Mutated Human P-Selectin Glycoprotein Ligand-1 and Viral Protein-1 of Enterovirus 71 Interactions on Au Nanoplasmonic Substrate for Specific Recognition by Surface-Enhanced Raman Spectroscopy
Abstract
1. Introduction
2. Experimental Section
2.1. Fabrication of the Au Nanoporous SERS Substrate
2.2. Molecular Probe Detection
2.3. Protein Immobilization on Plasmonic Au Nanoporous Substrate
2.3.1. Protein Preparation
2.3.2. Self-Assembled Monolayers of Proteins on Au Nanoporous Substrate
3. Results and Discussion
3.1. Characterization of the Plasmonic Au Nanoporous Substrate
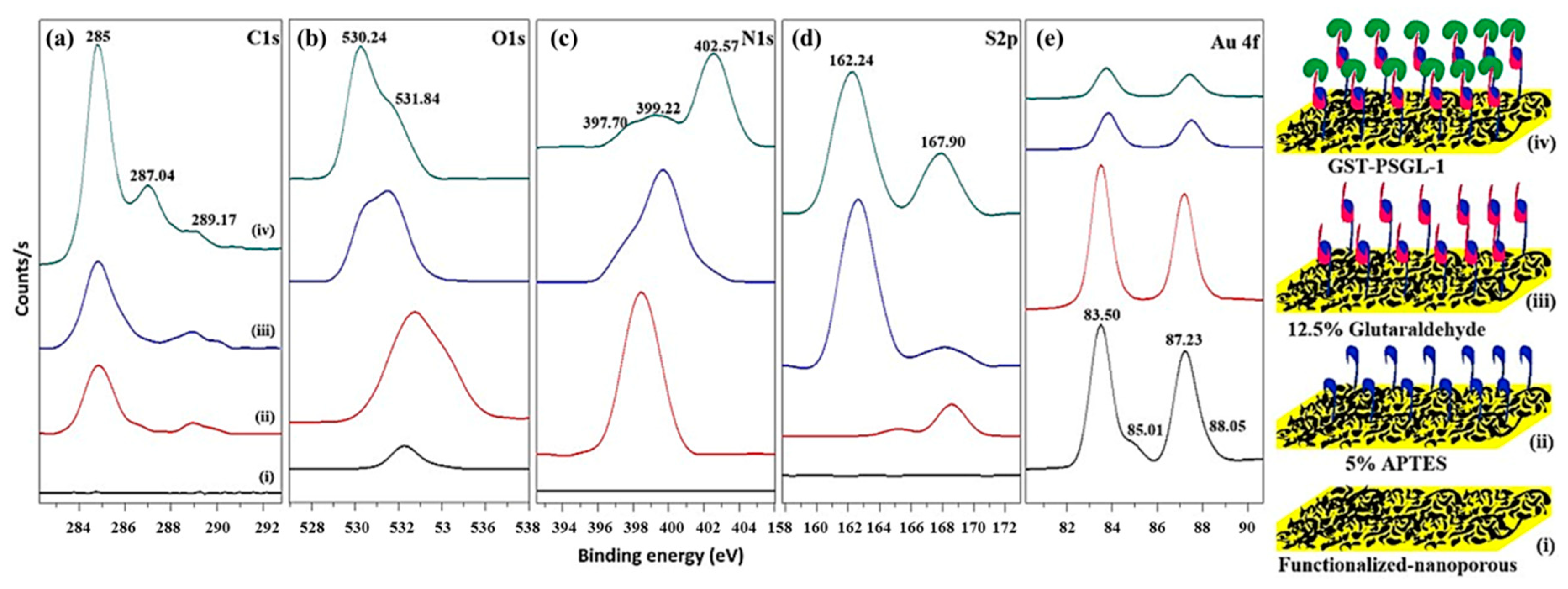
3.2. Chemisorption of Protein-Immobilized Plasmonic Au Nanoporous Substrate
3.3. Protein-Protein Interactions on the Plasmonic Au Nanoporous Substrate
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bettelheim, F.R. Tyrosine-O-sulfate in a peptide from fibrinogen. J. Am. Chem. Soc. 1954, 76, 2838–2839. [Google Scholar] [CrossRef]
- Moore, K.L. The biology and enzymology of protein tyrosine O-sulfation. J. Biol. Chem. 2003, 278, 24243–24246. [Google Scholar] [CrossRef]
- Kehoe, J.W.; Bertozzi, C.R. Tyrosine sulfation: A modulator of extracellular protein–protein interactions. Chem. Biol. 2000, 7, R57–R61. [Google Scholar] [CrossRef]
- Huang, B.Y.; Chen, P.C.; Chen, B.H.; Wang, C.C.; Liu, H.F.; Chen, Y.Z.; Chen, C.S.; Yang, Y.S. High-throughput screening of sulfated proteins by using a genome-wide proteome microarray and protein tyrosine sulfation system. Anal. Chem. 2017, 89, 3278–3284. [Google Scholar] [CrossRef]
- Monigatti, F.; Hekking, B.; Steen, H. Protein sulfation analysis-a primer. Biochim. Biophys. Acta (BBA) Proteins Proteom. 2006, 1764, 1904–1913. [Google Scholar] [CrossRef]
- Seibert, C.; Sakmar, T.P. Toward a framework for sulfoproteomics: Synthesis and characterization of sulfotyrosine-containing peptides. Pept. Sci. 2008, 90, 459–477. [Google Scholar] [CrossRef]
- Seibert, C.; Cadene, M.; Sanfiz, A.; Chait, B.T.; Sakmar, T.P. Tyrosine sulfation of CCR5 N-terminal peptide by tyrosylprotein sulfotransferases 1 and 2 follows a discrete pattern and temporal sequence. Proc. Natl. Acad. Sci. USA 2002, 99, 11031–11036. [Google Scholar] [CrossRef]
- Angiari, S.; Constantin, G. Regulation of T cell trafficking by the T cell immunoglobulin and mucin domain 1 glycoprotein. Trends Mol. Med. 2014, 20, 675–684. [Google Scholar] [CrossRef]
- Dong, J.F.; Ye, P.; Schade, A.J.; Gao, S.; Romo, G.M.; Turner, N.T.; López, J.A. Tyrosine sulfation of glycoprotein Ibα role of electrostatic interactions in von willebrand factor binding. J. Biol. Chem. 2011, 276, 16690–16694. [Google Scholar] [CrossRef]
- Farzan, M.; Mirzabekov, T.; Kolchinsky, P.; Wyatt, R.; Cayabyab, M.; Gerard, N.P.; Choe, H. Tyrosine sulfation of the amino terminus of CCR5 facilitates HIV-1 entry. Cell 1999, 96, 667–676. [Google Scholar] [CrossRef]
- Hsu, W.; Rosenquist, G.L.; Ansari, A.A.; Gershwin, M.E. Autoimmunity and tyrosine sulfation. Autoimmun. Rev. 2005, 4, 429–435. [Google Scholar] [CrossRef]
- Braun, P.; Gingras, A.C. History of protein–protein interactions: From egg-white to complex networks. Proteomics 2012, 12, 1478–1498. [Google Scholar] [CrossRef]
- Huang, C.C.; Venturi, M.; Majeed, S.; Moore, M.J.; Phogat, S.; Zhang, M.Y.; Wyatt, R. Structural basis of tyrosine sulfation and VH-gene usage in antibodies that recognize the HIV type 1 coreceptor-binding site on gp120. Proc. Natl. Acad. Sci. USA 2004, 101, 2706–2711. [Google Scholar] [CrossRef]
- Farzan, M.; Babcock, G.J.; Vasilieva, N.; Wright, P.L.; Kiprilov, E.; Mirzabekov, T.; Choe, H. The role of post-translational modifications of the CXCR4 amino terminus in stromal-derived factor 1α association and HIV-1 entry. J. Biol. Chem. 2002, 277, 29484–29489. [Google Scholar] [CrossRef]
- Nishimura, Y.; Shimojima, M.; Tano, Y.; Miyamura, T.; Wakita, T.; Shimizu, H. Human P-selectin glycoprotein ligand-1 is a functional receptor for enterovirus 71. Nat. Med. 2009, 15, 794. [Google Scholar] [CrossRef]
- Nishimura, Y.; Wakita, T.; Shimizu, H. Tyrosine sulfation of the amino terminus of PSGL-1 is critical for enterovirus 71 infection. PLoS Pathog. 2010, 6, 1174. [Google Scholar] [CrossRef]
- Chen, B.H.; Wang, C.C.; Lu, L.Y.; Hung, K.S.; Yang, Y.S. Fluorescence assay for protein post-translational tyrosine sulfation. Anal. Bioanal. Chem. 2013, 405, 1425–1429. [Google Scholar] [CrossRef]
- Goldberg, M.E.; Djavadi-Ohaniance, L. Methods for measurement of antibody/antigen affinity based on ELISA and RIA. Curr. Opin. Immunol. 1993, 5, 278–281. [Google Scholar] [CrossRef]
- Robinson, M.R.; Moore, K.L.; Brodbelt, J.S. Direct identification of tyrosine sulfation by using ultraviolet photodissociation mass spectrometry. J. Am. Soc. Mass Spectrom. 2014, 25, 1461–1471. [Google Scholar] [CrossRef]
- Itkonen, O.; Helin, J.; Saarinen, J.; Kalkkinen, N.; Ivanov, K.I.; Stenman, U.H.; Valmu, L. Mass spectrometric detection of tyrosine sulfation in human pancreatic trypsinogens, but not in tumor-associated trypsinogen. FEBS J. 2008, 275, 289–301. [Google Scholar] [CrossRef]
- Wang, C.C.; Sivashanmugan, K.; Chen, C.K.; Hong, J.R.; Sung, W.I.; Liao, J.D.; Yang, Y.S. Specific unbinding forces between mutated human P-selectin glycoprotein ligand-1 and viral protein-1 measured using force spectroscopy. J. Phys. Chem. Lett. 2017, 8, 5290–5295. [Google Scholar] [CrossRef]
- Pence, I.; Mahadevan-Jansen, A. Clinical instrumentation and applications of Raman spectroscopy. Chem. Soc. Rev. 2016, 45, 1958–1979. [Google Scholar] [CrossRef]
- Sato, H.; Maeda, Y.; Ishigaki, M.; Andriana, B.B. Biomedical Applications of Raman Spectroscopy. In Encyclopedia of Analytical Chemistry: Applications, Theory and Instrumentation; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006; pp. 1–12. [Google Scholar]
- Luo, S.C.; Sivashanmugan, K.; Liao, J.D.; Yao, C.K.; Peng, H.C. Nanofabricated SERS-active substrates for single-molecule to virus detection in vitro: A review. Biosens. Bioelectron. 2014, 61, 232–240. [Google Scholar] [CrossRef]
- Sivashanmugan, K.; Huang, W.L.; Lin, C.H.; Liao, J.D.; Lin, C.C.; Su, W.C.; Wen, T.C. Bimetallic nanoplasmonic gap-mode SERS substrate for lung normal and cancer-derived exosomes detection. J. Taiwan Inst. Chem. Eng. 2017, 80, 149–155. [Google Scholar] [CrossRef]
- Sivashanmugan, K.; Liao, J.D.; You, J.W.; Wu, C.L. Focused-ion-beam-fabricated Au/Ag multilayered nanorod array as SERS-active substrate for virus strain detection. Sensors Actuat. B Chem. 2013, 181, 361–367. [Google Scholar] [CrossRef]
- Sivashanmugan, K.; Liu, P.C.; Tsai, K.W.; Chou, Y.N.; Lin, C.H.; Chang, Y.; Wen, T.C. An anti-fouling nanoplasmonic SERS substrate for trapping and releasing a cationic fluorescent tag from human blood solution. Nanoscale 2017, 9, 2865–2874. [Google Scholar] [CrossRef]
- Pallaoro, A.; Mirsafavi, R.Y.; Culp, W.T.; Braun, G.B.; Meinhart, C.D.; Moskovits, M. Screening for canine transitional cell carcinoma (TCC) by SERS-based quantitative urine cytology. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 1279–1287. [Google Scholar] [CrossRef]
- Wang, H.N.; Fales, A.M.; Vo-Dinh, T. Plasmonics-based SERS nanobiosensor for homogeneous nucleic acid detection. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 811–814. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, A.; Kim, G.H.; Rhim, W.K.; Hartman, K.L.; Nam, J.M. Myoglobin and polydopamine-engineered raman nanoprobes for detecting, imaging, and monitoring reactive oxygen species in biological samples and living cells. Small 2017, 13, 1701584. [Google Scholar] [CrossRef]
- Shin, S.; Lee, J.; Lee, S.; Kim, H.; Seo, J.; Kim, D.; Lee, T. A droplet-based high-throughput SERS platform on a droplet-guiding-track-engraved superhydrophobic substrate. Small 2017, 13, 1602865. [Google Scholar] [CrossRef]
- Xue, L.; Xie, W.; Driessen, L.; Domke, K.F.; Wang, Y.; Schlücker, S.; Steinhart, M. Advanced SERS sensor based on capillarity-assisted preconcentration through gold nanoparticle-decorated porous nanorods. Small 2017, 13, 1603947. [Google Scholar] [CrossRef]
- Arnob, M.M.P.; Zhao, F.; Zeng, J.; Santos, G.M.; Li, M.; Shih, W.C. Laser rapid thermal annealing enables tunable plasmonics in nanoporous gold nanoparticles. Nanoscale 2014, 6, 12470–12475. [Google Scholar] [CrossRef]
- Seker, E.; Reed, M.L.; Begley, M.R. Nanoporous gold: Fabrication, characterization, and applications. Materials 2009, 2, 2188–2215. [Google Scholar] [CrossRef]
- Polat, O.; Seker, E. Effect of surface–molecule interactions on molecular loading capacity of nanoporous gold thin films. J. Phys. Chem. C 2016, 120, 19189–19194. [Google Scholar] [CrossRef]
- Ke, X.; Xu, Y.; Yu, C.; Zhao, J.; Cui, G.; Higgins, D.; Wu, G. Pd-decorated three-dimensional nanoporous Au/Ni foam composite electrodes for H2O2 reduction. J. Mater. Chem. A 2014, 2, 16474–16479. [Google Scholar] [CrossRef]
- Dorofeeva, T.S.; Seker, E. In situ electrical modulation and monitoring of nanoporous gold morphology. Nanoscale 2016, 8, 19551–19556. [Google Scholar] [CrossRef]
- Ishida, S.; Anno, Y.; Takeuchi, M.; Matsuoka, M.; Takei, K.; Arie, T.; Akita, S. Highly photosensitive graphene field-effect transistor with optical memory function. Sci. Rep. 2015, 5, 15491. [Google Scholar] [CrossRef]
- Kim, W.K.; Lee, J.L. Effect of oxygen plasma treatment on reduction of contact resistivity at pentacene/Au interface. Appl. Phys. Lett. 2006, 88, 262102. [Google Scholar] [CrossRef]
- Liu, W.; Sun, D.; Fu, J.; Yuan, R.; Li, Z. Assembly of evenly distributed Au nanoparticles on thiolated reduced graphene oxide as an active and robust catalyst for hydrogenation of 4-nitroarenes. RSC Adv. 2014, 4, 11003–11011. [Google Scholar] [CrossRef]
- Pasternack, R.M.; Rivillon, A.S.; Chabal, Y.J. Attachment of 3-(aminopropyl) triethoxysilane on silicon oxide surfaces: Dependence on solution temperature. Langmuir 2008, 24, 12963–12971. [Google Scholar] [CrossRef]
- Jastrzebska, M.; Wrzalik, R.; Kocot, A.; Zalewska-Rejdak, J.; Cwalina, B. Raman spectroscopic study of glutaraldehyde-stabilized collagen and pericardium tissue. J. Biomater. Sci. Polym. Ed. 2003, 14, 185–197. [Google Scholar] [CrossRef]
- Satheeshkumar, E.; Karuppaiya, P.; Sivashanmugan, K.; Chao, W.T.; Tsay, H.S.; Yoshimura, M. Biocompatible 3D SERS substrate for trace detection of amino acids and melamine. Pectroc. Acta Pt. A-Molec. Biomolec. Spectr. 2017, 181, 91–97. [Google Scholar] [CrossRef]
- Madzharova, F.; Heiner, Z.; Kneipp, J. Surface enhanced hyper-Raman scattering of the amino acids tryptophan, Histidine, Phenylalanine, and Tyrosine. J. Phys. Chem. C 2017, 121, 1235–1242. [Google Scholar] [CrossRef]
- Hernández, B.; Coïc, Y.M.; Pflüger, F.; Kruglik, S.G.; Ghomi, M. All characteristic Raman markers of tyrosine and tyrosinate originate from phenol ring fundamental vibrations. J. Raman Spectrosc. 2016, 47, 210–220. [Google Scholar] [CrossRef]
- Fischer, W.B.; Eysel, H.H. Polarized Raman spectra and intensities of aromatic amino acids phenylalanine, tyrosine and tryptophan. Spectroc. Acta Pt. A-Molec. Biomolec. Spectr. 1992, 48, 725–732. [Google Scholar] [CrossRef]
- Bugaev, K.O.; Zelenina, A.A.; Volodin, V.A. Vibrational spectroscopy of chemical species in silicon and silicon-rich nitride thin films. J. Spectrosc. 2012, 2012, 281851. [Google Scholar] [CrossRef]
- Li, Y.T.; Li, D.W.; Cao, Y.; Long, Y.T. Label-free in-situ monitoring of protein tyrosine nitration in blood by surface-enhanced Raman spectroscopy. Biosens. Bioelectron. 2015, 69, 1–7. [Google Scholar] [CrossRef]
- Brewster, V.L.; Ashton, L.; Goodacre, R. Monitoring the glycosylation status of proteins using Raman spectroscopy. Anal. Chem. 2011, 83, 6074–6081. [Google Scholar] [CrossRef]
- Davies, H.S.; Singh, P.; Deckert-Gaudig, T.; Deckert, V.; Rousseau, K.; Ridley, C.E.; Blanch, E.W. Secondary structure and glycosylation of mucus glycoproteins by Raman spectroscopies. Anal. Chem. 2016, 88, 11609–11615. [Google Scholar] [CrossRef]
- Ravikumar, B.; Rajaram, R.K.; Ramakrishnan, V. Raman and IR spectral studies of L-phenylalanine L-phenylalaninium dihydrogenphosphate and DL-phenylalaninium dihydrogenphosphate. J. Raman Spectrosc. 2006, 37, 597–605. [Google Scholar] [CrossRef]
- Arp, Z.; Autrey, D.; Laane, J.; Overman, S.A.; Thomas, G.J. Tyrosine Raman signatures of the filamentous virus Ff are diagnostic of non-hydrogen-bonded phenoxyls: Demonstration by Raman and infrared spectroscopy of p-cresol vapor. Biochemistry 2001, 40, 2522–2529. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Martin, S.J.; Chinoy, Z.S.; Liu, L.; Rittgers, B.; Dluhy, R.A.; Boons, G.J. Label-free detection of glycan–protein interactions for array development by surface-enhanced Raman spectroscopy (SERS). Chem.-Eur. J. 2016, 22, 11180–11185. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sivashanmugan, K.; Lee, H.; Liao, J.-D.; Wang, C.-C.; Lin, C.-H.; Yang, Y.-S.; Sitjar, J. Mutated Human P-Selectin Glycoprotein Ligand-1 and Viral Protein-1 of Enterovirus 71 Interactions on Au Nanoplasmonic Substrate for Specific Recognition by Surface-Enhanced Raman Spectroscopy. Coatings 2020, 10, 403. https://doi.org/10.3390/coatings10040403
Sivashanmugan K, Lee H, Liao J-D, Wang C-C, Lin C-H, Yang Y-S, Sitjar J. Mutated Human P-Selectin Glycoprotein Ligand-1 and Viral Protein-1 of Enterovirus 71 Interactions on Au Nanoplasmonic Substrate for Specific Recognition by Surface-Enhanced Raman Spectroscopy. Coatings. 2020; 10(4):403. https://doi.org/10.3390/coatings10040403
Chicago/Turabian StyleSivashanmugan, Kundan, Han Lee, Jiunn-Der Liao, Chen-Chu Wang, Chen-Hsueh Lin, Yuh-Shyong Yang, and Jaya Sitjar. 2020. "Mutated Human P-Selectin Glycoprotein Ligand-1 and Viral Protein-1 of Enterovirus 71 Interactions on Au Nanoplasmonic Substrate for Specific Recognition by Surface-Enhanced Raman Spectroscopy" Coatings 10, no. 4: 403. https://doi.org/10.3390/coatings10040403
APA StyleSivashanmugan, K., Lee, H., Liao, J.-D., Wang, C.-C., Lin, C.-H., Yang, Y.-S., & Sitjar, J. (2020). Mutated Human P-Selectin Glycoprotein Ligand-1 and Viral Protein-1 of Enterovirus 71 Interactions on Au Nanoplasmonic Substrate for Specific Recognition by Surface-Enhanced Raman Spectroscopy. Coatings, 10(4), 403. https://doi.org/10.3390/coatings10040403






