Abstract
UV–curable polyacrylate is widely used in free–radical type UV–cure coating systems, the disadvantages of which including poor thermal stability and UV resistance can be overcome through chemical modification by silicone. However, it is a remarkable fact that the strategies for fabrication UV–cured silicone modified polyacrylates are somewhat complicated and the price of the products may be much expensive than pure UV–cured polyacrylates. In this work, an easy fabrication method to prepare inexpensive UV–cured transparent silicone modified polyacrylate coatings with good adhesion and UV resistance performance was developed from copolymers of acylates and thiol silicone resin by UV initiated thiol–ene click reaction without UV initiator. The striking results with a high application value should be emphasized that when the amount of thiol silicone resin is only one wt.% of the copolymer of acrylates, the UV–cured coatings obtained exhibit fairly good performance. These coatings prepared exhibit transparency higher than 96% (800 nm), adhesion property to glass slides can reach grade 0, pencil hardness can reach 6H, water absorption is less than 0.16%. In particular, it is observed obviously that the silicone modified polyacrylate coatings exhibit better UV resistance performance than the coating prepared with only copolymers of acrylates initiated by UV initiator 1173. It is proved that it is actually an easy fabrication method to prepare inexpensive UV–cured transparent silicone modified polyacrylate coatings with high performance by UV initiated thiol–ene click reaction of copolymers of acylates and thiol silicone resin.
1. Introduction
UV curing technology has been extensively employed in various fields including coatings, inks, adhesion and so forth for energy saving, fast curing speed and mild curing conditions [1,2,3]. UV–curable polyacrylate is widely used in free–radical type UV–cure coating systems owning to their convenience to be designed in various molecular structures with various properties, together with their relatively low cost [4,5,6]. Nevertheless, some defects of polyacrylate, including poor thermal stability and UV resistance, need to be improved for further applications. Improvement in thermal stability and UV resistance of polyacrylates can be achieved by copolymerization with silicone materials [7,8], however, it is a remarkable fact that the strategies for fabrication UV–cured silicone modified polyacrylates reported by these references are somewhat complicated and the price of the products may be much expensive than pure UV–cured polyacrylates because the cost of the silicone raw materials are to some extent more expensive than acrylates. Therefore, it looks wonderful to fabrication silicone modified polyacrylates possessing the advantages of both polyacrylate and silicone material using a little few silicone raw materials with a small increase in costs.
It is convinced that UV photoinitiators will increase the cost and the risks of turning yellow of the UV–cured materials. As reported, the thiol–ene reaction initiated by UV light has attracted much attention in polymer science for their virtues such as high activity at mild condition, without photoinitiator and rather tolerant to various functional groups [9,10,11,12,13]. From this point, the attempts to develop silicone modified polyacrylates by UV initiated thiol–ene reaction also have potentials to reduce the cost and the risks of turning yellow of the products.
To fabricate a UV–cured silicone modified acrylate coating, methyl methacrylate, butyl methacrylate and styrene were copolymerized with traditional free radical polymerization initiated by azodiisobutyronitrile (AIBN), then the copolymers were cured with thiol silicone resins through UV initiated thiol–ene reaction. The properties of the coatings including thermal stability, transparency, pencil hardness, adhesion property, water absorption and surface water contact angle were also studied. The results with a high application value should be emphasized that when the amount of thiol silicone resin is only one wt.% of the copolymer of acrylates, the UV–cured coatings obtained exhibit fairly good performance. It is observed the pencil hardness of the material can reach 6H, the water absorption is less than 0.16%, the transmittance is as high as 96% (800 nm), the initial thermal decomposition temperature is 200–230 °C, and the adhesive to the glass slide can reach 0 grade. In particular, it is observed obviously that the silicone modified polyacrylate coatings exhibit better UV resistance performance than the coating prepared with only copolymers of acrylates initiated by UV initiator 1173. It is proved that it is actually an easy fabrication method to prepare inexpensive UV–cured transparent silicone modified polyacrylate coatings with high performance by UV initiated thiol–ene click reaction of copolymers of acylates and thiol silicone resin.
2. Materials and Methods
2.1. Materials
Acrylic acid, hydroxypropyl methacrylate, styrene and n−dodecanethiol were purchased from Beijing HWRK Chem Co., Ltd. (Beijing, China) Methyl methacrylate, butyl acrylate, azodiisobutyronitrile, UV initiator 1173 and isopropanol were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). All these chemicals were analytical grade purity and used directly without prior purification. Thiol silicone resins were prepared according to references [1,2,3].
2.2. Synthesis of Copolymers of Acrylate Monomers and Styrene
Various copolymers of acrylate monomers and styrene with various of styrene content were synthesized according to the procedure shown in Scheme S1. The too lower molecular weight of the copolymers will lead to the UV–cured system to easy to flow while the too higher molecular weight of the copolymers will result in the UV–cured system is too thick to flow, which are unfavorable for the fabrication of the coatings. Therefore, the molecular weight of the copolymers are controlled to be about 2.5 × 104–4.0 × 104 as shown in Figure S1. To a typical process of prepare copolymers of acrylate monomers and styrene, a mixture of 6.25 g (0.0867 mol) acrylic acid, 6.25 g (0.0480 mol) hydroxypropyl methacrylate, 9.2 g (0.0883 mol) styrene, 10.52 g (0.1051 mol) methyl methacrylate and 15.78 g (0.1110 mol) butyl acrylate was added into a three-necked bottle containing 1 g azodiisobutyronitrile, 1 g n−dodecanethiol and 50 g isopropanol at 80 °C for 3 h, then kept at 80 °C for another 2 h. The pale yellow liquid of copolymer was produced after the mixture was treated under 80 °C/130 mm Hg for 2 h to take off residue of raw materials.
FT–IR analysis of the copolymers was carried out as shown in Figure S2. The absorption at 3500 cm−1 is characteristic peak of the association of carboxyl group from acrylic acid with hydroxypropyl from hydroxypropyl methacrylate. The absorption at 1730 cm−1 is characteristic peak of carbonyl group in copolymer and the weak absorption at 1621 cm−1 is characteristic peaks of terminal acrylate group in the copolymer. The absorptions at 3030–2845 cm−1 and 1942–1801 cm−1 are attributed to be characteristic peaks of unsaturated hydrocarbon bond and stretching vibration peaks for C=H in benzene ring from styrene, respectively.
1H–NMR analysis was performed to determine the chemical structure of the copolymers (Figure S3) and the chemical shifts are ascribed as following: 1H−NMR (400 MHz, CDCl3) δ (ppm) 6.8–7.3 (3H,–C6H5 of styrene), 0.8–1.0 (3H, −CH3), 3.3–3.8(3H, −OCH3 of acrylate derivatives), 1.4–1.7 (2H, −CH2– of acrylate derivatives).
2.3. Preparation of Transparent Materials
The mixtures of copolymers of acrylate monomers and styrene and thiol silicone resin were deposited on slides and treated similar to references [1,2,3] and the coatings with about 0.2 mm were cured by UV as shown in Scheme S2.
2.4. Characterization
SEC measurements of copolymers from acrylate monomers and styrene were performed on a Waters 150−C in THF at 25 °C using commercial polystyrene standards for calibration. 1H NMR spectrum of copolymers from acrylate monomers and styrene was recorded with a Bruker AVANCE AV400 (400 MHz, Bruker, Billerica, MA, USA) spectrometer. The sample was dissolved in CDCl3 with 0.03% v/v tetramethylsilane. FT–IR spectra were recorded on a Nicolet 700 spectrometer (Nicolet Co., Ltd., Madison, WI, USA). Thermogravimetric analysis (TGA) was performed on a TG 209C apparatus (NETZSCH−Gerätebau GmbH, Selb, Germany) under nitrogen and these samples were heated from 25 °C to 600 °C at a rate of 10 °C min−1. The pencil hardness, degree of curing contents, adhesion properties, water absorption and surface water contact angle were test similar to references [1,2].
3. Results and Discussion
3.1. UV Curing Behavior
3.1.1. Curing Time
First, the effect of curing time was studied as shown in Table 1. The speed of UV curing reaction is quite fast because the pencil hardness of coating reaches H when the coating was cured for only 10 s. The pencil hardness of the coatings increase from H to 6H when the curing time increases from 10 s to 60 s. The coatings with so much high pencil hardness will be confronted with less risk of being scratched. It is observed that the coatings exhibit relatively low water contact angle (76°–84°) and water absorption (0.02 wt.%–0.13 wt.%).

Table 1.
Effect of UV-curing time.
It can be seen from the FT−IR analysis given Figure 1 that the characteristic absorption at 3500 cm−1 of the association of carboxyl group from acrylic acid with hydroxypropyl from hydroxypropyl methacrylate and characteristic absorption at 1621 cm−1 of terminal acrylate group gradually turn weaken until they disappear when the curing time prolonged from 10 s to 60 s. It can be concluded that the coating was cured completely by UV for 60 s.
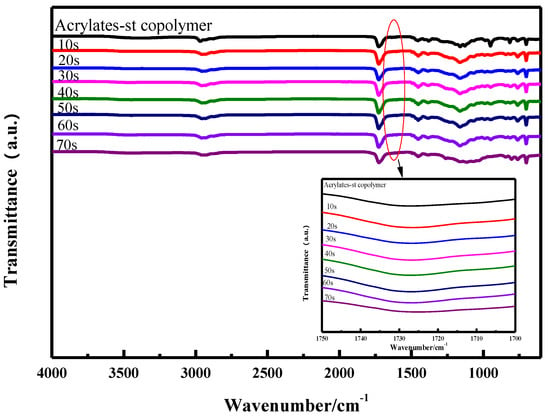
Figure 1.
FT−IR spectra for copolymer of acrylate and styrene, the coatings cured for different times.
3.1.2. The Amount of Styrene to Prepare the Copolymers
The amounts of styrene to prepare the copolymers were investigated as shown in Table 2 and Figure 2. The pencil hardness of the coatings increased with the increasing of the amount of styrene, however, the water contact angle and water absorption are affected by the amount of styrene slightly. The temperature at which the coatings start decomposition with mass loss of 5% (Td5) and 10% (Td10) are in the range of 154.0–192.2 °C and 228.9–281.2 °C, respectively, which increase with the increment of the amount of styrene. It should be pointed out that thermal stability of the coating prepared from the copolymer with 18 wt.% styrene monomer. With a comprehensive consideration of hardness and thermal stability of coatings, the amount of styrene is 18.0 wt.% may be more appropriate.

Table 2.
Effect of styrene amounts on the coatings.
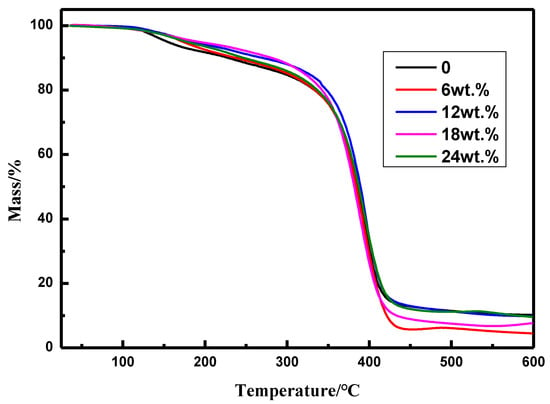
Figure 2.
TGA analysis for the coatings.
3.1.3. The Thiol Content of Silicone Resin
The thiol content of silicone resins also exhibits a great impact on the performance of coatings. It can be seen that when the thiol content of silicone resin is 5 mmol g−1, the pencil hardness of the coating is the highest (Table 3). From this point, the suitable amount of thiol content may be 5 mmol g−1.

Table 3.
Impact of thiol content of silicone resin.
3.1.4. The Amount of Thiol Silicone Resin
The amount of thiol silicone resin in the UV-cured system also can influence the properties of the coatings significantly (Table 4). When the amount of thiol silicone resin increased from 0.6 wt.% to 1 wt.%, the pencil hardness of the coatings increased from F to 4H, while the amount of thiol silicone resin higher than 1 wt.% will lead to a decline of pencil hardness. It may be ascribed to be the excess silicone resin acts as a kind of plasticizer. It must be point out that the suitable amount of thiol silicone resin is not higher than 1 wt.%, which means that the coating prepared will be cheaper than the coatings prepared with pure silicone materials. Therefore, the coating prepared by this method is quite suitable for industrial production.

Table 4.
Impact of the amount of thiol silicone resin on the coatings.
3.2. Performance of UV–cured Coatings
3.2.1. Transparency
The transparency of polymer materials is quite important when they are used in optical devices [14,15,16,17]. It is shown in Figure 3 and Figure 4 that the coatings prepared from thiol silicone resins with various of thiol content have good transparency. However, the transmittance of the coatings cured with thiol silicone resin is relatively lower than the coating of the copolymer cured with 1173, these coatings obtained exhibit a quite high transparency as high as 84.7–91.3%. The relatively lower transmittance of coatings prepared with higher amount of thiol silicone resins may lie in the relatively poor compatibility of thiol silicone resins than UV initiator 1173.

Figure 3.
Photos of coatings prepared in Table 3 and the coating cured with UV initiator 1173.
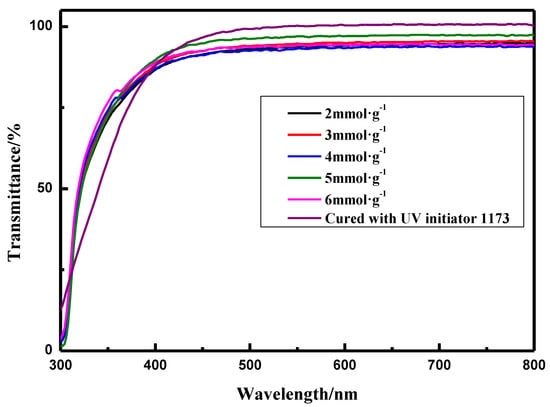
Figure 4.
Transparent of coatings prepared in Table 3 and the coating cured with UV initiator 1173.
3.2.2. Thermal Stability
Silicone materials acting as auxiliary materials may be endure much higher temperature because electronic devices will generate heat during the course of operation. Therefore, thermal stability of silicone materials may be quite important and the thermal stability of the coatings prepared with various amounts of thiol silicone resins was investigated by TGA and DTG shown in Figure 5 and Figure 6. As shown by Figure 5, the Td5 of the obtained UV–cured materials is fairly high (180.8–252 °C), which increases with the increment of the amount of thiol silicone resin when the amount of silicone resin is in the range of 0.4 wt.%–5 wt.%. From DTG curves in Figure 6, it can be seen there two stages in the thermal degradation procedure of UV-cured coatings: The initiate degradation took place at about 161 °C and maximum degradation speed took place are about 400 °C at carbonization stage. A conclusion can be made that thermal stability of UV–cured materials is improved by the addition of silicone resin.
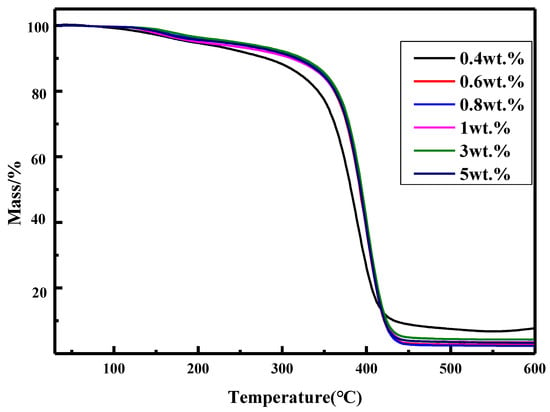
Figure 5.
TGA curves for the coatings (entries 2–7 in Table 4).
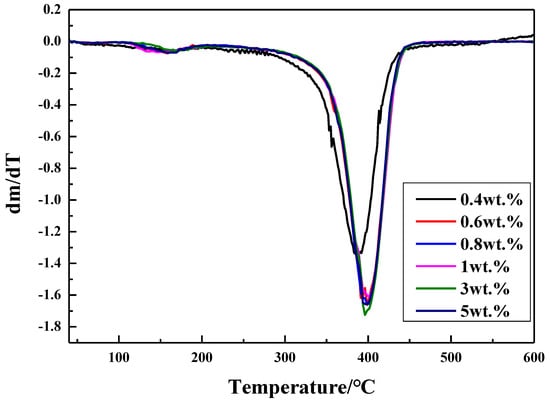
Figure 6.
DTG curves for the coatings (entries 2–7 in Table 4).
3.2.3. Adhesive Property
Coating’s adhesion property on substrate is crucial and the adhesive property on glass slides was investigated by a cross cut test according to reference [1,2] as shown in Figure 7. Obviously, when the coating was cured with UV initiator 1173, the adhesion property of which is poorer (Grade 3) than those of the other coatings because the separated area is about 15–35%. It also can be seen from Figure 7 that coatings prepared from 1 wt.% thiol silicone resin; the adhesion property increased with the increment of content of thiol for the silicone resins. The adhesion property for the coatings prepared from thiol silicone resin with thiol content of 2 and 3 mmol g−1 are grade 2 because the separated area is about 5–15% and that of the coating prepared from thiol silicone resin with thiol content of 4 mmol g−1 is grade 1 because the separated area is about 0–5%. By contrast, the adhesion property for the coatings prepared from thiol silicone resin with thiol content of 5 and 6 mmol g−1 are grade 0 because the separated area is 0%, especially, there is no scratch mark at all for the coating prepared from thiol silicone resin with thiol content of 5 mmol g−1 after cross test experiment.
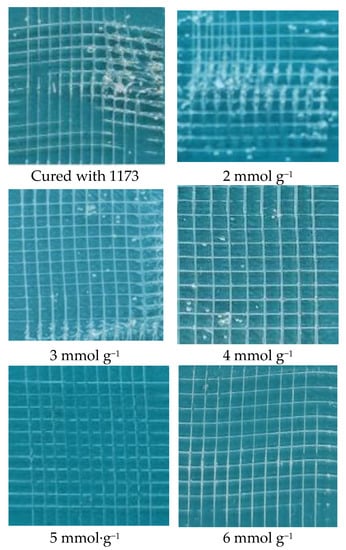
Figure 7.
Photos of adhesive tests of coatings cured with 1 wt.% thiol silicone resin with different amount of thiol content.
The effect of the amounts of thiol silicone resin on the adhesion properties of the coatings also were explored as exhibited by Figure 8. The adhesion property for the coatings prepared with 0.4 wt.%, 0.6 wt.%, 3 wt.% and 5 wt.% of thiol silicone resin is grade 1 for the separated area is about 0–5% while the adhesion property of the coatings prepared with 0.8 wt.% and 1 wt.% of thiol silicone resin is grade 0 for there is no separated area. Generally speaking, the adhesion property for the coating prepared from 1 wt.% thiol silicone resin with thiol content of 5 mmol g−1.
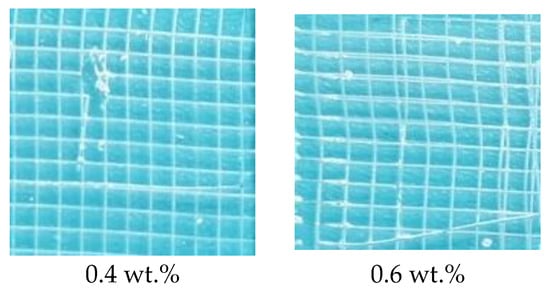
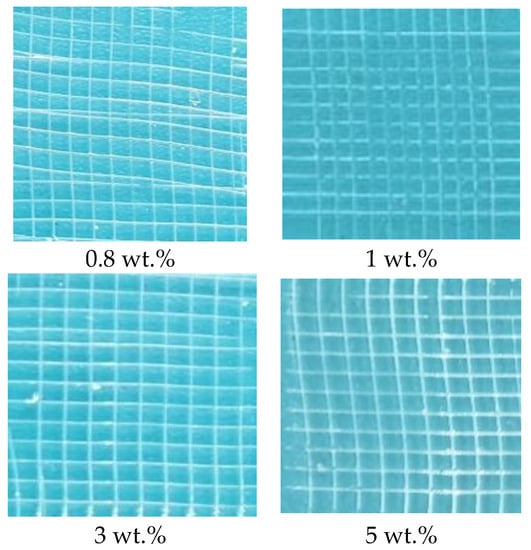
Figure 8.
Photos of adhesive tests of coatings cured with various amount of thiol silicone resin with thiol content of 5 mmol g−1.
3.2.4. UV Resistance for the Coatings
The UV resistance for the coatings is significant important because if the coatings with relatively poor UV resistance are exposed to UV for a long time, they may turn yellow and even loss of use value due to degradation [2,18]. The UV resistance performance of the UV–cured coatings (Entry 1–7 in Table 4) was evaluated in a UV test chamber of 10.6 mw cm−2. First, the coatings were aged at room temperature as shown in Figure 9a, it reveals that the coating prepared from copolymer cured by UV initiator 1173 begin to turn yellow after being aged about 20 min. By contrast, among coatings cured with various amount of thiol silicone resin, only the coating prepared with 0.4 wt.% thiol silicone resin turned slightly yellow and the other coatings do not turn yellow even after being aged for 35 min. To explore the effect of elevated temperature on the UV resistance performance, the coatings were exposed to UV (10.6 mw cm−2) at 100 °C (Figure 9b). The coating prepared with 1173 began to turn yellow after being exposed to UV only for 6 min at 100 °C while the silicone modified coatings with 0.4 wt.%–0.6 wt.% thiol silicone resin began to turn yellow after being aged for about 12 min, the other coatings still did not turn yellow after being aged for 21 min. Comparing the results in Figure 9a,b, it can be convinced that elevated temperature will be bad for the UV resistance of silicone modified coatings because the coatings have more risks to be decomposed at high temperature.


Figure 9.
Photos of coatings (entries 1–7 in Table 4) before and after UV-aging. (a) Before and after UV–aging at room temperature; (b) Before and after UV–aging at 100 °C.
To further study the performance influenced by UV–aging, the transmittance of these coatings before and after UV–aging was measured and shown in Figure 10. Interestingly, the transmittance of the coating cured with UV initiator 1173 decreases obviously from about 100% to 90.3% and 83.5% after being aged at room temperature for 35 min and aged at 100 °C for 21 min, respectively, while that of the coatings prepared from various of amounts of thiol silicone resins is still in the range of 96–99% and 88–93% after being aged at room temperature for 35 min and aged at 100 °C for 21 min, respectively. When it comes to the transmittance of silicone modified coatings before UV-aging, the higher amount of thiol silicone resin used, the lower the transmittance of the coatings is, which may be due to the relative poor compatibility of silicone resin and copolymer of acylate. On the contrary, the higher amount of thiol silicone resin used, the higher the transmittance of the coatings after being aged is, which may be due to the good UV resistance ability of silicone resin component.
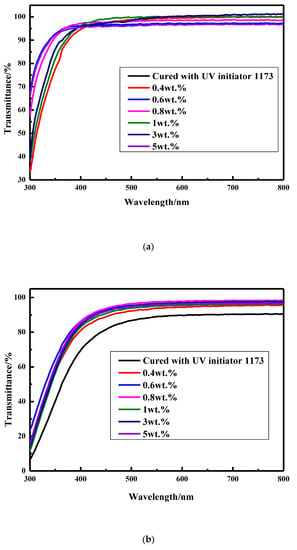
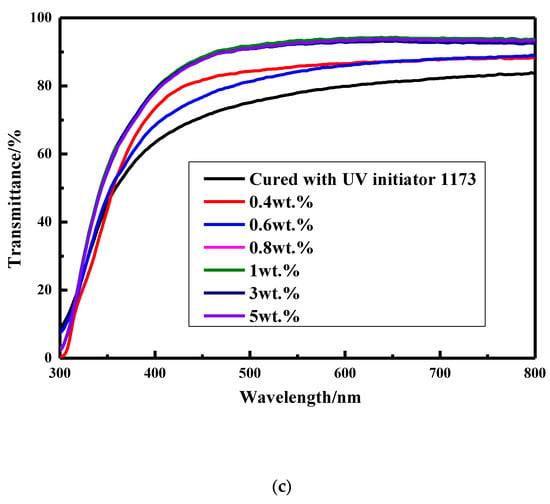
Figure 10.
Transmittance of coatings before and after UV–aging. (a) Transmittance of the coatings before UV–aging; (b) transmittance of the coatings after UV–aging at room temperature for 35 min. (c) transmittance of the coatings after UV–aging at 100 °C for 21 min.
In view of the results above, it is believed that the coatings cured with thiol silicone resin have superior UV resistance than the coating cured by UV initiator 1173.
4. Conclusions
An easy fabrication method to prepare inexpensive UV–cured transparent silicone modified polyacrylate coatings with good adhesion and UV resistance performance was developed. When the amount of thiol silicone with thiol content of five millimoles per gram is only one wt.% of copolymers of acylates and the coatings were cured for 60 s, the coatings obtained exhibit superior performance. The coatings prepared exhibit transparency higher than 96% (800 nm), adhesion property to glass slides can reach grade 0, pencil hardness can reach 6H, water absorption is less than 0.16%. In particular, it is observed obviously that the silicone modified polyacrylate coatings exhibit better UV resistance performance than the coating prepared with only copolymers of acrylates initiated by UV initiator 1173. This easy fabrication method will promote us to explore industry production of this kind of transparent silicone modified polyacrylate coatings.
Supplementary Materials
The following are available online at https://www.mdpi.com/2079-6412/10/7/675/s1, Figure S1: SEC curves of copolymers of acrylate; Figure S2: FT–IR analysis of copolymers of acrylate; Figure S3: The 1H–NMR spectra of copolymers of acrylate.
Author Contributions
Conceptualization, X.Y.; formal analysis F.C., L.Z., X.J., and X.Y.; investigation, X.J. and Y.F.; data curation, F.C., L.Z., and X.H.; writing—original draft preparation, X.Y.; writing—review and editing, X.Y.; project administration, G.L. and X.Y.; funding acquisition, X.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Open Fund of the Collaborative Innovation Center for Fluorosilicon Fine Chemicals and Materials Manufacturing of Zhejiang Province, Grant No. FSi2019A007.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Jiao, X.J.; Zhang, T.X.; Cheng, F.; Fan, Y.X.; Liu, J.L.; Lai, G.Q.; Wu, Y.F.; Yang, X.F. UV-cured coatings prepared with sulfhydryl terminated branched polyurethane and allyl terminated hyperbranched polycarbosilane. Coatings 2020, 10, 350. [Google Scholar] [CrossRef]
- Liu, J.L.; Jiao, X.J.; Cheng, F.; Fan, Y.X.; Wu, Y.F.; Yang, X.F. Fabrication and performance of UV cured transparent silicone modified polyurethane–acrylate coatings with high hardness, good thermal stability and adhesion. Prog. Org. Coat. 2020, 144, 10567. [Google Scholar] [CrossRef]
- Wu, Y.F.; Liu, J.L.; Jiao, X.J.; Cheng, F.; Lai, G.Q.; Yang, X.F. UV–cured transparent flexible silicone materials with high tensile strength. ACS Omega 2020, 5, 6199–6206. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.Q.; Liu, W.Q.; Wang, H.L.; Sun, Y.; Wang, S.J. A novel mono-methacryloyloxy terminated fluorinated macromonomer used for the modification of UV curable acrylic copolymers. J. Appl. Polym. Sci. 2016, 133, 43116. [Google Scholar] [CrossRef]
- Yu, F.Y.; Gao, J.; Liu, C.P.; Chen, Y.K.; Zhong, G.; Hodges, C.; Chen, M.F.; Zhang, H.G. Preparation and UV aging of nano–SiO2/fluorinated polyacrylate polyurethane hydrophobic composite coating. Prog. Org. Coat. 2020, 141, 105556. [Google Scholar] [CrossRef]
- Rengasamy, S.; Mannari, V. Development of soy–based UV–curable acrylate oligomers and study of their film properties. Prog. Org. Coat. 2013, 76, 78–85. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, C.P.; Gao, J.; Chen, Y.K.; Yu, F.Y.; Chen, M.F.; Zhang, H.G. A novel hydrophobic coating film of water–borne fluoro-silicon polyacrylate polyurethane with properties governed by surface self–segregation. Prog. Org. Coat. 2019, 134, 134–144. [Google Scholar] [CrossRef]
- Darras, V.; Fichet, O.; Perrot, F.; Boileau, S.; Teyssie, D. Polysiloxane—poly(fluorinated acrylate) interpenetrating polymer networks: Synthesis and characterization. Polymer 2007, 48, 687–695. [Google Scholar] [CrossRef]
- Lowe, A.B.; Hoyle, C.E.; Bowman, C.N. Thiol–yne click chemistry: A powerful and versatile methodology for materials synthesis. J. Mater. Chem. 2010, 20, 4745–4750. [Google Scholar] [CrossRef]
- Rafael, A.; Pilar, J.M.; Jaime, G.R.; María–José, B.; Ángel, M. Thiol–ene click chemistry towards easy microarraying of half–antibodies. Chem. Commun. 2018, 54, 6144–6147. [Google Scholar] [CrossRef] [PubMed]
- Nakabayashi, K.; Sobu, S.; Kosuge, Y.; Mori, H. Synthesis and nanoimprinting of high refractive index and highly transparent polythioethers based on thiol–ene click chemistry. J. Polym. Sci. Part A Polym. Chem. 2018, 56, 2175–2182. [Google Scholar] [CrossRef]
- Miao, J.T.; Yuan, L.; Guan, Q.B.; Liang, G.Z.; Gu, A.J. Water–phase synthesis of a biobased allyl compound for building UV–curable flexible thiol−ene polymer networks with high mechanical strength and transparency. ACS Sustain. Chem. Eng. 2018, 6, 7902–7909. [Google Scholar] [CrossRef]
- Childress, K.K.; Alim, M.D.; Hernandez, J.J.; Stansbury, J.W.; Bowman, C.N. Additive manufacture of lightly crosslinked semicrystalline thiol–enes for enhanced mechanical performance. Polym. Chem. 2020, 11, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.F.; Shao, Q.; Yang, L.L.; Zhu, X.B.; Hua, X.L.; Zheng, Q.L.; Song, G.X.; Lai, G.Q. Preparation and performance of high refractive index silicone resin–type materials for the packaging of light–emitting diodes. J. Appl. Polym. Sci. 2013, 127, 1717–1724. [Google Scholar] [CrossRef]
- Oh, Y.S.; Yoon, I.S.; Lee, C.; Kim, S.H.; Ju, B.K.; Hong, J.M. Selective photonic sintering of Ag flakes embedded in silicone elastomers to fabricate stretchable conductors. J. Mater. Chem. C 2017, 5, 11733–11740. [Google Scholar] [CrossRef]
- Park, S.Y.; Lee, J.W.; Ko, H.H. Transparent and flexible surface–enhanced raman scattering (SERS) sensors based on gold nanostar arrays embedded in silicone rubber film. ACS Appl. Mater. Int. 2017, 9, 44088–44095. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.R.; Yang, W.; Zhang, Q.H.; Hui, H.H.; Wei, Y.W.; Wang, J.; Xu, Q.; Lei, X.Y.; Chen, J.J.; Zhu, J.L. Fabrication of UV–curable silicone coating with high transmittance and laser–induced damage threshold for high–power laser system. J. Sol Gel Sci. Technol. 2018, 88, 249–254. [Google Scholar] [CrossRef]
- Afshar, A.; Mihut, D.; Baqersad, J.; Hill, S. Study of metallic thin films on epoxy matrix as protective barrier to ultraviolet radiation. Surf. Coat. Tech. 2019, 367, 41–48. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).