Effect of Low-Concentration Rb+ Mixing on Semiconductor Majority Charge Carriers Type of Perovskite Light-Absorption Layer by Using Two-Step Spin-Coating Method
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
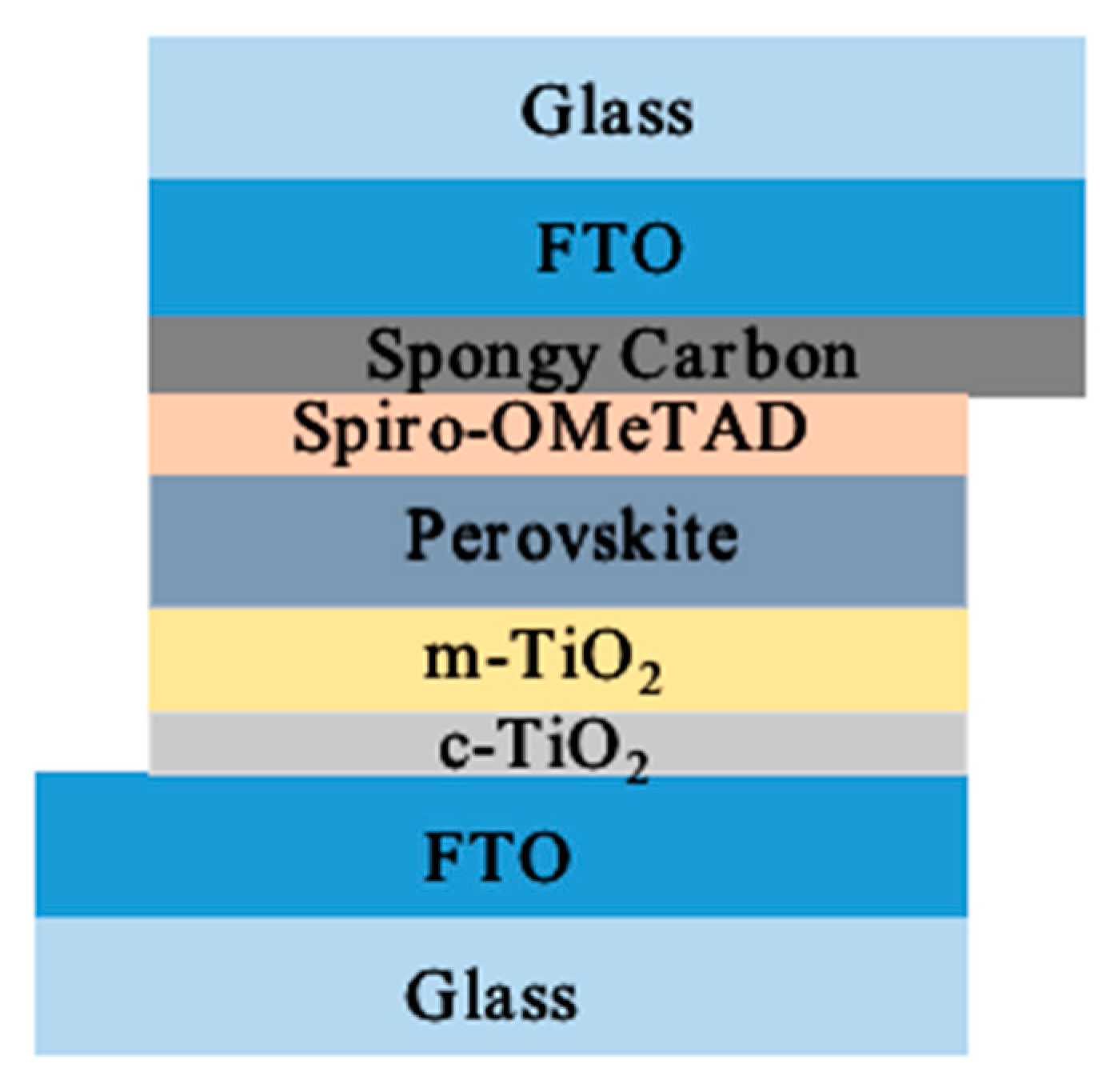
2.2. Device Fabrication
2.2.1. Cleaning of FTO Substrate
2.2.2. Fabrication of Electron Transport Layer (ETL)
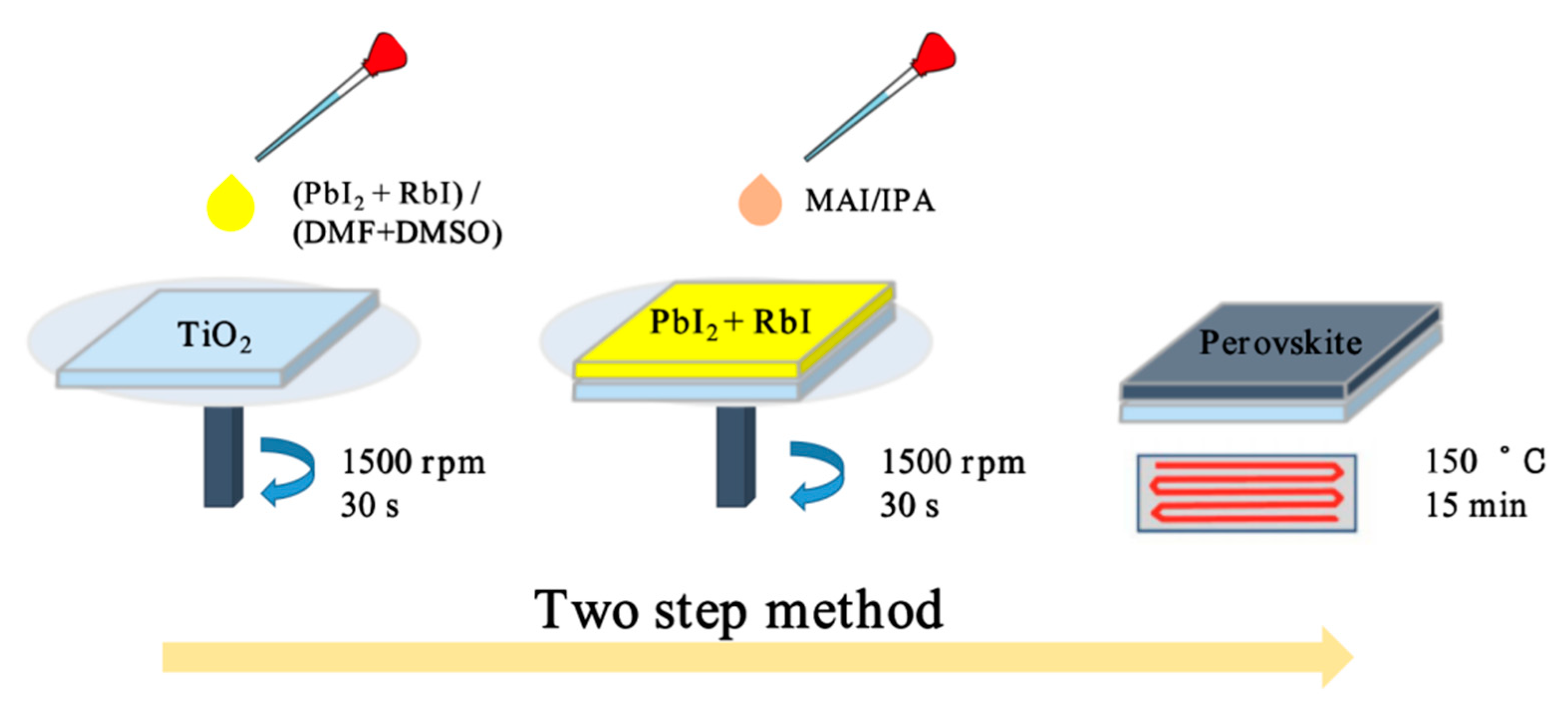
2.2.3. Fabrication of Perovskite Light-Absorption Layer
2.2.4. Fabrication of Hole Transport Layer (HTL)
2.2.5. Fabrication of Counter Electrode (CE)
2.3. Characterization
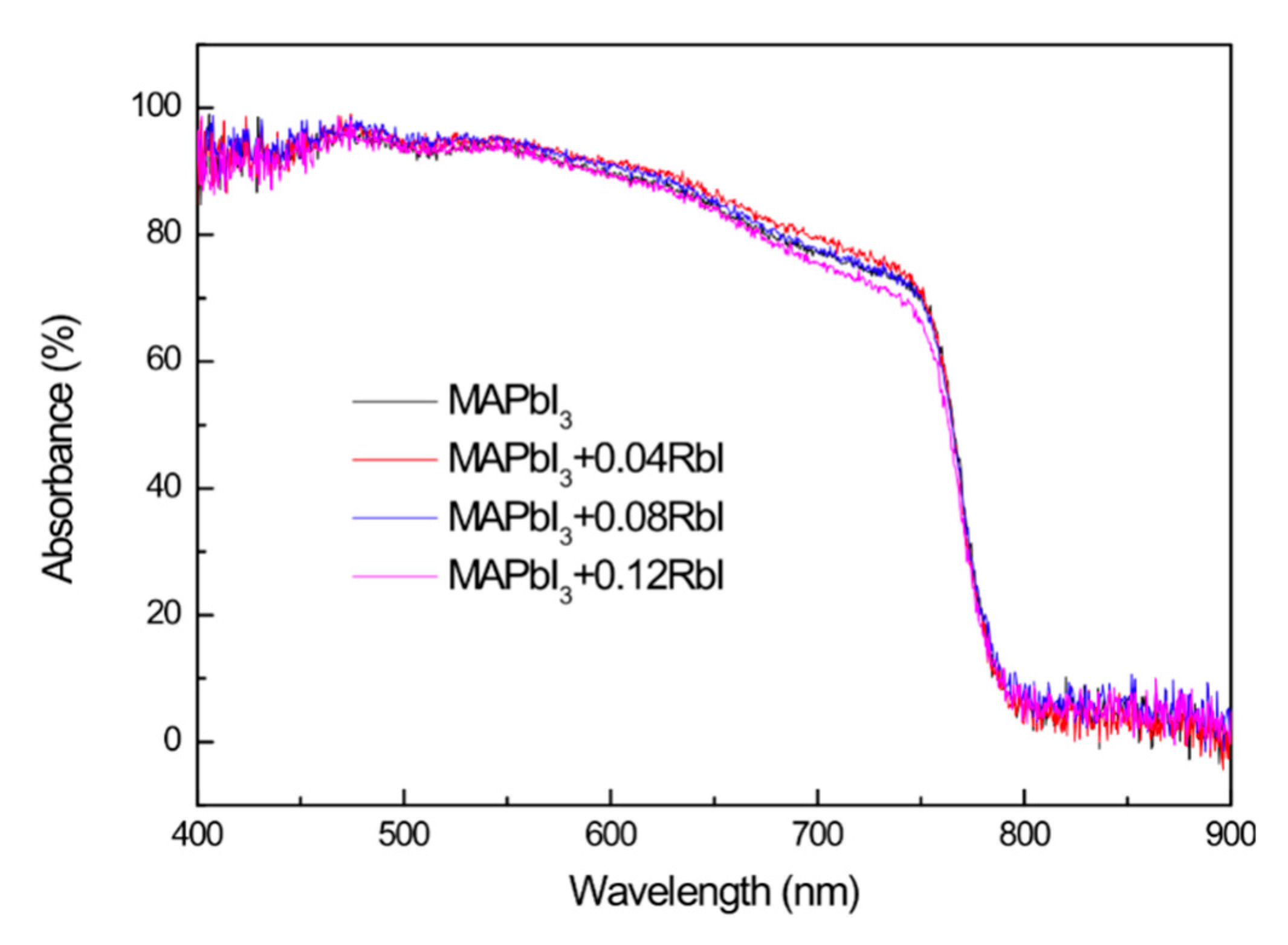
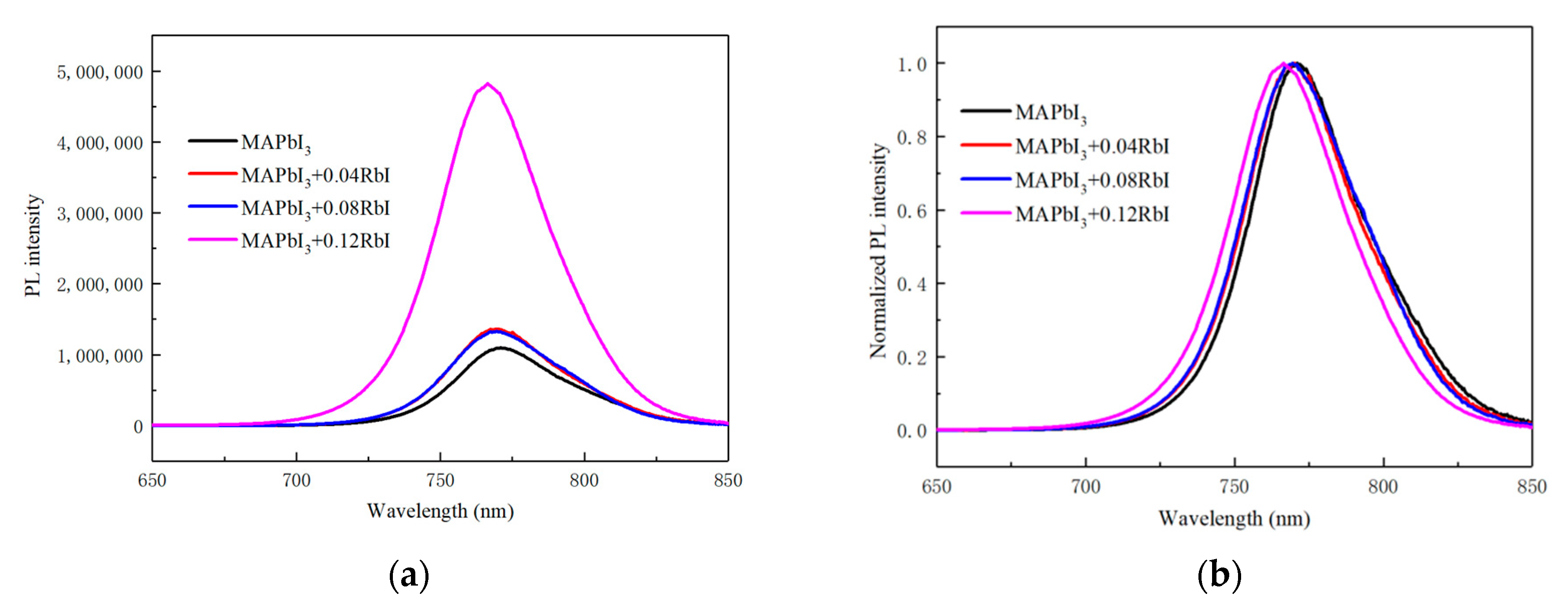
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Li, X.; Yang, J.; Jiang, Q.; Lai, H.; Li, S.; Tan, Y.; Chen, Y.; Li, S. Perovskite Solar Cells Employing an Eco-friendly and Low-cost Inorganic Hole Transport Layer for Enhanced Photovoltaic Performance and Operational Stability. J. Mater. Chem. A 2019, 7, 7065–7073. [Google Scholar] [CrossRef]
- Liu, X.; Cheng, Y.; Liu, C.; Zhang, T.; Zhang, N.; Zhang, S.; Chen, J.; Xu, Q.; Ouyang, J.; Gong, H. 20.7% Highly Reproducible Inverted Planar Perovskite Solar Cells with Enhanced Fill Factor and Eliminated Hysteresis. Energy Environ. Sci. 2019, 12, 1622–1633. [Google Scholar] [CrossRef]
- Liang, J.W.; Chen, Z.L.; Yang, G.; Wang, H.B.; Ye, F.H.; Tao, C.; Fang, G.J. Achieving High Open-Circuit Voltage on Planar Perovskite Solar Cells via Chlorine-Doped Tin Oxide Electron Transport Layers. ACS Appl. Mater. Interfaces 2019, 11, 23152–23159. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Fang, Y.; Shao, Y.; Mulligan, P.; Qiu, J.; Cao, L.; Huang, J. Electron-hole diffusion lengths > 175 um in solution-grown CH3NH3PbI3 single crystals. Science 2015, 347, 967–970. [Google Scholar] [CrossRef]
- Oga, H.; Saeki, A.; Ogomi, Y.; Hayase, S.; Seki, S. Improved Understanding of the Electronic and Energetic Landscapes of Perovskite Solar Cells: High Local Charge Carrier Mobility, Reduced Recombination, and Extremely Shallow Traps. J. Am. Chem. Soc. 2014, 136, 16948. [Google Scholar] [CrossRef]
- Yang, G.; Chen, C.; Yao, F.; Chen, Z.; Zhang, Q.; Zheng, X.; Ma, J.; Lei, H.; Qin, P.; Xiong, L.; et al. Effective Carrier-Concentration Tuning of SnO2 Quantum Dot Electron-Selective Layers for High-Performance Planar Perovskite Solar Cells. Adv. Mater. 2018, 30, 1706023.1–1706023.9. [Google Scholar] [CrossRef]
- Cao, B.; Yang, L.; Jiang, S.; Lin, H.; Wang, N.; Li, X. Flexible quintuple cation perovskite solar cells with high efficiency. J. Mater. Chem. A 2019, 7, 4960–4970. [Google Scholar] [CrossRef]
- Wojciechowski, K.; Stranks, S.D.; Abate, A.; Sadoughi, G.; Sadhanala, A.; Kopidakis, N.; Rumbles, G.; Li, C.Z.; Friend, R.H.; Jen, A.K.; et al. Heterojunction Modification for Highly Efficient Organic-Inorganic Perovskite Solar Cells. ACS Nano 2014, 8, 12701–12709. [Google Scholar] [CrossRef]
- Li, C.B.; Wang, A.; Xie, L.S.; Deng, X.Y.; Liao, K.J.; Yang, J.A.; Li, T.S.; Hao, F. Emerging alkali metal ion (Li+, Na+, K+ and Rb+) doped perovskite films for efficient solar cells: Recent advances and prospect. J. Mater. Chem. A 2019, 7, 42. [Google Scholar] [CrossRef]
- Bu, T.; Liu, X.; Li, J.; Li, W.; Huang, W.; Ku, Z.; Peng, Y.; Huang, F.; Cheng, Y.B.; Zhong, J. Sub-sized monovalent alkaline cations enhanced electrical stability for over 17% hysteresis-free planar perovskite solar mini-module. Electrochim. Acta 2019, 306, 635–642. [Google Scholar] [CrossRef]
- Jacobsson, T.J.; Svanstrm, S.; Andrei, V.; Rivett, J.P.H.; Kornienko, N.; Philippe, B.; Cappel, U.; Rensmo, H.; Deschler, F.; Boschloo, G. Extending the Compositional Space of Mixed Lead Halide Perovskites by Cs, Rb, K, and Na-Doping. J. Phys. Chem. C 2018, 122, 25. [Google Scholar] [CrossRef]
- Mahmud, M.A.; Elumalai, N.K.; Upama, M.B.; Wang, D.; Gonɕales, V.R.; Wright, M.; Xu, C.; Haque, F.; Uddin, A. Passivation of interstitial and vacancy mediated trap-states for efficient and stable triple-cation perovskite solar cells. J. Power Sources 2018, 383, 59–71. [Google Scholar] [CrossRef]
- Pham, H.T.; Duong, T.; Rickard, W.D.A.; Kremer, F.; Weber, K.J.; Wong-Leung, J. Understanding the Chemical and Structural Properties of Multiple-Cation Mixed Halide Perovskite. J. Phys. Chem. C 2019, 123, 26718–26726. [Google Scholar] [CrossRef]
- Zhang, M.; Yun, J.S.; Ma, Q.S.; Zheng, J.H.; Lau, C.F.J.; Deng, X.F.; Kim, J.; Kim, D.; Seidel, J.; Green, M.A.; et al. High-Efficiency Rubidium-Incorporated Perovskite Solar Cells by Gas Quenching. ACS Energy Lett. 2017, 2, 438–444. [Google Scholar] [CrossRef]
- Park, I.J.; Seo, S.; Park, M.A.; Lee, S.; Kim, D.H.; Zhu, K.; Shim, H.; Kim, J.Y. Effect of Rubidium Incorporation on the Structural, Electrical, and Photovoltaic Properties of Methylammonium Lead Iodide-Based Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2017, 9, 41898. [Google Scholar] [CrossRef] [PubMed]
- Saliba, M.; Matsui, T.; Domanski, K.; Seo, J.Y.; Ummadisingu, A.; Zakeeruddin, S.M.; Correa-Baena, J.P.; Tress, W.R.; Abate, A.; Hagfeldt, A.; et al. Incorporation of rubidium cations into perovskite solar cells improves photovoltaic performance. Science 2016, 354, 206–209. [Google Scholar] [CrossRef]
- Duong, T.; Mulmudi, H.K.; Shen, H.; Wu, Y.; Barugkin, C.; Mayon, Y.O.; Nguyen, H.T.; Macdonald, D.H.; Peng, J.; Lockrey, M.N.; et al. Structural engineering using rubidium iodide as a dopant under excess lead iodide conditions for high efficiency and stable perovskites. Nano Energy 2016, 30, 330–340. [Google Scholar] [CrossRef]
- Xiao, J.W.; Liang, Y.; Zhang, S.; Zhao, Y.; Li, Y.; Chen, Q. Stabilizing RbPbBr3 Perovskite Nanocrystals through Cs+ Substitution. Chem. A Eur. J. 2018, 25, 2597–2603. [Google Scholar] [CrossRef]
- Yao, F.; Gui, P.; Chen, C.; Li, B.; Li, R.; Tao, C.; Lin, Q.; Fang, G. High-Rubidium-Formamidinium-Ratio Perovskites for High-Performance Photodetection with Enhanced Stability. ACS Appl. Mater. Interfaces 2019, 11, 39875–39881. [Google Scholar] [CrossRef]
- Arjaan, K.; Loreta, A.M.; René, M.W. Perovskite Thin Film Materials Stabilized and Enhanced by Zinc (II) Doping. Appl. Sci. 2019, 9, 1678. [Google Scholar]
- Wang, Q.; Shao, Y.; Xie, H.; Lyu, L.; Liu, X.; Gao, Y.; Huang, J. Qualifying composition dependent p and n self-doping in CH3NH3PbI3. Appl. Phys. Lett. 2014, 105, 163508. [Google Scholar] [CrossRef]
- Cui, P.; Wei, D.; Ji, J.; Huang, H.; Jia, E.; Dou, S.; Wang, T.; Wang, W.; Li, M. Planar p–n homojunction perovskite solar cells with efficiency exceeding 21.3%. Nat. Energy 2019, 4, 150–159. [Google Scholar] [CrossRef]
- Bai, X.; Zou, X.P.; Zhu, J.L.; Pei, Y.X.; Yang, Y.; Jin, W.B.; Chen, D. Effect of Rb-doping on modulating grain shape and semiconductor properties of MAPbI3 perovskite layer. Mater. Lett. 2018, 21, 328–330. [Google Scholar] [CrossRef]
- Saleem, A.; Imran, M.; Arshad, M.; Kamboh, A.H.; Khan, N.A.; Haider, M.I. Investigation of Rbx(MA)1−xPbI3(x = 0, 0.1, 0.3, 0.5, 0.75, 1) perovskites as a potential source of P- and N-type materials for PN-junction solar cell. Appl. Phys. 2019, 125, 229. [Google Scholar] [CrossRef]
- Yao, Y.J.; Zou, X.P.; Cheng, J.; Ling, T.; Chang, C.C.; Chen, D. Impact of K+ Doping on Modulating Majority Charge Carrier Type and Quality of Perovskite Thin Films by Two-step Solution Method for Solar Cells. Coatings 2019, 9, 647. [Google Scholar] [CrossRef]
- Liu, X.X.; Li, B.C.; Zhang, N.D.; Yu, Z.M.; Sun, K.; Tang, B.S.; Shi, D.W.; Yao, H.Y.; Ouyang, J.Y.; Gong, H. Multifunctional RbCl dopants for efficient inverted planar perovskite solar cell with ultra-high fill factor, negligible hysteresis and improved stability. Nano Energy 2018, 53, 567–578. [Google Scholar] [CrossRef]
- Burschka, J.; Pellet, N.; Moon, S.J.; Humphry-Baker, R.; Gao, P.; Nazeeruddin, M.K.; Grätzel, M. Sequential deposition as a route to high-performance perovskite-sensitized solar cells. Nature 2013, 499, 316–319. [Google Scholar] [CrossRef]
- Im, J.H.; Kim, H.S.; Park, N.G. Morphology-photovoltaic property correlation in perovskite solar cells: One-step versus two-step deposition of CH3NH3PbI3. APL Mater. 2014, 2, 591–713. [Google Scholar] [CrossRef]
- Wang, M.H.; Feng, Y.L.; Bian, J.M.; Liu, H.Z.; Shi, Y.T. A comparative study of one-step and two-step approaches for MAPbI3 perovskite layer and its influence on the performance of mesoscopic perovskite solar cell. Chem. Phys. Lett. 2018, 692, 44–49. [Google Scholar] [CrossRef]
- Chang, C.C.; Zou, X.P.; Cheng, J.; Ling, T.; Yao, Y.Y.; Chen, D. Influence of Solution Deposition Process on Modulating Majority Charge Carrier Type and Quality of Perovskite Thin Films for Solar Cells. Materials 2019, 12, 2494. [Google Scholar] [CrossRef]
- Zhang, N.N.; Guo, Y.J.; Yin, X.; He, M.; Zou, X.P. Spongy carbon film deposited on a separated substrate as counter electrode for perovskite-based solar cell. Mater. Lett. 2016, 182, 248–252. [Google Scholar] [CrossRef]
- Yao, Y.J.; Zou, X.P.; Cheng, J.; Chen, D.; Chang, C.C.; Ling, T.; Ren, H.Y. Impact of Delay Time before Annealing MAI-PbI2-DMSO Intermediate Phase on Perovskite Film Quality and Photo-Physical Properties. Crystals 2019, 9, 151. [Google Scholar] [CrossRef]
- Ling, T.; Zou, X.P.; Cheng, J.; Yang, Y.; Ren, H.Y.; Chen, D. Modulating Surface Morphology Related to Crystallization Speed of Perovskite Grain and Semiconductor Properties of Optical Absorber Layer under Controlled Doping of Potassium Ions for Solar Cells. Materials 2018, 11, 1605. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Game, O.S.; Pang, S.; Padture, N.P. Microstructures of Organometal Trihalide Perovskites for Solar Cells: Their Evolution from Solutions and Characterization. J. Phys. Chem. Lett. 2015, 6, 4827–4839. [Google Scholar] [CrossRef]
- Zhou, Z.M.; Qiang, Z.Y.; Sakamaki, T.; Takei, I.; Shang, R.; Nakamura, E. Organic/Inorganic Hybrid p-Type Semiconductor Doping Affords Hole Transporting Layer Free Thin-Film Perovskite Solar Cells with High Stability. ACS Appl. Mater. Interfaces 2019, 11, 22603–22611. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.H.; Jeong, I.; Bae, S.; Son, H.J.; Lee, P.; Lee, J.; Lee, C.H.; Ko, M.J. Inorganic Rubidium Cation as an Enhancer for Photovoltaic Performance and Moisture Stability of HC(NH2)2PbI3 Perovskite Solar Cells. Adv. Funct. Mater. 2017, 27, 1605988.1–1605988.9. [Google Scholar] [CrossRef]
- Kim, H.; Byun, H.R.; Jeong, M.S. Synthesis and Characterization of Multiple-Cation Rb(MAFA)PbI3 Perovskite Single Crystals. Sci. Rep. 2019, 9, 2022. [Google Scholar] [CrossRef]

| Samples | Hall Parameters | ||
|---|---|---|---|
| Mob a (cm2/Vs) | N b (/cm3) | Types c | |
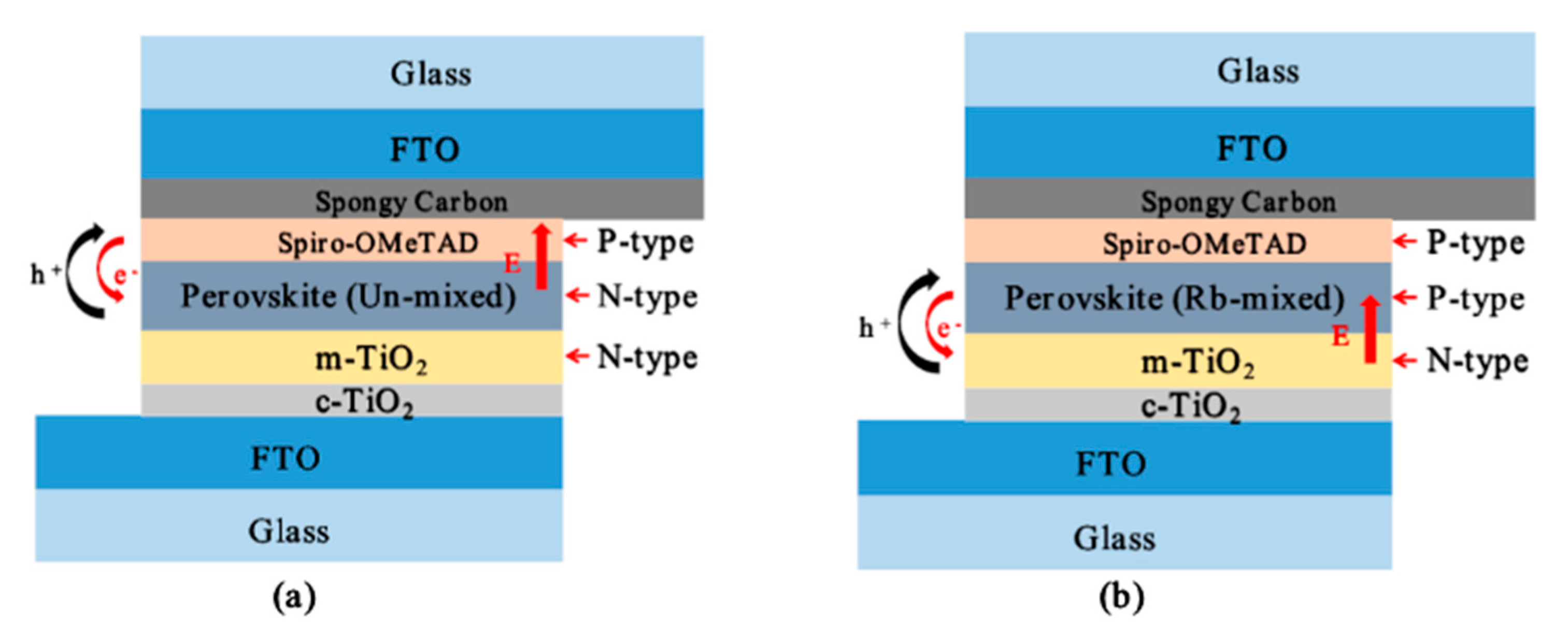
| MAPbI3 | 169 | −3.9 × 1012 | n |
| MAPbI3 + 0.04 RbI | 76.2 | +6.9 × 1012 | p |
| MAPbI3 + 0.08 RbI | 58.5 | +5.9 × 1013 | p |
| MAPbI3 + 0.12 RbI | 79.2 | +2.9 × 1013 | p |
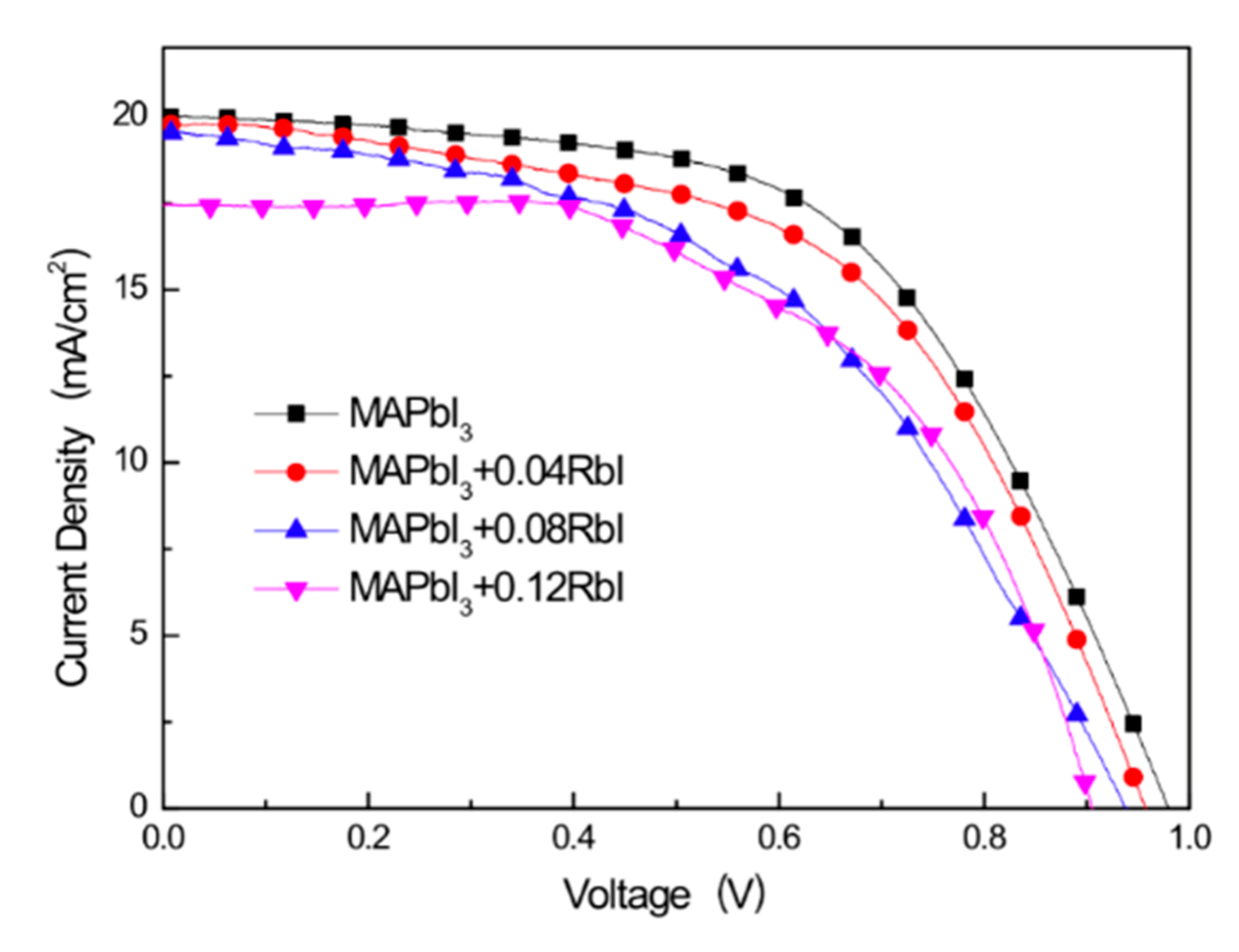
| Samples | VOCa (V) | JSCb (mA/cm2) | FF c (%) | PCE d (%) |
|---|---|---|---|---|
| MAPbI3 | 0.98 | 19.99 | 56.63 | 11.09 |
| MAPbI3 + 0.04 RbI | 0.96 | 19.77 | 55.07 | 10.41 |
| MAPbI3 + 0.08 RbI | 0.94 | 19.58 | 49.20 | 9.04 |
| MAPbI3 + 0.12 RbI | 0.91 | 17.47 | 56.24 | 8.90 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, B.; Zou, X.; Cheng, J.; Ling, T.; Yao, Y.; Chen, D.; Chang, C.; Yu, X.; Wang, J.; Zhou, Z.; et al. Effect of Low-Concentration Rb+ Mixing on Semiconductor Majority Charge Carriers Type of Perovskite Light-Absorption Layer by Using Two-Step Spin-Coating Method. Coatings 2020, 10, 627. https://doi.org/10.3390/coatings10070627
Liu B, Zou X, Cheng J, Ling T, Yao Y, Chen D, Chang C, Yu X, Wang J, Zhou Z, et al. Effect of Low-Concentration Rb+ Mixing on Semiconductor Majority Charge Carriers Type of Perovskite Light-Absorption Layer by Using Two-Step Spin-Coating Method. Coatings. 2020; 10(7):627. https://doi.org/10.3390/coatings10070627
Chicago/Turabian StyleLiu, Baoyu, Xiaoping Zou, Jin Cheng, Tao Ling, Yujun Yao, Dan Chen, Chuangchuang Chang, Xing Yu, Junqi Wang, Zixiao Zhou, and et al. 2020. "Effect of Low-Concentration Rb+ Mixing on Semiconductor Majority Charge Carriers Type of Perovskite Light-Absorption Layer by Using Two-Step Spin-Coating Method" Coatings 10, no. 7: 627. https://doi.org/10.3390/coatings10070627
APA StyleLiu, B., Zou, X., Cheng, J., Ling, T., Yao, Y., Chen, D., Chang, C., Yu, X., Wang, J., Zhou, Z., & Li, G. (2020). Effect of Low-Concentration Rb+ Mixing on Semiconductor Majority Charge Carriers Type of Perovskite Light-Absorption Layer by Using Two-Step Spin-Coating Method. Coatings, 10(7), 627. https://doi.org/10.3390/coatings10070627




