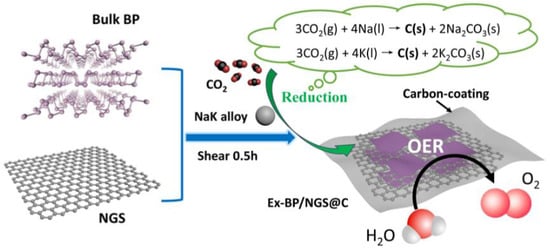
Robust Carbon-Stabilization of Few-Layer Black Phosphorus for Superior Oxygen Evolution Reaction
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of NGS
2.2. Synthesis of Ex-BP
2.3. Synthesis of Ex-BP/NGS
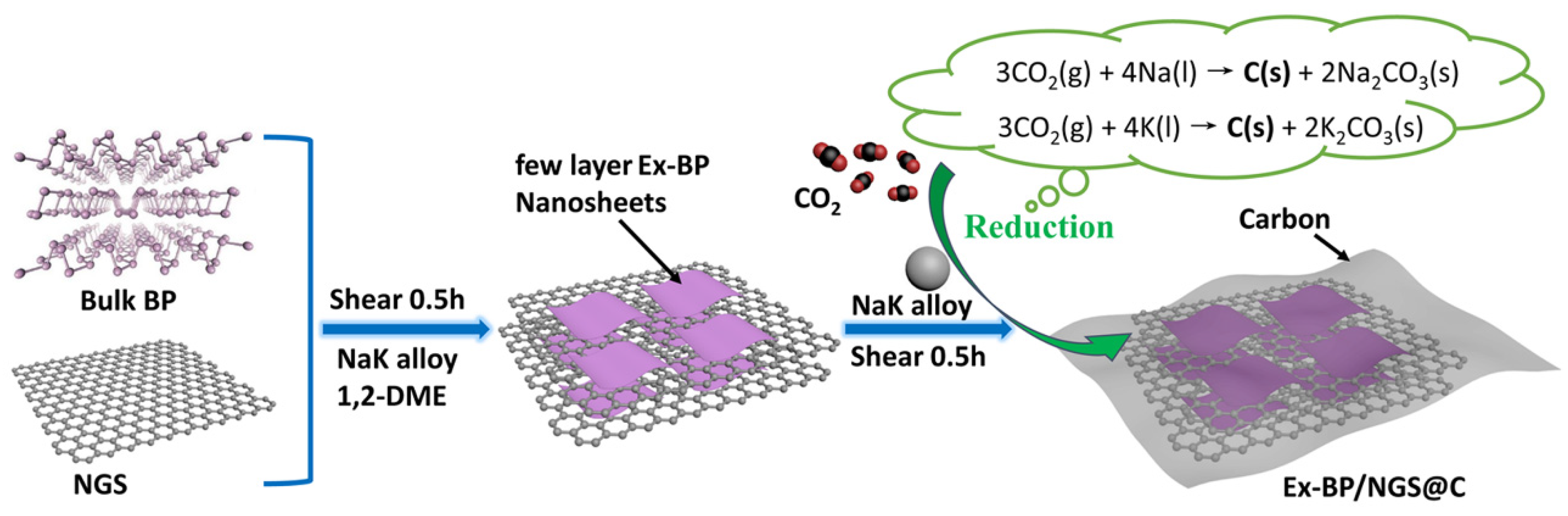
2.4. Synthesis of Ex-BP/NGS@C
2.5. Product Characterization
2.6. Electrochemical Measurements
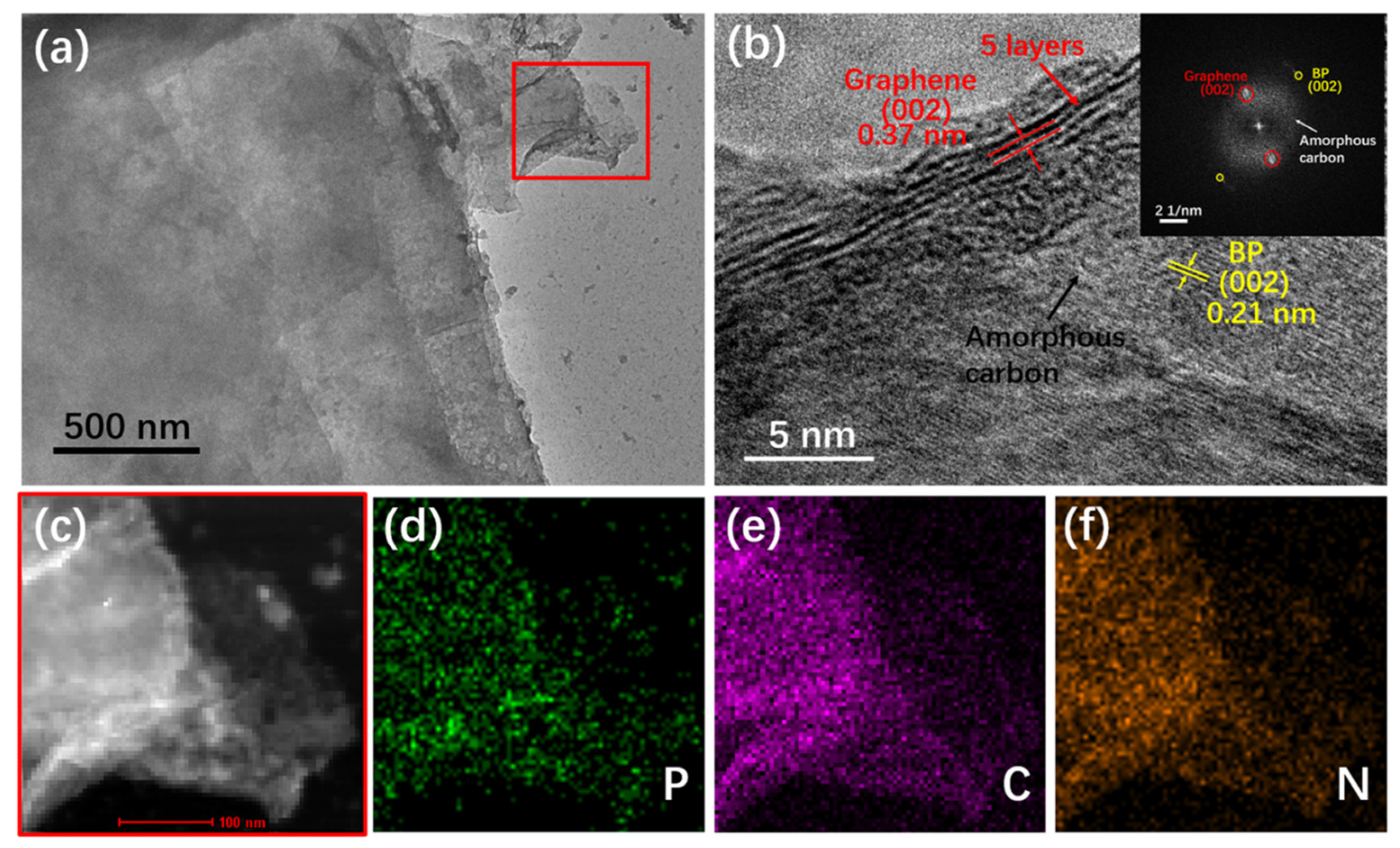
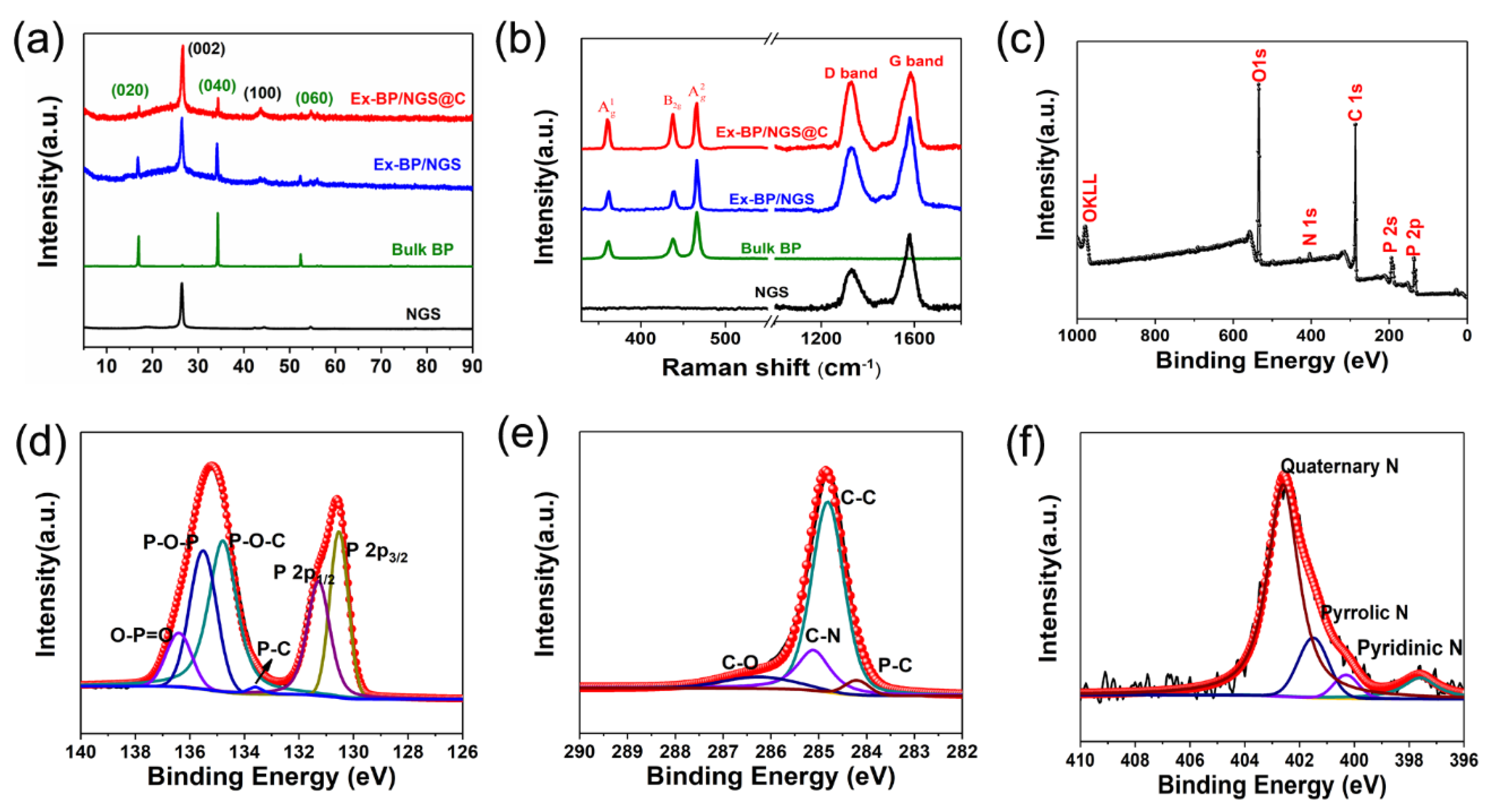
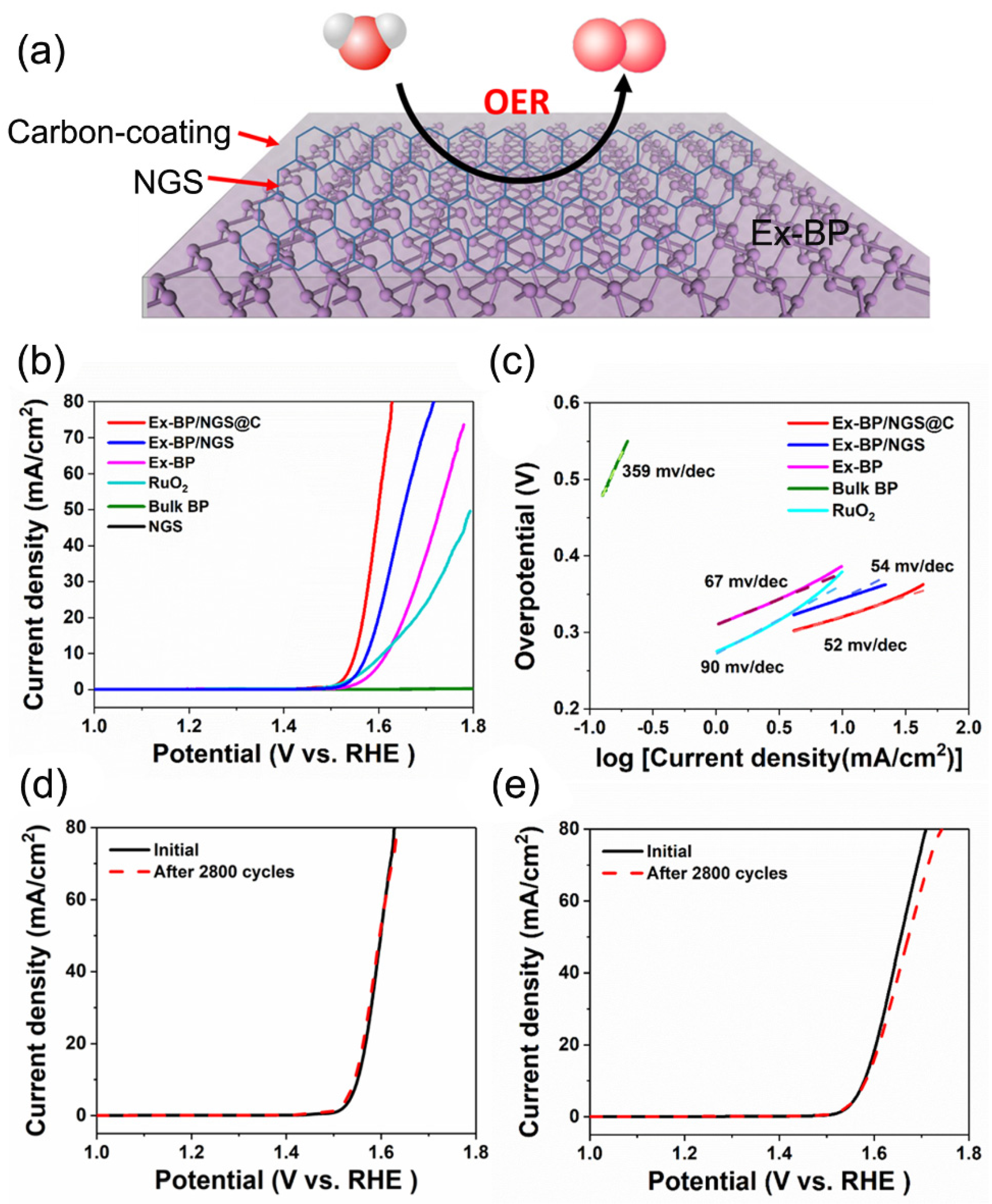
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Choi, J.W.; Aurbach, D. Promise and reality of post-lithium-ion batteries with high energy densities. Nat. Rev. Mater. 2016, 1. [Google Scholar] [CrossRef]
- Wang, H.; Yu, D.; Kuang, C.; Cheng, L.; Li, W.; Feng, X.; Zhang, Z.; Zhang, X.; Zhang, Y. Alkali metal anodes for rechargeable batteries. Chem 2019, 5, 313–338. [Google Scholar] [CrossRef]
- Liu, D.; Li, X.; Chen, S.; Yan, H.; Wang, C.; Wu, C.; Haleem, Y.A.; Duan, S.; Lu, J.; Ge, B.; et al. Atomically dispersed platinum supported on curved carbon supports for efficient electrocatalytic hydrogen evolution. Nat. Energy 2019, 4, 512–518. [Google Scholar] [CrossRef]
- Zhang, J.; Ren, M.; Wang, L.; Li, Y.; Yakobson, B.I.; Tour, J.M. Oxidized laser-induced graphene for efficient oxygen electrocatalysis. Adv. Mater. 2018, 30, 1707319. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Lee, B.; Ju, H.; Lee, S.W.; Kim, J. Reducing the barrier energy of self-reconstruction for anchored cobalt nanoparticles as highly active oxygen evolution electrocatalyst. Adv. Mater. 2019, 31, 1901977. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Lin, M.; Li, J.; Huang, L.; Zhuang, Z.; Lin, C.; Zhou, L.; Mai, L. Metal-organic framework derived carbon-confined Ni2P nanocrystals supported on graphene for an efficient oxygen evolution reaction. Chem. Commun. 2017, 53, 8372–8375. [Google Scholar] [CrossRef] [PubMed]
- Seitz, L.C.; Dickens, C.F.; Nishio, K.; Hikita, Y.; Montoya, J.; Doyle, A.; Kirk, C.; Vojvodic, A.; Hwang, H.Y.; Norskov, J.K.; et al. A highly active and stable IrOx/SrIrO3 catalyst for the oxygen evolution reaction. Science 2016, 353, 1011–1014. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, X.; Cheng, H.; Liu, J.; Shao, M.; Wei, M.; Evans, D.G.; Zhang, H.; Duan, X. Confined synthesis of 2D nanostructured materials toward electrocatalysis. Adv. Energy Mater. 2020, 10, 1900486. [Google Scholar] [CrossRef]
- Chen, Y.C.; Lu, A.Y.; Lu, P.; Yang, X.; Jiang, C.M.; Mariano, M.; Kaehr, B.; Lin, O.; Taylor, A.; Sharp, I.D.; et al. Structurally deformed MoS2 for electrochemically stable, thermally resistant, and highly efficient hydrogen evolution reaction. Adv. Mater. 2017, 29, 1703863. [Google Scholar] [CrossRef]
- García de Arquer, F.P.; Bushuyev, O.S.; De Luna, P.; Dinh, C.T.; Seifitokaldani, A.; Saidaminov, M.I.; Tan, C.S.; Quan, L.N.; Proppe, A.; Kibria, M.G.; et al. 2D Metal oxyhalide-derived catalysts for efficient CO2 electroreduction. Adv. Mater. 2018, 30, 6–11. [Google Scholar] [CrossRef]
- Rubio, R.; Santander, J.; Fonseca, L.; Sabate, N.; Gracia, I.; Cane, C.; Udina, S.; Marco, S. Non-selective NDIR array for gas detection. Sens. Actuators B Chem. 2007, 127, 69–73. [Google Scholar] [CrossRef]
- Yuan, Z.; Li, J.; Yang, M.; Fang, Z.; Jian, J.; Yu, D.; Chen, X.; Dai, L. Ultrathin black phosphorus-on-nitrogen doped graphene for efficient overall water splitting: Dual modulation roles of directional interfacial charge transfer. J. Am. Chem. Soc. 2019, 141, 4972–4979. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Jin, B.; Park, C.; Cho, Y.; Song, X.; Shi, X.; Zhang, S.; Kim, W.; Zeng, H.; Park, J.H. Black phosphorene as a hole extraction layer boosting solar water splitting of oxygen evolution catalysts. Nat. Commun. 2019, 10, 2001. [Google Scholar] [CrossRef] [PubMed]
- Shi, F.; Geng, Z.; Huang, K.; Liang, Q.; Zhang, Y.; Sun, Y.; Cao, J.; Feng, S. Cobalt nanoparticles/black phosphorus nanosheets: An efficient catalyst for electrochemical oxygen evolution. Adv. Sci. 2018, 5. [Google Scholar] [CrossRef]
- Wang, J.; Liu, D.; Huang, H.; Yang, N.; Yu, B.; Wen, M.; Wang, X.; Chu, P.K.; Yu, X.F. In-plane black phosphorus/dicobalt phosphide heterostructure for efficient electrocatalysis. Angew. Chem. Int. Ed. 2018, 57, 2600–2604. [Google Scholar] [CrossRef]
- Brent, J.R.; Ganguli, A.K.; Kumar, V.; Lewis, D.J.; McNaughter, P.D.; O’Brien, P.; Sabherwal, P.; Tedstone, A.A. On the stability of surfactant-stabilised few-layer black phosphorus in aqueous media. RSC Adv. 2016, 6, 86955–86958. [Google Scholar] [CrossRef]
- Tan, S.J.R.; Abdelwahab, I.; Chu, L.; Poh, S.M.; Liu, Y.; Lu, J.; Chen, W.; Loh, K.P. Quasi-monolayer black phosphorus with high mobility and air stability. Adv. Mater. 2018, 30, 1704619. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, N.; Tao, H.; Yan, C.; Huang, J.; Liu, T.; Robertson, A.W.; Texter, J.; Wang, J.; Sun, Z. Exfoliation of STable 2D Black Phosphorus for Device Fabrication. Chem. Mater. 2017, 29, 6445–6456. [Google Scholar] [CrossRef]
- Edmonds, M.T.; Tadich, A.; Carvalho, A.; Ziletti, A.; O’Donnell, K.M.; Koenig, S.P.; Coker, D.F.; Özyilmaz, B.; Neto, A.H.C.; Fuhrer, M.S. Creating a stable oxide at the surface of black phosphorus. ACS Appl. Mater. Interfaces 2015, 7, 14557–14562. [Google Scholar] [CrossRef]
- Gusmão, R.; Sofer, Z.; Pumera, M. Functional protection of exfoliated black phosphorus by noncovalent modification with anthraquinone. ACS Nano 2018, 12, 5666–5673. [Google Scholar] [CrossRef]
- Abate, Y.; Akinwande, D.; Gamage, S.; Wang, H.; Snure, M.; Poudel, N.; Cronin, S.B. Recent progress on stability and passivation of black phosphorus. Adv. Mater. 2018, 30, 1704749. [Google Scholar] [CrossRef] [PubMed]
- Walia, S.; Balendhran, S.; Ahmed, T.; Singh, M.; El-Badawi, C.; Brennan, M.D.; Weerathunge, P.; Karim, M.N.; Rahman, F.; Rassell, A.; et al. Ambient protection of few-layer black phosphorus via sequestration of reactive oxygen species. Adv. Mater. 2017, 29, 1700152. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Zhou, Y.; Zhou, X.; Zhang, T.; Wang, C.; Yuen, R.K.K.; Hu, W.; Hu, Y. Air-stable polyphosphazene-functionalized few-layer black phosphorene for flame retardancy of epoxy resins. Small 2019, 15, 1805175. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.; Zhang, M.; Zhang, H.; Liu, X.; Feng, H. Room-temperature carbon coating on MoS2/graphene hybrids with carbon dioxide for enhanced sodium storage. Carbon 2019, 153, 217–224. [Google Scholar] [CrossRef]
- Wu, X.; Wang, Z.; Yu, M.; Xiu, L.; Qiu, J. Stabilizing the mxenes by carbon nanoplating for developing hierarchical nanohybrids with efficient lithium storage and hydrogen evolution capability. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef]
- Park, J.E.; Jang, Y.S.; Bae, T.S.; Lee, M.H. Multi-walled carbon nanotube coating on alkali treated TiO2 nanotubes surface for improvement of biocompatibility. Coatings 2018, 8, 159. [Google Scholar] [CrossRef]
- Feng, H.; Hu, Z.; Liu, X. Facile and efficient exfoliation of inorganic layered materials using liquid alkali metal alloys. Chem. Commun. 2015, 51, 10961–10964. [Google Scholar] [CrossRef]
- Feng, H.; Cheng, R.; Zhao, X.; Duan, X.; Li, J. A low-temperature method to produce highly reduced graphene oxide. Nat. Commun. 2013, 4, 1539. [Google Scholar] [CrossRef]
- Brent, J.R.; Savjani, N.; Lewis, E.A.; Haigh, S.J.; Lewis, D.J.; O’Brien, P. Production of few-layer phosphorene by liquid exfoliation of black phosphorus. Chem. Commun. 2014, 50, 13338–13341. [Google Scholar] [CrossRef]
- Sun, J.; Lee, H.W.; Pasta, M.; Yuan, H.; Zheng, G.; Sun, Y.; Li, Y.; Cui, Y. A phosphorene-graphene hybrid material as a high-capacity anode for sodium-ion batteries. Nat. Nanotechnol. 2015, 10, 980–985. [Google Scholar] [CrossRef]
- Favron, A.; Gaufrès, E.; Fossard, F.; Phaneuf-Laheureux, A.L.; Tang, N.Y.W.; Lévesque, P.L.; Loiseau, A.; Leonelli, R.; Francoeur, S.; Martel, R. Photooxidation and quantum confinement effects in exfoliated black phosphorus. Nat. Mater. 2015, 14, 826–832. [Google Scholar] [CrossRef]
- Kuntz, K.L.; Wells, R.A.; Hu, J.; Yang, T.; Dong, B.; Guo, H.; Woomer, A.H.; Druffel, D.L.; Alabanza, A.; Tománek, D.; et al. Control of surface and edge oxidation on phosphorene. ACS Appl. Mater. Interfaces 2017, 9, 9126–9135. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Chen, H.; Xu, Q.; Liu, H.; Wang, Y.-G.; Xia, Y. Black phosphorus stabilizing Na2Ti3O7/C each other with an improved electrochemical property for sodium-ion storage. ACS Appl. Mater. Interfaces 2018, 10, 37163–37171. [Google Scholar] [CrossRef]
- Sun, J.; Zheng, G.; Lee, H.W.; Liu, N.; Wang, H.; Yao, H.; Yang, W.; Cui, Y. Formation of stable phosphorus-carbon bond for enhanced performance in black phosphorus nanoparticle-graphite composite battery anodes. Nano Lett. 2014, 14, 4573–4580. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Zhou, J.; Qi, X.; Liu, Y.; Huang, Z.; Li, Z.; Ge, Y.; Dhanabalan, S.C.; Ponraj, J.S.; Wang, S.; et al. Few-layer black phosphorus nanosheets as electrocatalysts for highly efficient oxygen evolution reaction. Adv. Energy Mater. 2017, 7, 1700396. [Google Scholar] [CrossRef]
- Jiang, Q.; Xu, L.; Chen, N.; Zhang, H.; Dai, L.; Wang, S. Facile synthesis of black phosphorus: An efficient electrocatalyst for the oxygen evolving reaction. Angew. Chem. Int. Ed. 2016, 55, 13849–13853. [Google Scholar] [CrossRef]
- Liang, Y.; Li, Y.; Wang, H.; Zhou, J.; Wang, J.; Regier, T.; Dai, H. Co3O4 nanocrystals on graphene as a synergistic catalyst for oxygen reduction reaction. Nat. Mater. 2011, 10, 780–786. [Google Scholar] [CrossRef]
- Prasannachandran, R.; Vineesh, T.V.; Anil, A.; Krishna, B.M.; Shaijumon, M.M. Functionalized phosphorene quantum dots as efficient electrocatalyst for oxygen evolution reaction. ACS Nano 2018, 12, 11511–11519. [Google Scholar] [CrossRef]

| Sample | Onset Potential (V) | Potential at the Current Density of 10 mA/cm2 (V) | Tafel Slope (mV dec−1) | Electrolyte | References |
|---|---|---|---|---|---|
| Ex-BP/NGS@C | 1.43 V vs. RHE | 1.55 V vs. RHE | 52 | 1 M KOH. | This work |
| Few-Layer BP | 1.45V vs. RHE | - | 88 | 1 M KOH. | [35] |
| BP-CNT | 1.49 V vs. RHE | 1.6 V vs. RHE | 72.88 | 0.1 M KOH. | [36] |
| BP-Co2P | 1.53 V vs. RHE | 1.61 V vs. RHE | 78 | 1 M KOH. | [15] |
| Co/BP | 1.44 V vs. RHE | 1.54 V vs. RHE | 61 | 1 M KOH. | [36] |
| Co3O4/N-RGO | 1.5 V vs. RHE | 1.54 V vs. RHE | 67 | 1 M KOH. | [37] |
| FPQD | - | 1.66 V vs. RHE | 49 | 1 M NaOH. | [38] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Zhu, W.; Yang, X.; Feng, M.; Feng, H. Robust Carbon-Stabilization of Few-Layer Black Phosphorus for Superior Oxygen Evolution Reaction. Coatings 2020, 10, 695. https://doi.org/10.3390/coatings10070695
Zhang M, Zhu W, Yang X, Feng M, Feng H. Robust Carbon-Stabilization of Few-Layer Black Phosphorus for Superior Oxygen Evolution Reaction. Coatings. 2020; 10(7):695. https://doi.org/10.3390/coatings10070695
Chicago/Turabian StyleZhang, Mengjie, Wenchang Zhu, Xingzhe Yang, Meng Feng, and Hongbin Feng. 2020. "Robust Carbon-Stabilization of Few-Layer Black Phosphorus for Superior Oxygen Evolution Reaction" Coatings 10, no. 7: 695. https://doi.org/10.3390/coatings10070695
APA StyleZhang, M., Zhu, W., Yang, X., Feng, M., & Feng, H. (2020). Robust Carbon-Stabilization of Few-Layer Black Phosphorus for Superior Oxygen Evolution Reaction. Coatings, 10(7), 695. https://doi.org/10.3390/coatings10070695