Effect of SiO2 Sol/Silane Emulsion in Reducing Water and Chloride Ion Penetration in Concrete
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Concrete
2.1.2. Preparation of Triethoxy(Isobutyl)Silane Emulsion and SiO2 Sol
2.2. Formation of Hydrophobic Layer on Concrete Surface
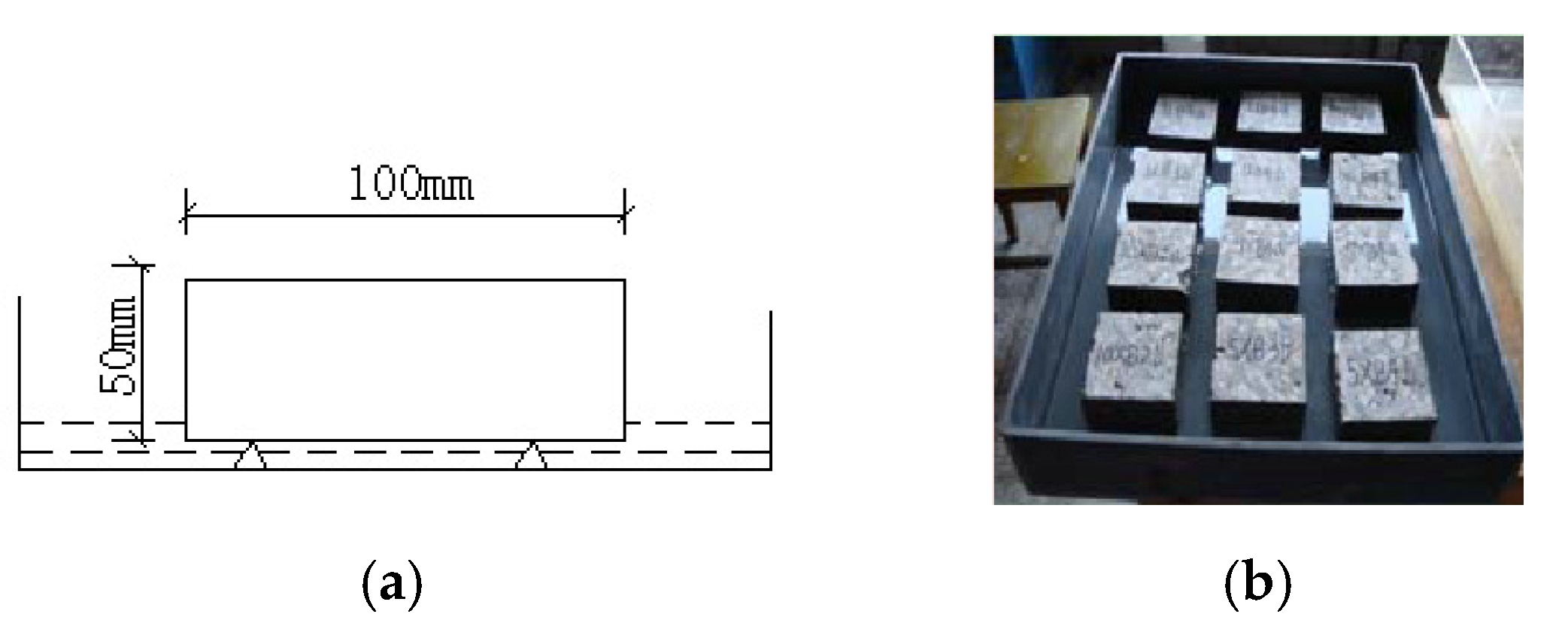
2.3. Test Process and Method
2.3.1. Capillary Water Absorption
2.3.2. Chloride Penetration
2.3.3. Chloride Diffusion Coefficients
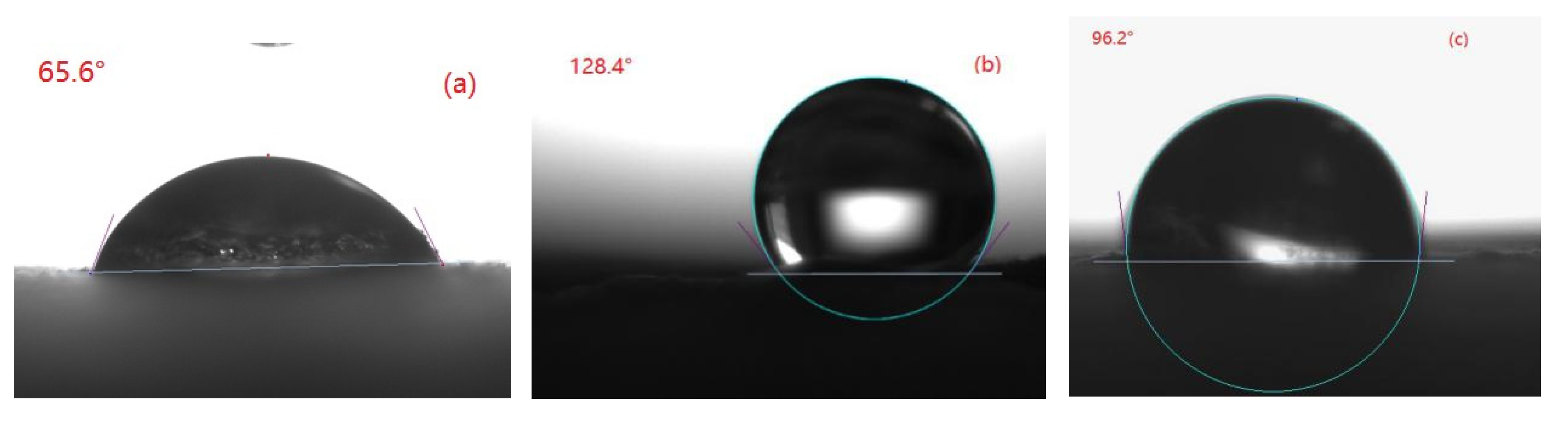
2.3.4. Contact Angle Measurement
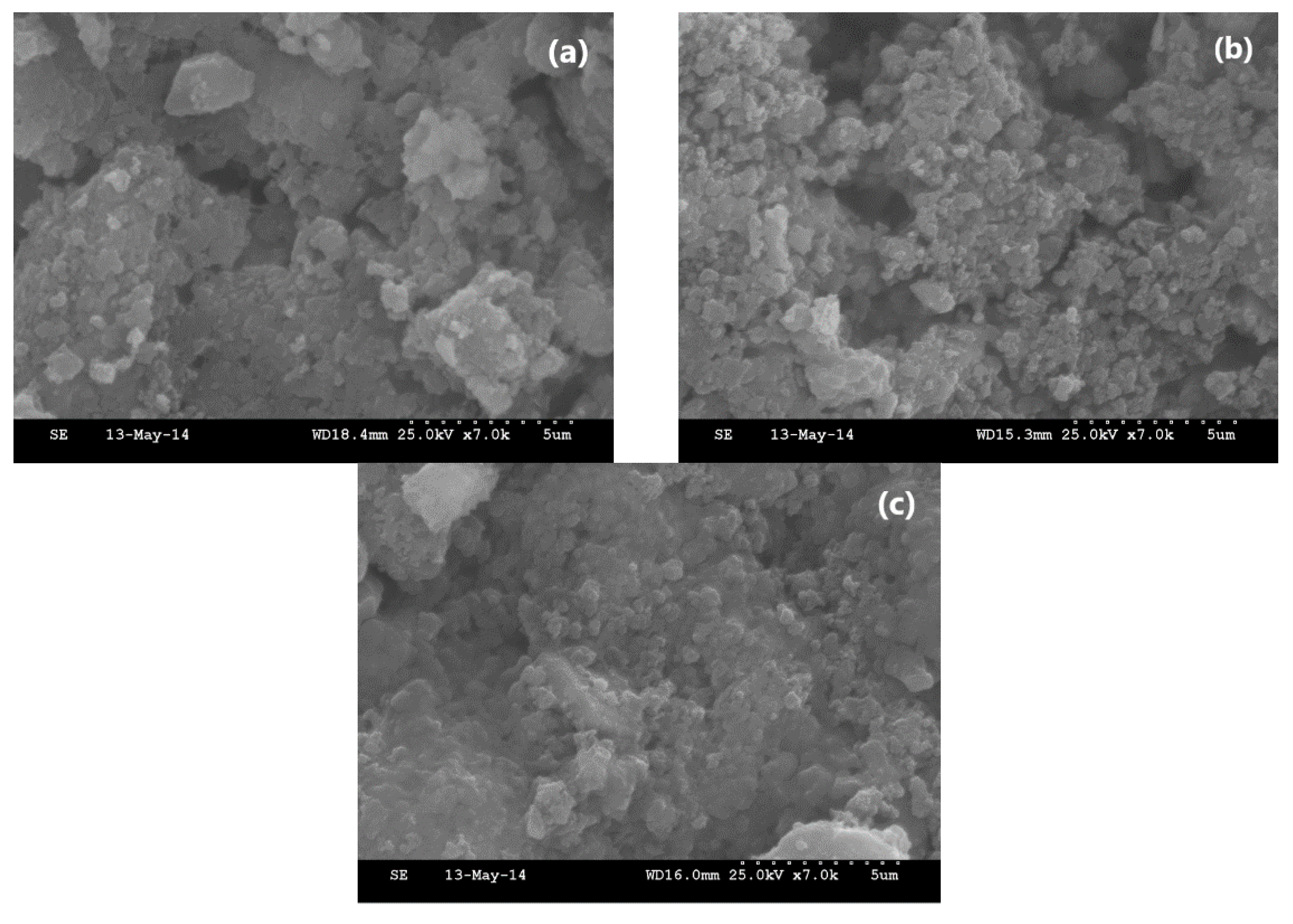
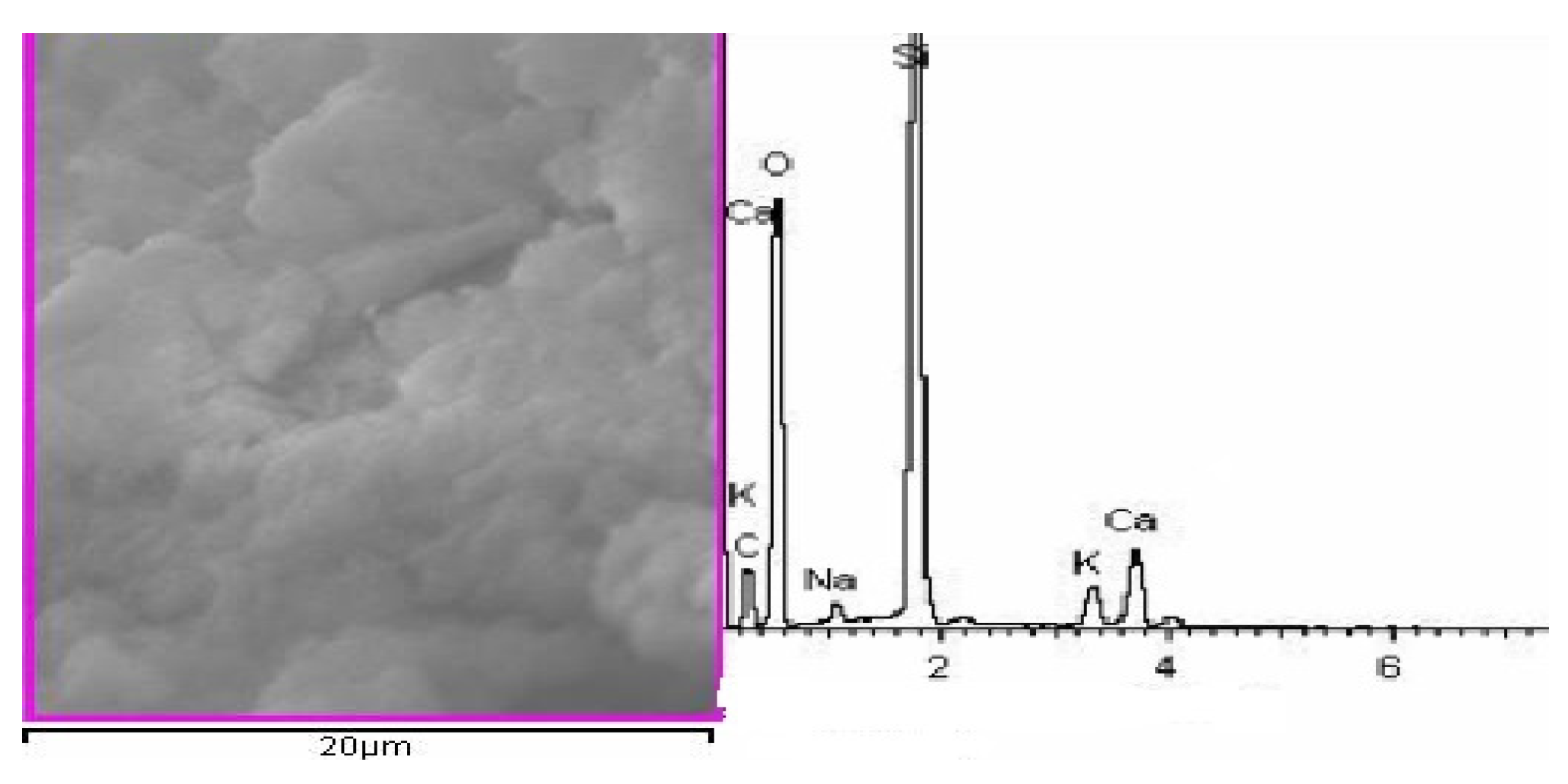
2.3.5. SEM
3. Results and Discussion
3.1. Effect of SiO2 Sol/Silane Emulsion on Water Contact Angle on Concrete
3.2. Effect of SiO2 Sol/Silane Emulsion on Water Absorption in Concrete
3.3. Effect of SiO2 Sol/Silane Emulsion on Chloride Ion Diffusion in Concrete
3.4. Microstructure Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dareyni, M.; Moghaddam, A.M.; Delarami, A. Effect of cationic asphalt emulsion as an admixture on transport properties of roller-compacted concrete. Constr. Build. Mater. 2018, 163, 724–733. [Google Scholar] [CrossRef]
- Wasim, M.; Ngo, T.D.; Abid, M. Investigation of long-term corrosion resistance of reinforced concrete structures constructed with various types of concretes in marine and various climate environments. Constr. Build. Mater. 2020, 237, 117701. [Google Scholar] [CrossRef]
- Chang, H.; Jin, Z.; Zhao, T.; Wang, B.; Li, Z.; Liu, J. Capillary suction induced water absorption and chloride transport in non-saturated concrete: The influence of humidity, mineral admixtures and sulfate ions. Constr. Build. Mater. 2020, 236, 117581. [Google Scholar] [CrossRef]
- Rossi, E.; Polder, R.; Copuroglu, O.; Nijland, T.; Šavija, B. The influence of defects at the steel/concrete interface for chloride-induced pitting corrosion of naturally-deteriorated 20-years-old specimens studied through X-ray computed tomography. Constr. Build. Mater. 2020, 235, 117474. [Google Scholar] [CrossRef]
- Balestra, C.E.T.; Nakano, A.Y.; Savaris, G.; Medeiros-Junior, R.A. Reinforcement corrosion risk of marine concrete structures evaluated through electrical resistivity: Proposal of parameters based on field structures. Ocean Eng. 2019, 187, 106167. [Google Scholar] [CrossRef]
- Li, G.; Yue, J.; Guo, C.; Ji, Y. Influences of modified nanoparticles on hydrophobicity of concrete with organic film coating. Constr. Build. Mater. 2018, 169, 1–7. [Google Scholar] [CrossRef]
- Wang, Q.; Li, S.; Pan, S.; Guo, Z. Synthesis and properties of a silane and copolymer-modified graphene oxide for use as a water-reducing agent in cement pastes. New Carbon Mater. 2018, 33, 131–139. [Google Scholar] [CrossRef]
- Cai, Y.; Hou, P.; Duan, C.; Zhang, R.; Zhou, Z.; Cheng, X.; Shah, S. The use of tetraethyl orthosilicate silane (TEOS) for surface-treatment of hardened cement-based materials: A comparison study with normal treatment agents. Constr. Build. Mater. 2016, 117, 144–151. [Google Scholar] [CrossRef]
- Herb, H.; Gerdes, A.; Brenner-Weiß, G. Characterization of silane-based hydrophobic admixtures in concrete using TOF-MS. Cement Concr. Res. 2015, 70, 77–82. [Google Scholar] [CrossRef]
- Shea, W.; Du, Y.; Miao, C.; Liu, J.; Zhao, G.; Jiang, J.; Zhang, Y. Application of organic- and nanoparticle-modified foams in foamed concrete: Reinforcement and stabilization mechanisms. Cement Concr. Res. 2018, 106, 12–22. [Google Scholar] [CrossRef]
- Shen, L.; Jiang, H.; Wang, T.; Chen, K.; Zhang, H. Performance of silane-based surface treatments for protecting degraded historic concrete. Prog. Org. Coat. 2019, 129, 209–216. [Google Scholar] [CrossRef]
- Zhu, Y.-G.; Kou, S.-C.; Poon, C.-S.; Dai, J.-G.; Li, Q.-Y. Influence of silane-based water repellent on the durability properties of recycled aggregate concrete. Cem. Concr. Compos. 2013, 35, 32–38. [Google Scholar] [CrossRef]
- Sudbrink, B.; Moradllo, M.K.; Hu, Q.; Ley, M.T.; Davis, J.M.; Materer, N.; Apblett, A. Imaging the presence of silane coatings in concrete with micro X-ray fluorescence. Cem. Concr. Res. 2017, 92, 121–127. [Google Scholar] [CrossRef]
- Moradllo, M.K.; Sudbrink, B.; Ley, M.T. Determining the effective service life of silane treatments in concrete bridge decks. Constr. Build. Mater. 2016, 116, 121–127. [Google Scholar] [CrossRef]
- Christodoulou, C.; Goodier, C.I.; Austin, S.A.; Webb, J.; Glass, G.K. Long-term performance of surface impregnation of reinforced concrete structures with silane. Constr. Build. Mater. 2013, 48, 708–716. [Google Scholar] [CrossRef]
- Xue, X.; Li, Y.; Yang, Z.; He, Z.; Dai, J.-G.; Xu, L.; Zhang, W. A systematic investigation of the waterproofing performance and chloride resistance of a self-developed waterborne silane-based hydrophobic agent for mortar and concrete. Constr. Build. Mater. 2017, 155, 939–946. [Google Scholar] [CrossRef]
- Petcherdchoo, A.; Chindaprasirt, P. Exponentially aging functions coupled with time-dependent chloride transport model for predicting service life of surface-treated concrete in tidal zone. Cem. Concr. Res. 2019, 120, 1–12. [Google Scholar] [CrossRef]
- Liu, J.; Cai, J.; Shi, L.; Liu, J.; Zhou, X.; Mu, S.; Hong, J. The inhibition behavior of a water-soluble silane for reinforcing steel in 3.5% NaCl saturated Ca(OH)2 solution. Constr. Build. Mater. 2018, 189, 95–101. [Google Scholar] [CrossRef]
- Aguiar, J.B.; Júnior, C. Carbonation of surface protected concrete. Constr. Build. Mater. 2013, 49, 478–483. [Google Scholar] [CrossRef]
- Tittarelli, F.; Moriconi, G. The effect of silane-based hydrophobic admixture on corrosion of reinforcing steel in concrete. Cem. Concr. Res. 2008, 38, 1354–1357. [Google Scholar] [CrossRef]
- Guo, T.; Weng, X. Evaluation of the freeze-thaw durability of surface-treated airport pavement concrete under adverse conditions. Constr. Build. Mater. 2019, 206, 519–530. [Google Scholar] [CrossRef]
- Karthick, S.; Park, D.-J.; Lee, Y.S.; Saraswathy, V.; Lee, H.-S.; Jang, H.-O.; Choi, H.-J. Development of water-repellent cement mortar using silane enriched with nanomaterials. Prog. Org. Coat. 2018, 125, 48–60. [Google Scholar] [CrossRef]
- Pan, X.; Shi, Z.; Shi, C.; Ling, T.-C.; Li, N. A review on concrete surface treatment part I: Types and mechanisms. Constr. Build. Mater. 2017, 132, 578–590. [Google Scholar] [CrossRef]
- Pan, X.; Shi, Z.; Shi, C.; Ling, T.C.; Li, N. A review on surface treatment for concrete–part 2: Performance. Constr. Build. Mater. 2017, 133, 81–90. [Google Scholar] [CrossRef]
- Hou, P.K.; Kawashima, S.; Wang, K.J.; Corr, D.J.; Qian, J.S.; Shah, S.P. Effects of colloidal nanosilica on rheological and mechanical properties of fly ash-cement mortar. Cement Concr. Compos. 2013, 35, 12–22. [Google Scholar] [CrossRef]
- Mei, J.; Tan, H.; Li, H.; Ma, B.; Liu, X.; Jiang, W.; Zhang, T.; Li, X. Effect of sodium sulfate and nano-SiO2 on hydration and microstructure of cementitious materials containing high volume fly ash under steam curing. Constr. Build. Mater. 2018, 163, 812–825. [Google Scholar] [CrossRef]
- Liu, M.; Tan, H.; He, X. Effects of nano-SiO2 on early strength and microstructure of steam-cured high volume fly ash cement system. Constr. Build. Mater. 2019, 194, 350–359. [Google Scholar] [CrossRef]
- Liu, X.; Ma, B.; Tan, H.; Zhang, T.; Mei, J.; Qi, H.; Chen, P.; Wang, J. Effects of colloidal nano-SiO2 on the immobilization of chloride ions in cement-fly ash system. Cem. Concr. Compos. 2020, 110, 103596. [Google Scholar] [CrossRef]
- Kumar, S.; Sirajudeen; Sivaranjani; Vani; Ali, N.; Begum, S.; Rahman, Z. Characterization, properties and microstructure studies of cement mortar incorporating nano-SiO2. Mater. Today Proc. 2020, in press. [Google Scholar] [CrossRef]
- Wang, C.; Yang, H.; Chen, F.; Peng, L.; Gao, H.-F.; Zhao, L.-P. Influences of VTMS/SiO2 ratios on the contact angle and morphology of modified super-hydrophobic silicon dioxide material by vinyl trimethoxy silane. Results Phys. 2018, 10, 891–902. [Google Scholar] [CrossRef]
- Pantoja, M.; Abenojar, J.; Martinez, M.A. Influence of the type of solvent on the development of superhydrophobicity from silane-based solution containing nanoparticles. Appl. Surf. Sci. 2017, 397, 87–94. [Google Scholar] [CrossRef]
- UNI EN 1015-18:2004 Methods of Test for Mortar for Masonry-Determination of Water Absorption Coefficient Due to Capillary Action of Hardened Mortar; Italian Standards: Milano, Italy, 2004.
- Zhang, Y.; Li, S.; Zhang, W.; Chen, X.; Hou, D.; Zhao, T.; Li, X. Preparation and mechanism of graphene oxide/isobutyltriethoxysilane composite emulsion and its effects on waterproof performance of concrete. Constr. Build. Mater. 2019, 208, 343–349. [Google Scholar] [CrossRef]
- British Standard BS EN 14629:2007 Products and Systems for the Protection and Repair of Concrete Structures-Test Methods; Determination of Chloride Content in Hardened Concrete; British Standards Institution: London, UK, 2007.
- ASTM C1202-97 Standard Test Method for Electrical Indication of Concrete’s Ability to Resist Chloride Ion Penetration; ASTM: West Conshohocken, PA, USA, 1997.
- Cengiz, U.; Cansoy, C.E. Applicability of Cassie–Baxter equation for superhydrophobic fluoropolymer–silica composite films. Appl. Surf. Sci. 2015, 335, 99–106. [Google Scholar] [CrossRef]
- Hou, P.; Kawashima, S.; Kong, D.; Corr, D.J.; Qian, J.; Shah, S.P. Modification effects of colloidal nanoSiO2 on cement hydration and its gel property. Compos. Part B 2013, 45, 440–448. [Google Scholar] [CrossRef]
- Ardalan, R.B.; Jamshidi, N.; Arabameri, H.; Joshaghani, A.; Mehrinejad, M.; Sharafi, P. Enhancing the permeability and abrasion resistance of concrete using colloidal nano-SiO2 oxide and spraying nanosilicon practices. Constr. Build. Mater. 2017, 146, 128–135. [Google Scholar] [CrossRef]
- Ren, J.; Lai, Y.; Gao, J. Exploring the influence of SiO2 and TiO2 nanoparticles on the mechanical properties of concrete. Constr. Build. Mater. 2018, 175, 277–285. [Google Scholar] [CrossRef]
- Nazari, A.; Riahi, S. Microstructural, thermal, physical and mechanical behavior of the self compacting concrete containing SiO2 nanoparticles. Mater. Sci. Eng. A 2010, 527, 7663–7672. [Google Scholar] [CrossRef]
- Balapour, M.; Joshaghani, A.; Althoey, F. Nano-SiO2 contribution to mechanical, durability, fresh and microstructural characteristics of concrete: A review. Constr. Build. Mater. 2018, 181, 27–41. [Google Scholar] [CrossRef]




| Strength Grade | Cement | Sand | Gravel | Water | Superplasticizer | Water Cement Ratio (W/C) | Compressive Strength (MPa) |
|---|---|---|---|---|---|---|---|
| C50 | 380.0 | 579.0 | 1269.0 | 152.0 | 1% | 0.4 | 52.6 |
| C40 | 320.0 | 653.0 | 1267.0 | 160.0 | 0.8% | 0.5 | 44.7 |
| Form | Appearance | pH Value | Stability | Silane Content | Viscosity (mPa·s) | Note |
|---|---|---|---|---|---|---|
| Isobutyl-triethoxy silane emulsion | Milk white, no precipitation | 7.2 | >6 months | 50.5% | 5.1 | Available water dilution, silane emulsion |
| SiO2 sol | transparent, yellowish, no precipitation | 6.0 | >12 months | – | 5.5 | Available water dilution, SiO2 sol |
| Type | SiO2 Sol | Silane Emulsion |
|---|---|---|
| S0 | 0 | 600 |
| S1 | 100 | 500 |
| S2 | 200 | 400 |
| S3 | 300 | 300 |
| Type | C50 | C40 |
|---|---|---|
| untreated | 45.83 | 53.71 |
| S0 | 12.06 | 18.64 |
| S1 | 10.53 | 17.20 |
| S2 | 10.75 | 14.89 |
| S3 | 8.95 | 14.31 |
| W/C | Chloride Diffusion Coefficient/(10−12 m2·s−1) | Penetration Depth/mm | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Untreated | S0 | S1 | S2 | S3 | Untreated | S0 | S1 | S2 | S3 | |
| C50 | 9.2 | 1.6 | 1.4 | 1.1 | 1.1 | 17.35 | 8.32 | 7.38 | 6.47 | 6.23 |
| C40 | 10.4 | 1.8 | 1.7 | 1.5 | 1.2 | 18.46 | 10.52 | 8.21 | 7.72 | 6.57 |
| Sample | Content of Si (%) | |
|---|---|---|
| Spot Scanning | Surface Scanning | |
| Without Treatment | 4.04 | 4.81 |
| Treated By SiO2 Sol | 7.34 | 7.21 |
| Treated By SiO2 Sol/Silane Emulsion (S3) | 14.12 | 10.23 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geng, Y.; Li, S.; Hou, D.; Chen, X.; Jin, Z. Effect of SiO2 Sol/Silane Emulsion in Reducing Water and Chloride Ion Penetration in Concrete. Coatings 2020, 10, 682. https://doi.org/10.3390/coatings10070682
Geng Y, Li S, Hou D, Chen X, Jin Z. Effect of SiO2 Sol/Silane Emulsion in Reducing Water and Chloride Ion Penetration in Concrete. Coatings. 2020; 10(7):682. https://doi.org/10.3390/coatings10070682
Chicago/Turabian StyleGeng, Yongjuan, Shaochun Li, Dongshuai Hou, Xu Chen, and Zuquan Jin. 2020. "Effect of SiO2 Sol/Silane Emulsion in Reducing Water and Chloride Ion Penetration in Concrete" Coatings 10, no. 7: 682. https://doi.org/10.3390/coatings10070682
APA StyleGeng, Y., Li, S., Hou, D., Chen, X., & Jin, Z. (2020). Effect of SiO2 Sol/Silane Emulsion in Reducing Water and Chloride Ion Penetration in Concrete. Coatings, 10(7), 682. https://doi.org/10.3390/coatings10070682




