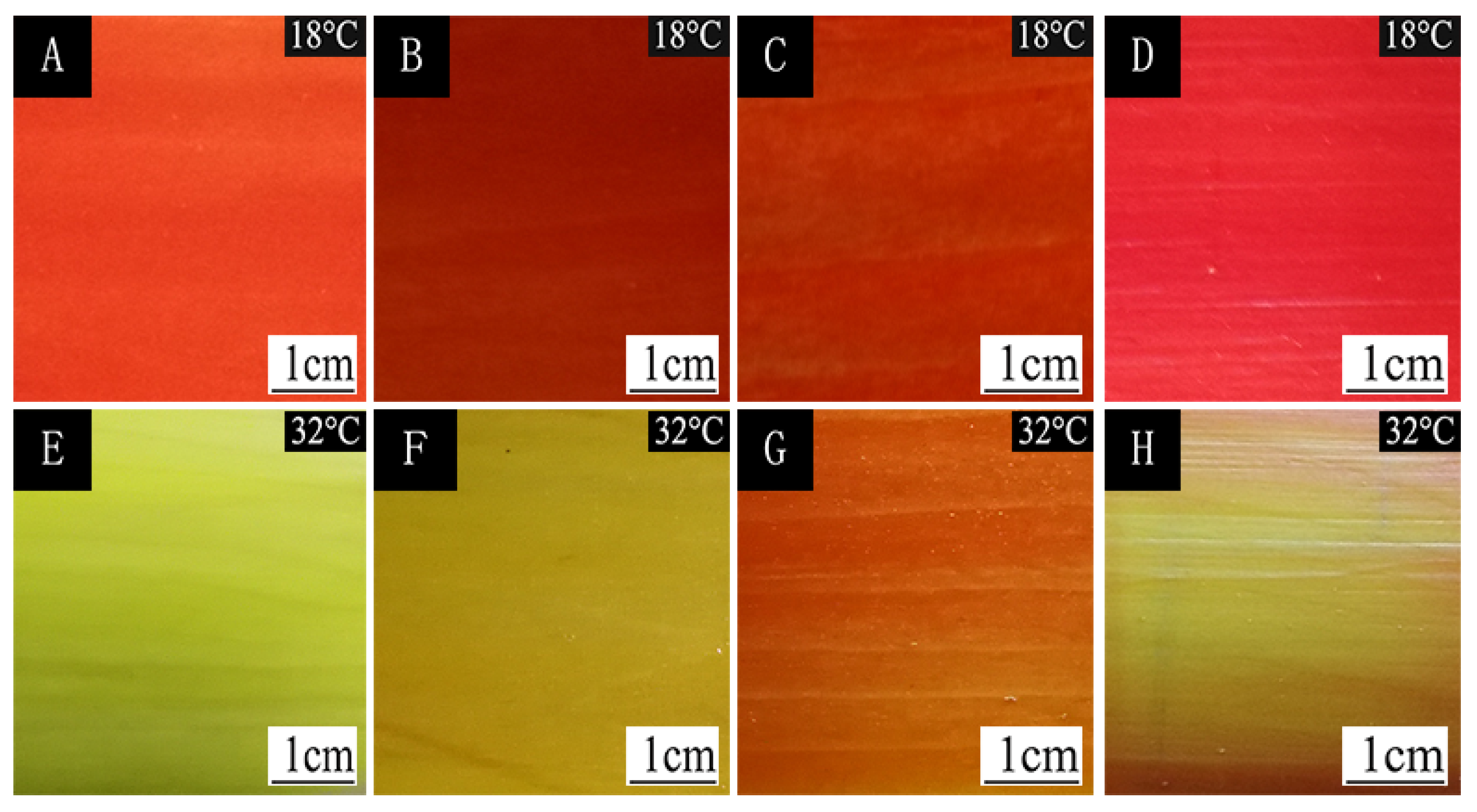
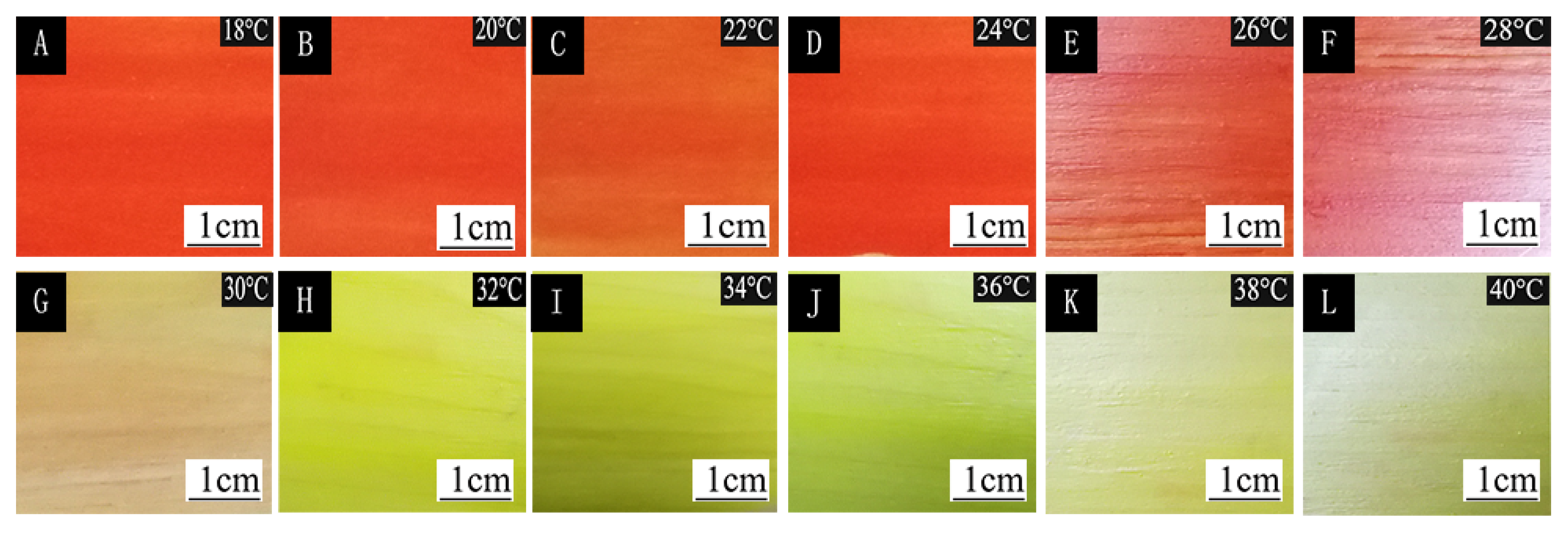
| Sample | Chroma Value | 18 °C | 20 °C | 22 °C | 24 °C | 26 °C | 28 °C | 30 °C | 32 °C | 34 °C | 36 °C | 38 °C | 40 °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| #1 | L* | 51.1 ± 1.2 | 51.3 ± 1.2 | 50.9 ± 1.2 | 51.0 ± 1.3 | 50.8 ± 1.2 | 50.9 ± 1.2 | 51.8 ± 1.3 | 77.4 ± 1.9 | 90.5 ± 2.2 | 95.9 ± 2.4 | 97.5 ± 2.4 | 98.1 ± 2.4 |
| a* | 64.0 ± 1.6 | 63.6 ± 1.5 | 63.5 ± 1.5 | 63.8 ± 1.6 | 63.1 ± 1.5 | 62.9 ± 1.5 | 60.3 ± 1.5 | 15.9 ± 0.4 | −6.0 ± 0.1 | −18.8 ± 0.4 | −22.2 ± 0.5 | −23.7 ± 0.5 | |
| b* | 41.7 ± 1.0 | 42.5 ± 1.0 | 41.5 ± 1.0 | 41.7 ± 1.0 | 41.3 ± 1.0 | 42.5 ± 1.0 | 42.9 ± 1.0 | 69.7 ± 1.7 | 89.0 ± 2.2 | 99.1 ± 2.4 | 103.4 ± 2.5 | 101.7 ± 2.5 | |
| c | 76.4 ± 1.9 | 76.5 ± 1.8 | 75.8 ± 1.9 | 76.2 ± 1.9 | 75.5 ± 1.8 | 75.9 ± 1.9 | 74.1 ± 1.8 | 71.5 ± 1.7 | 89.2 ± 2.2 | 100.9 ± 2.5 | 105.7 ± 2.6 | 104.4 ± 2.6 | |
| H | 33.1 ± 0.8 | 33.7 ± 0.8 | 33.1 ± 0.8 | 33.1 ± 0.8 | 33.1 ± 0.8 | 34.0 ± 0.8 | 35.4 ± 0.8 | 77.1 ± 1.9 | 93.8 ± 2.3 | 100.7 ± 2.5 | 102.1 ± 2.5 | 103.1 ± 2.5 | |
| #2 | L* | 53.6 ± 1.3 | 53.7 ± 1.3 | 53.8 ± 1.3 | 53.8 ± 1.3 | 53.7 ± 1.3 | 53.0 ± 1.3 | 54.1 ± 1.3 | 66.0 ± 1.6 | 94.8 ± 2.3 | 95.0 ± 2.3 | 95.0 ± 2.3 | 94.7 ± 2.3 |
| a* | 59.2 ± 1.4 | 59.8 ± 1.5 | 59.2 ± 1.4 | 59.0 ± 1.5 | 58.7 ± 1.4 | 58.3 ± 1.4 | 56.7 ± 1.4 | 35.8 ± 0.9 | −18.9 ± 0.4 | −19.2 ± 0.4 | −19.8 ± 0.5 | −19.1 ± 0.4 | |
| b* | 47.5 ± 1.1 | 46.5 ± 1.1 | 47.0 ± 1.1 | 46.9 ± 1.2 | 46.7 ± 1.1 | 46.2 ± 1.1 | 46.9 ± 1.1 | 53.4 ± 1.3 | 88.5 ± 2.2 | 88.3 ± 2.2 | 89.1 ± 2.2 | 88.5 ± 2.2 | |
| c | 76.0 ± 1.9 | 75.7 ± 1.9 | 75.6 ± 1.8 | 75.4 ± 1.9 | 75.0 ± 1.8 | 74.4 ± 1.8 | 73.6 ± 1.8 | 64.3 ± 1.6 | 90.5 ± 2.2 | 90.4 ± 2.2 | 91.3 ± 2.2 | 90.5 ± 2.2 | |
| H | 38.7 ± 0.9 | 37.8 ± 0.9 | 38.4 ± 0.9 | 38.4 ± 1.0 | 38.5 ± 0.9 | 38.4 ± 0.9 | 39.5 ± 0.9 | 56.1 ± 1.4 | 102.0 ± 2.5 | 102.2 ± 2.5 | 102.5 ± 2.5 | 102.2 ± 2.5 | |
| #3 | L* | 51.2 ± 1.2 | 51.6 ± 1.3 | 51.6 ± 1.2 | 51.8 ± 1.3 | 51.6 ± 1.2 | 51.8 ± 1.3 | 77.8 ± 1.9 | 97.1 ± 2.4 | 97.8 ± 2.4 | 97.5 ± 2.4 | 98.2 ± 2.4 | 97.3 ± 2.4 |
| a* | 63.0 ± 1.5 | 62.8 ± 1.6 | 63.3 ± 1.5 | 62.9 ± 1.6 | 62.1 ± 1.5 | 61.4 ± 1.5 | 14.7 ± 0.3 | −23.0 ± 0.5 | −23.9 ± 0.6 | −24.0 ± 0.6 | −24.6 ± 0.6 | −24.3 ± 0.6 | |
| b* | 47.0 ± 1.1 | 47.8 ± 1.2 | 46.5 ± 1.1 | 47.3 ± 1.2 | 46.8 ± 1.1 | 47.4 ± 1.1 | 76.3 ± 1.9 | 101.5 ± 2.5 | 102.7 ± 2.5 | 101.8 ± 2.5 | 104.0 ± 2.6 | 100.4 ± 2.5 | |
| c | 78.6 ± 1.9 | 79.0 ± 2.0 | 78.5 ± 1.9 | 78.7 ± 2.0 | 77.8 ± 1.9 | 77.6 ± 1.9 | 77.8 ± 1.9 | 104.1 ± 2.6 | 105.4 ± 2.6 | 104.6 ± 2.6 | 106.9 ± 2.6 | 103.3 ± 2.5 | |
| H | 36.7 ± 0.9 | 37.2 ± 0.9 | 36.3 ± 0.9 | 36.9 ± 0.9 | 37.0 ± 0.9 | 37.6 ± 0.9 | 79.0 ± 1.9 | 102.7 ± 2.5 | 103.1 ± 2.5 | 103.2 ± 2.5 | 103.3 ± 2.5 | 103.6 ± 2.5 | |
| #4 | L* | 49.9 ± 1.2 | 50.0 ± 1.3 | 50.0 ± 1.2 | 49.8 ± 1.3 | 49.9 ± 1.2 | 49.7 ± 1.2 | 55.2 ± 1.3 | 93.5 ± 2.3 | 96.5 ± 2.4 | 97.2 ± 2.4 | 97.0 ± 2.4 | 97.2 ± 2.4 |
| a* | 62.2 ± 1.5 | 62.5 ± 1.6 | 61.9 ± 1.5 | 61.8 ± 1.5 | 62.0 ± 1.5 | 60.9 ± 1.5 | 53.9 ± 1.3 | −15.8 ± 0.4 | −21.9 ± 0.5 | −23.7 ± 0.5 | −24.0 ± 0.6 | −24.2 ± 0.6 | |
| b* | 40.3 ± 1.0 | 40.5 ± 1.0 | 41.5 ± 1.0 | 40.1 ± 1.0 | 40.2 ± 1.0 | 41.0 ± 1.0 | 44.7 ± 1.1 | 100.0 ± 2.5 | 105.1 ± 2.6 | 106.9 ± 2.6 | 106.2 ± 2.6 | 107.1 ± 2.6 | |
| c | 74.1 ± 1.8 | 74.5 ± 1.8 | 74.6 ± 1.8 | 73.7 ± 1.9 | 73.9 ± 1.8 | 73.4 ± 1.8 | 70.1 ± 1.7 | 101.2 ± 2.5 | 107.4 ± 2.6 | 109.5 ± 2.7 | 108.9 ± 2.7 | 109.8 ± 2.7 | |
| H | 32.9 ± 0.8 | 32.9 ± 0.8 | 33.8 ± 0.8 | 32.9 ± 0.8 | 32.9 ± 0.8 | 33.9 ± 0.8 | 39.6 ± 0.9 | 98.9 ± 2.4 | 101.7 ± 2.5 | 102.5 ± 2.5 | 102.7 ± 2.5 | 102.7 ± 2.5 |
| Sample | Number of Primers | Number of Topcoats | Adding Way of Color Changing Powder | Color Difference |
|---|---|---|---|---|
| #1 | 2 | 2 | Topcoat Addition | 61.6 ± 1.9 |
| #2 | 2 | 3 | Primer Addition | 27.1 ± 0.3 |
| #3 | 3 | 2 | Primer Addition | 111.7 ± 1.4 |
| #4 | 3 | 3 | Topcoat Addition | 107.5 ± 0.6 |
| Mean 1 | 44.35 | 86.65 | 84.55 | – |
| Mean 2 | 109.60 | 67.30 | 69.40 | – |
| Range | 65.25 | 19.35 | 15.15 | – |
| Variance | 4257.56 | 374.42 | 229.52 | – |
| Sample | Number of Primers | Number of Topcoats | Adding Way of Color Changing Powder | Gloss 60° (%) |
|---|---|---|---|---|
| #1 | 2 | 2 | Topcoat Addition | 14.2 ± 0.4 |
| #2 | 2 | 3 | Primer Addition | 69.0 ± 0.4 |
| #3 | 3 | 2 | Primer Addition | 70.4 ± 1.5 |
| #4 | 3 | 3 | Topcoat Addition | 15.3 ± 0.1 |
| Mean 1 | 41.60 | 42.30 | 14.75 | – |
| Mean 2 | 42.85 | 42.15 | 69.70 | – |
| Range | 1.25 | 0.15 | 54.95 | – |
| Variance | 1.56 | 0.02 | 3019.50 | – |
| Sample | Chroma Value | 18 °C | 18 °C after NaCl | 18 °C after Detergent | 18 °C after Ethanol | 18 °C after Red Ink | 32 °C | 32 °C after NaCl | 32 °C after Detergent | 32 °C after Ethanol | 32 °C after Red Ink |
|---|---|---|---|---|---|---|---|---|---|---|---|
| #1 | L* | 51.1 ± 1.2 | 50.9 ± 1.2 | 50.4 ± 1.2 | 50.0 ± 1.2 | 53.5 ± 1.3 | 77.4 ± 1.9 | 85.4 ± 2.1 | 76.3 ± 1.9 | 87.1 ± 2.1 | 64.3 ± 1.6 |
| a* | 64.0 ± 1.6 | 62.2 ± 1.5 | 62.7 ± 1.5 | 63.3 ± 1.5 | 66.4 ± 1.6 | 15.9 ± 0.4 | −10.5 ± 0.2 | 10.6 ± 0.2 | −15.6 ± 0.3 | 35.4 ± 0.8 | |
| b* | 41.7 ± 1.0 | 41.5 ± 1.0 | 41.2 ± 1.0 | 41.4 ± 1.0 | 40.9 ± 1.0 | 69.7 ± 1.7 | 78.0 ± 1.9 | 68.1 ± 1.7 | 80.6 ± 2.0 | 51.4 ± 1.2 | |
| c | 76.4 ± 1.9 | 74.1 ± 1.8 | 74.6 ± 1.8 | 75.7 ± 1.8 | 77.2 ± 1.9 | 71.5 ± 1.7 | 78.7 ± 1.9 | 69.0 ± 1.73 | 82.1 ± 2.0 | 62.3 ± 1.5 | |
| H | 33.1 ± 0.8 | 34.4 ± 0.8 | 33.5 ± 0.8 | 33.1 ± 0.8 | 32.0 ± 0.8 | 77.1 ± 1.9 | 97.6 ± 2.4 | 81.1 ± 2.0 | 101.0 ± 2.5 | 55.3 ± 1.3 | |
| #2 | L* | 53.6 ± 1.3 | 53.5 ± 1.3 | 52.8 ± 1.3 | 53.0 ± 1.3 | 45.2 ± 1.1 | 66.0 ± 1.6 | 81.5 ± 2.0 | 64.8 ± 1.6 | 76.2 ± 1.9 | 53.2 ± 1.3 |
| a* | 59.2 ± 1.4 | 58.9 ± 1.4 | 59.6 ± 1.4 | 58.4 ± 1.4 | 66.1 ± 1.6 | 35.8 ± 0.9 | −2.2 ± 0.1 | 20.1 ± 0.5 | 1.9 ± 0.1 | 61.5 ± 1.5 | |
| b* | 47.5 ± 1.1 | 46.3 ± 1.1 | 46.5 ± 1.1 | 46.9 ± 1.1 | 43.7 ± 1.1 | 53.4 ± 1.3 | 71.3 ± 1.7 | 52.7 ± 1.3 | 67.2 ± 1.6 | 46.1 ± 1.1 | |
| c | 76.0 ± 1.9 | 72.7 ± 1.8 | 75.2 ± 1.8 | 73.7 ± 1.8 | 80.8 ± 2.0 | 64.3 ± 1.6 | 71.3 ± 1.7 | 56.1 ± 1.4 | 67.3 ± 1.6 | 76.9 ± 1.9 | |
| H | 38.7 ± 0.9 | 38.5 ± 0.9 | 37.5 ± 0.9 | 37.5 ± 0.9 | 29.9 ± 0.7 | 56.1 ± 1.4 | 91.8 ± 2.3 | 69.0 ± 1.73 | 88.3 ± 2.2 | 36.8 ± 0.9 | |
| #3 | L* | 51.2 ± 1.2 | 51.8 ± 1.3 | 51.4 ± 1.2 | 51.4 ± 1.2 | 50.3 ± 1.2 | 97.1 ± 2.4 | 82.7 ± 2.0 | 71.2 ± 1.7 | 66.7 ± 1.6 | 48.2 ± 1.2 |
| a* | 63.0 ± 1.5 | 62.3 ± 1.5 | 63.5 ± 1.5 | 61.9 ± 1.5 | 67.2 ± 1.6 | −23.0 ± 0.5 | −7.8 ± 0.2 | 16.5 ± 0.4 | −5.2 ± 0.1 | 62.1 ± 1.5 | |
| b* | 47.0 ± 1.1 | 47.3 ± 1.2 | 47.2 ± 1.1 | 46.5 ± 1.1 | 46.6 ± 1.1 | 101.5 ± 2.5 | 75.1 ± 1.8 | 64.5 ± 1.6 | 63.7 ± 1.5 | 43.3 ± 1.0 | |
| c | 78.6 ± 1.9 | 78.3 ± 1.9 | 79.2 ± 1.9 | 76.4 ± 1.9 | 81.8 ± 2.0 | 104.1 ± 2.6 | 75.5 ± 1.8 | 66.6 ± 1.6 | 63.9 ± 1.6 | 75.7 ± 1.8 | |
| H | 36.7 ± 0.9 | 37.1 ± 0.9 | 36.6 ± 0.9 | 37.6 ± 0.9 | 34.7 ± 0.8 | 102.7 ± 2.5 | 95.9 ± 2.4 | 75.6 ± 1.8 | 94.6 ± 2.3 | 34.8 ± 0.8 | |
| #4 | L* | 49.9 ± 1.2 | 49.8 ± 1.2 | 50.4 ± 1.2 | 49.6 ± 1.2 | 46.8 ± 1.1 | 93.5 ± 2.3 | 72.2 ± 1.8 | 50.3 ± 1.2 | 46.1 ± 1.1 | 71.8 ± 1.8 |
| a* | 62.2 ± 1.5 | 61.8 ± 1.5 | 61.7 ± 1.5 | 61.4 ± 1.5 | 68.7 ± 1.7 | −15.8 ± 0.4 | 16.4 ± 0.4 | 59.4 ± 1.4 | 45.3 ± 1.1 | 30.6 ± 0.7 | |
| b* | 40.3 ± 1.0 | 40.1 ± 1.0 | 40.5 ± 1.0 | 39.5 ± 0.9 | 36.7 ± 0.9 | 100.0 ± 2.5 | 29.0 ± 0.7 | 41.6 ± 1.0 | 47.9 ± 1.2 | 65.4 ± 1.6 | |
| c | 74.1 ± 1.8 | 73.7 ± 1.8 | 73.9 ± 1.8 | 72.8 ± 1.8 | 77.9 ± 1.9 | 101.2 ± 2.5 | 33.3 ± 0.8 | 72.5 ± 1.8 | 65.9 ± 1.6 | 72.2 ± 1.8 | |
| H | 32.9 ± 0.8 | 32.9 ± 0.8 | 33.2 ± 0.8 | 32.1 ± 0.8 | 28.1 ± 0.7 | 98.9 ± 2.4 | 60.5 ± 1.5 | 35.0 ± 0.8 | 46.5 ± 1.1 | 64.9 ± 1.6 |
| Level | Finish Film Change |
|---|---|
| 1 | No Mark |
| 2 | Slightly Discolored Impression |
| 3 | Slight Discoloration or Noticeable Discoloration |
| 4 | Obvious Changes, Bubbling, Wrinkles, etc. |
| Sample | NaCl (Level) | Detergent (Level) | Ethanol (Level) | Red Ink (Level) |
|---|---|---|---|---|
| #1 | 1 ± 0 | 1 ± 0 | 1 ± 0 | 3 ± 0 |
| #2 | 1 ± 0 | 1 ± 0 | 1 ± 0 | 3 ± 0 |
| #3 | 1 ± 0 | 1 ± 0 | 1 ± 0 | 3 ± 0 |
| #4 | 1 ± 0 | 1 ± 0 | 1 ± 0 | 3 ± 0 |
| Sample | 20° Gloss (%) | 60° Gloss (%) | 85° Gloss (%) |
|---|---|---|---|
| #5 | 2.7 ± 0 | 12.9 ± 0 | 32.2 ± 0.9 |
| #6 | 3.4 ± 0 | 16.3 ± 0.4 | 39.5 ± 0.6 |
| #7 | 2.8 ± 0 | 14.2 ± 0.4 | 31.0 ± 0.9 |
| #8 | 2.8 ± 0 | 17.6 ± 0.6 | 43.2 ± 0.9 |
| #9 | 3.9 ± 0 | 16.2 ± 0.2 | 40.8 ± 1.3 |
| #10 | 4.1 ± 0 | 17.7 ± 0.6 | 40.2 ± 1.4 |
| Sample | Adhesion (Level) | Impact Resistance (N·cm) | NaCl (Level) | Detergent (Level) | Ethanol (Level) | Red Ink (Level) |
|---|---|---|---|---|---|---|
| #5 | 0 ± 0 | 70.0 ± 0 | 1 ± 0 | 1 ± 0 | 1 ± 0 | 3 ± 0 |
| #6 | 0 ± 0 | 70.0 ± 0 | 1 ± 0 | 1 ± 0 | 1 ± 0 | 3 ± 0 |
| #7 | 0 ± 0 | 70.0 ± 0 | 1 ± 0 | 1 ± 0 | 1 ± 0 | 3 ± 0 |
| #8 | 0 ± 0 | 70.0 ± 0 | 1 ± 0 | 1 ± 0 | 1 ± 0 | 3 ± 0 |
| #9 | 0 ± 0 | 70.0 ± 0 | 1 ± 0 | 1 ± 0 | 1 ± 0 | 3 ± 0 |
| #10 | 0 ± 0 | 70.0 ± 0 | 1 ± 0 | 1 ± 0 | 1 ± 0 | 3 ± 0 |
| Sample | Adhesion (Level) | Impact Resistance (N·cm) | NaCl (Level) | Detergent (Level) | Ethanol (Level) | Red Ink (Level) | Gloss 60° (%) | Color Difference |
|---|---|---|---|---|---|---|---|---|
| Traditional Water-Based Coating | 0 | 40.0 | 1 | 1 | 1 | 1 | 43.1 | 0.8 |
| Water-Based Coating with Thermochromic Ink | 0 | 50.0 | 1 | 1 | 1 | 3 | 55.6 | 21.4 |
| Color-Changing Powder Water-Based Coating | 0 | 70.0 | 1 | 1 | 1 | 3 | 14.2 | 61.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, X.; Chang, Y. Investigation of the Properties of Color-Changing Powder Water-Based Coating. Coatings 2020, 10, 815. https://doi.org/10.3390/coatings10090815
Yan X, Chang Y. Investigation of the Properties of Color-Changing Powder Water-Based Coating. Coatings. 2020; 10(9):815. https://doi.org/10.3390/coatings10090815
Chicago/Turabian StyleYan, Xiaoxing, and Yijuan Chang. 2020. "Investigation of the Properties of Color-Changing Powder Water-Based Coating" Coatings 10, no. 9: 815. https://doi.org/10.3390/coatings10090815
APA StyleYan, X., & Chang, Y. (2020). Investigation of the Properties of Color-Changing Powder Water-Based Coating. Coatings, 10(9), 815. https://doi.org/10.3390/coatings10090815




