Re-Oxidation of ZnO Clusters Grown on HOPG
Abstract
1. Introduction
2. Materials and Methods
3. Results
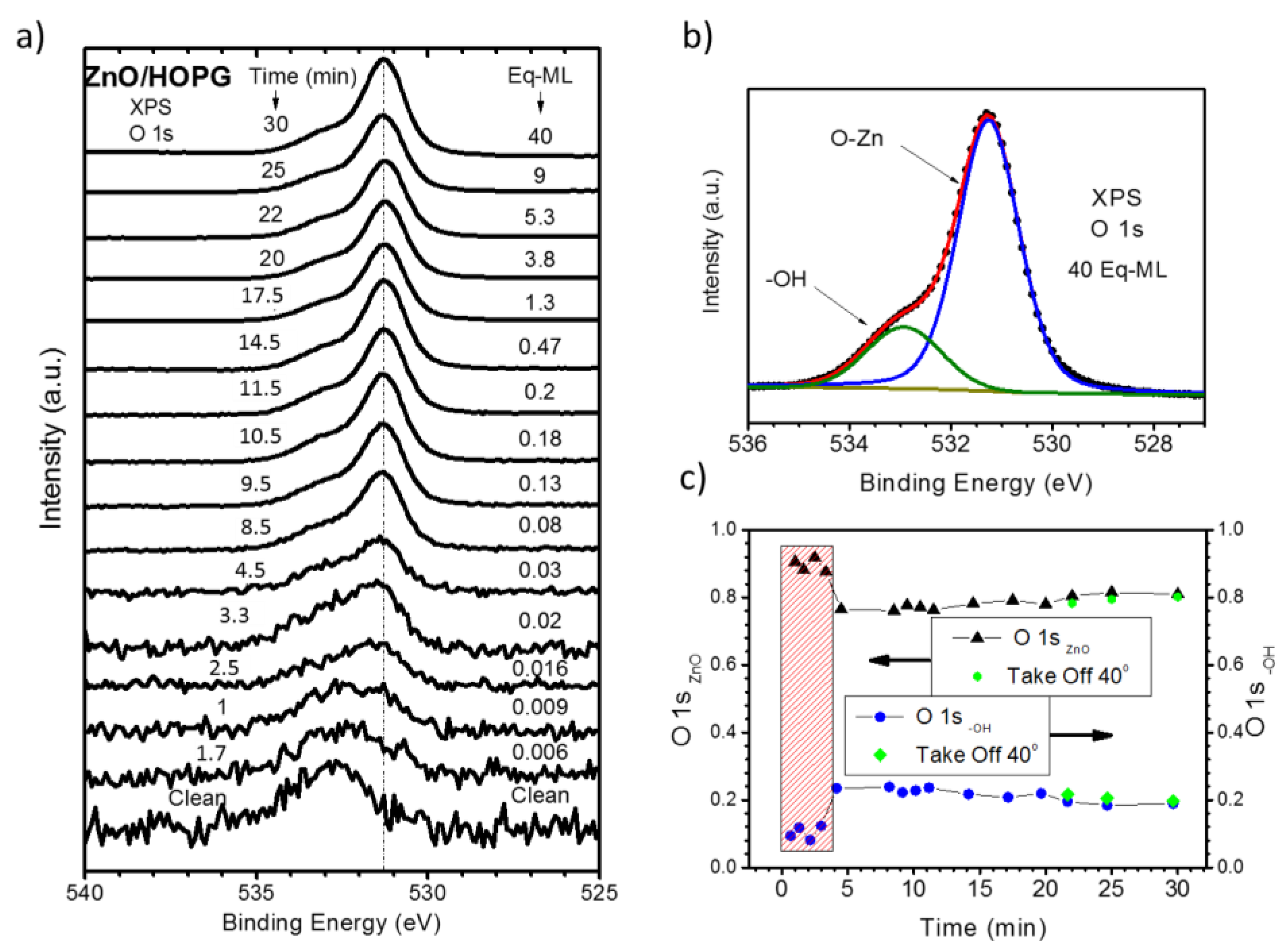
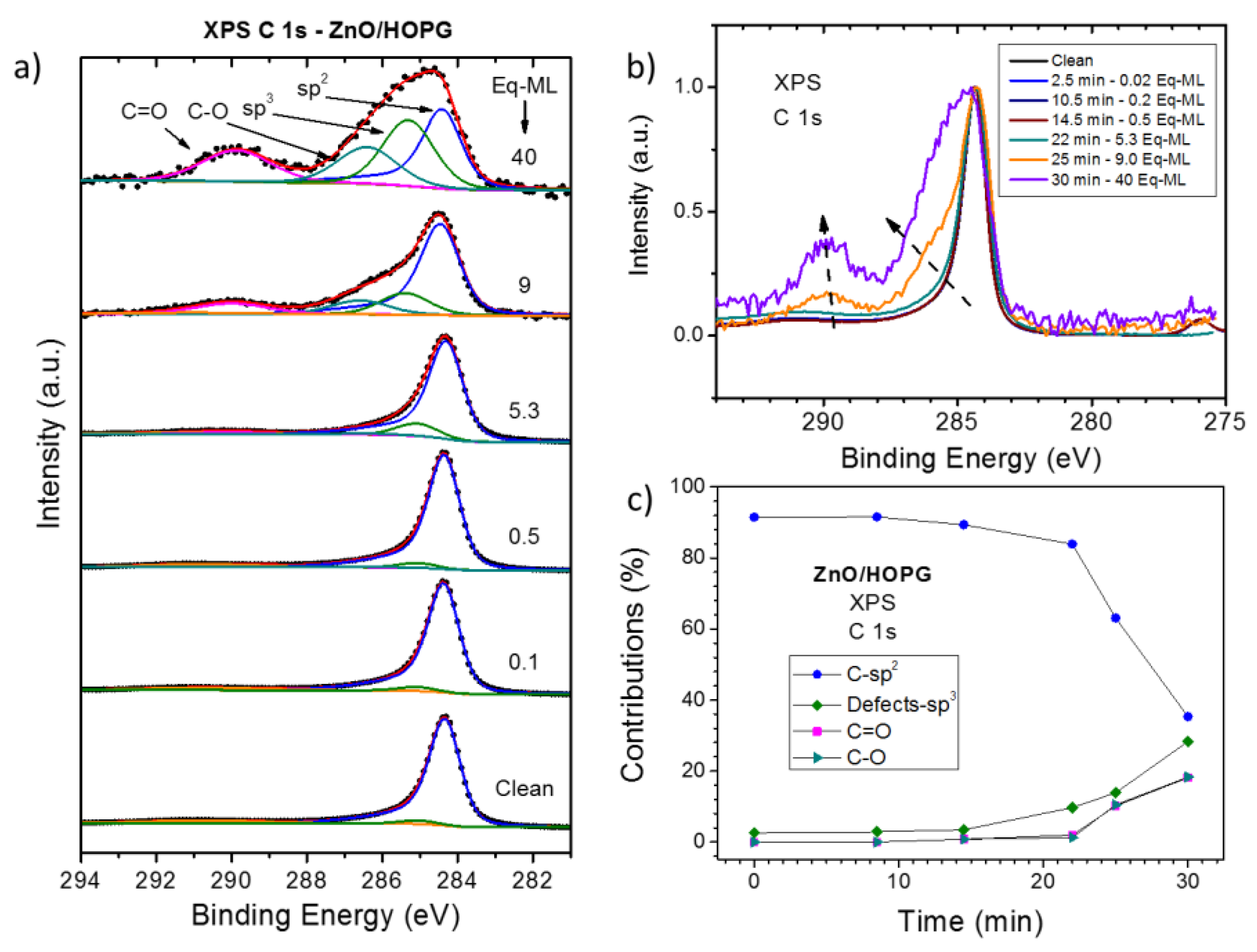
3.1. XPS Characterization of as Grown ZnO/HOPG
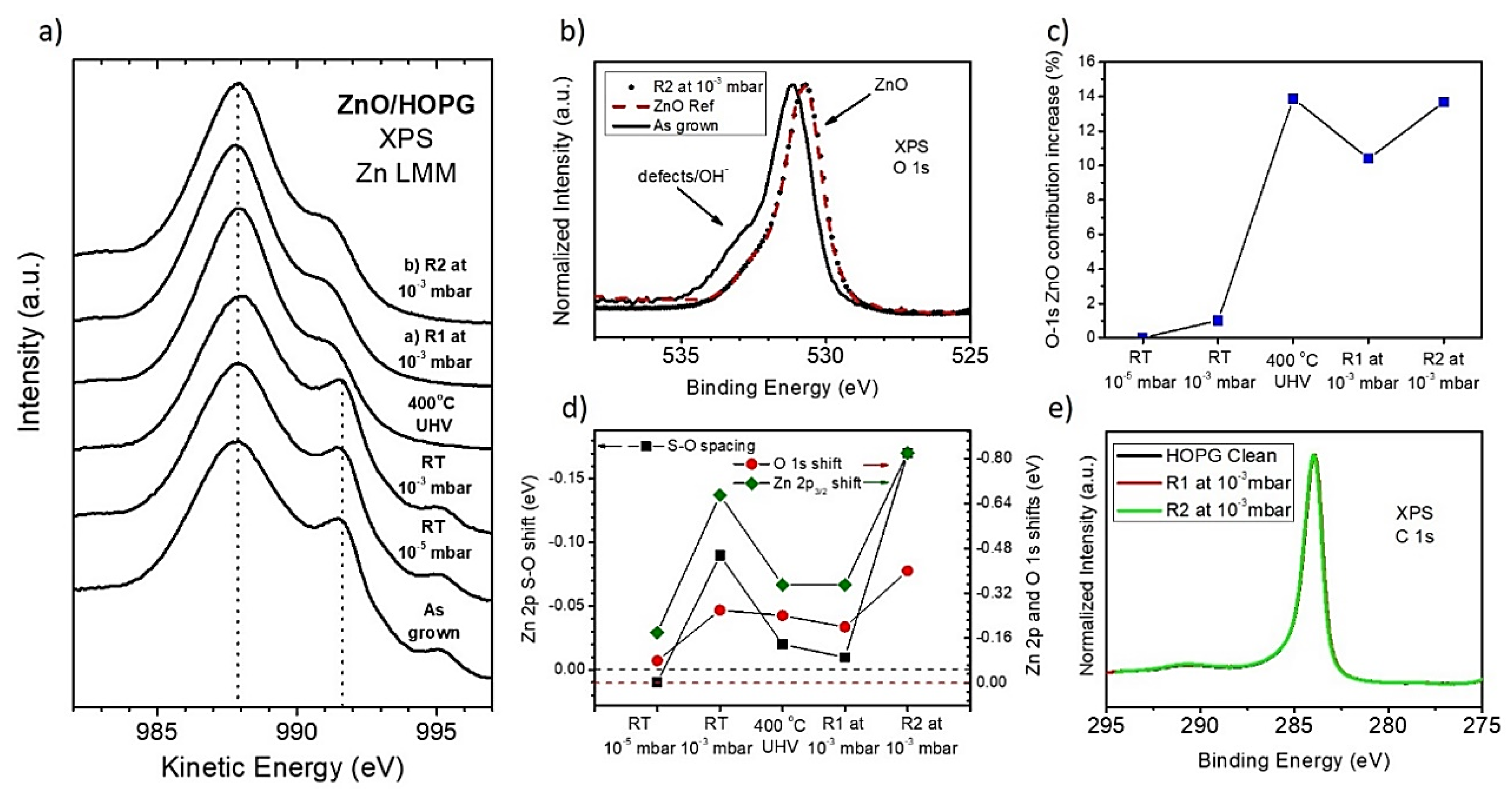
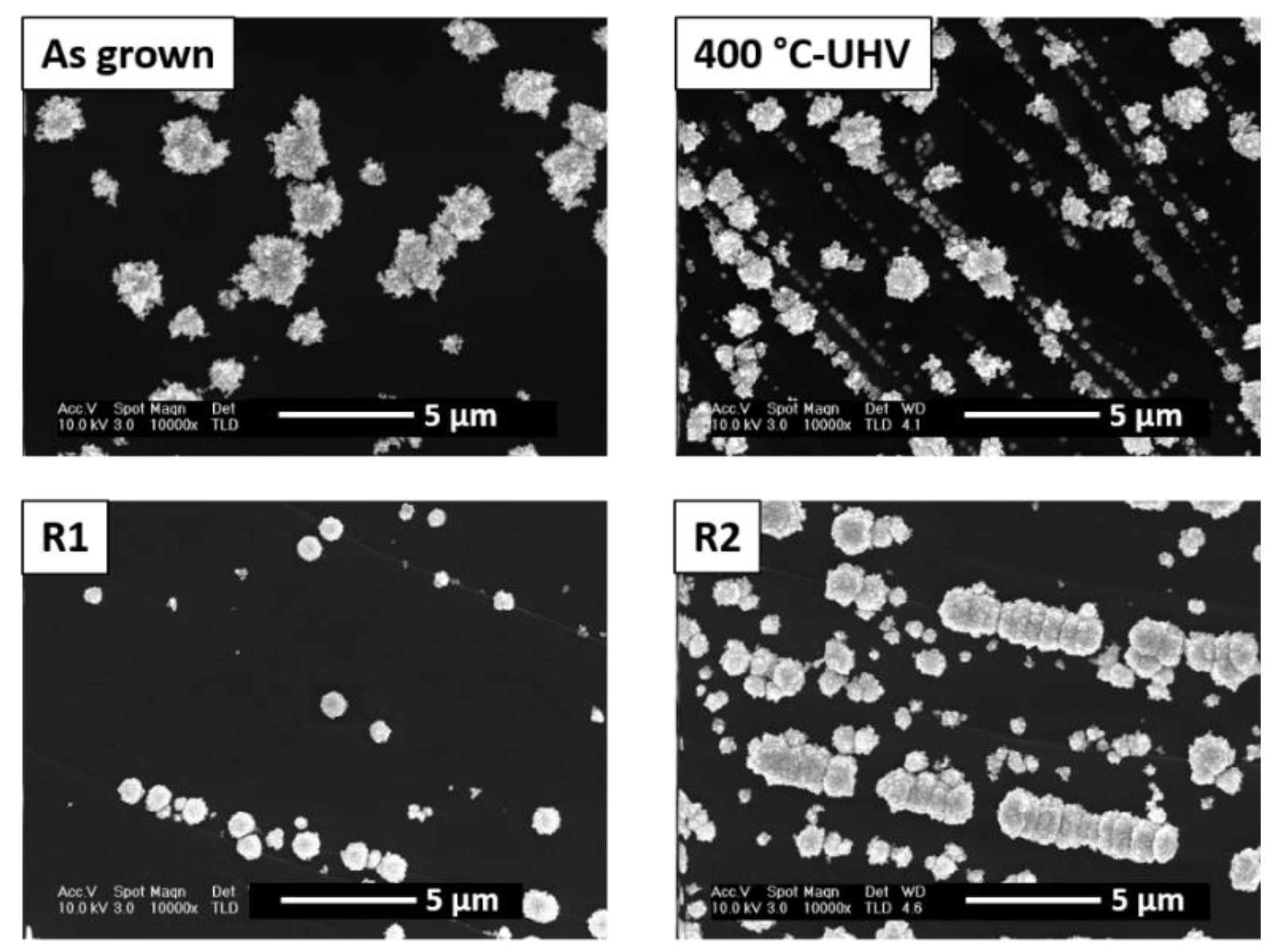
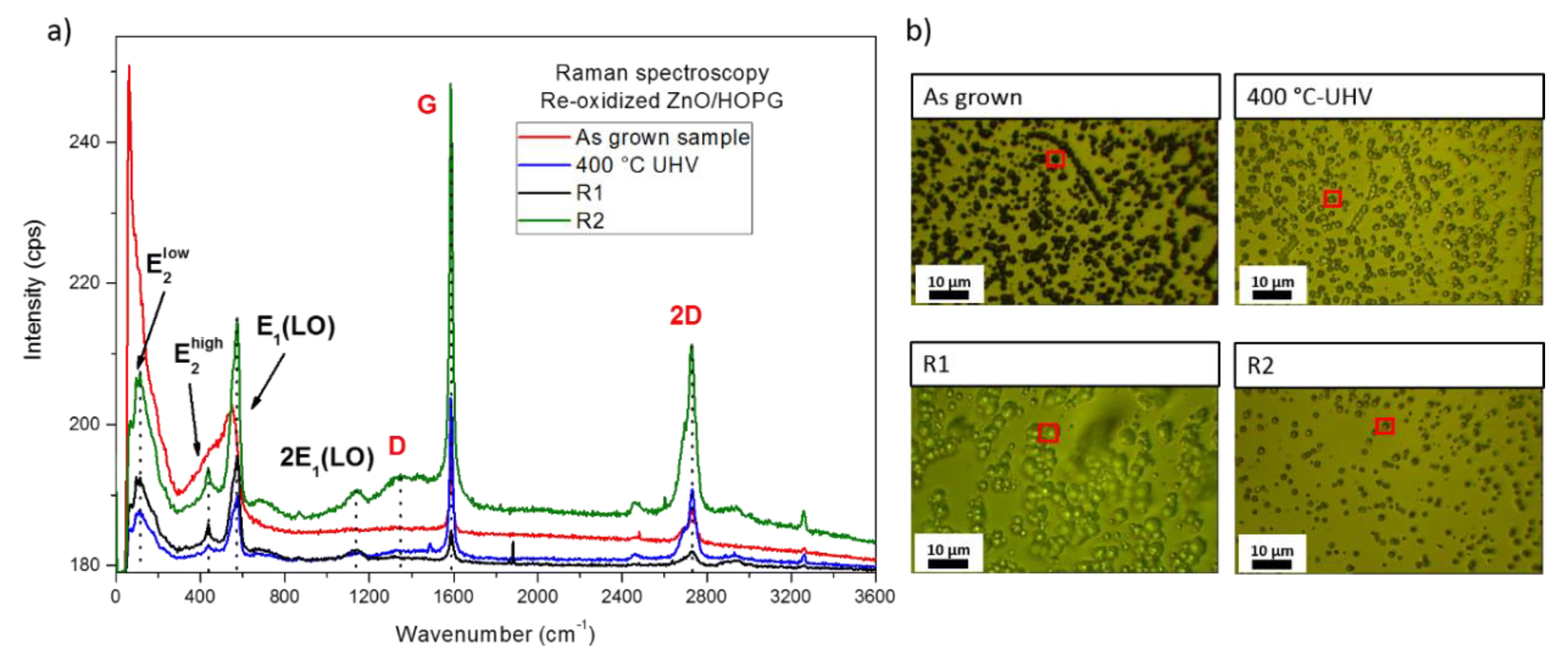
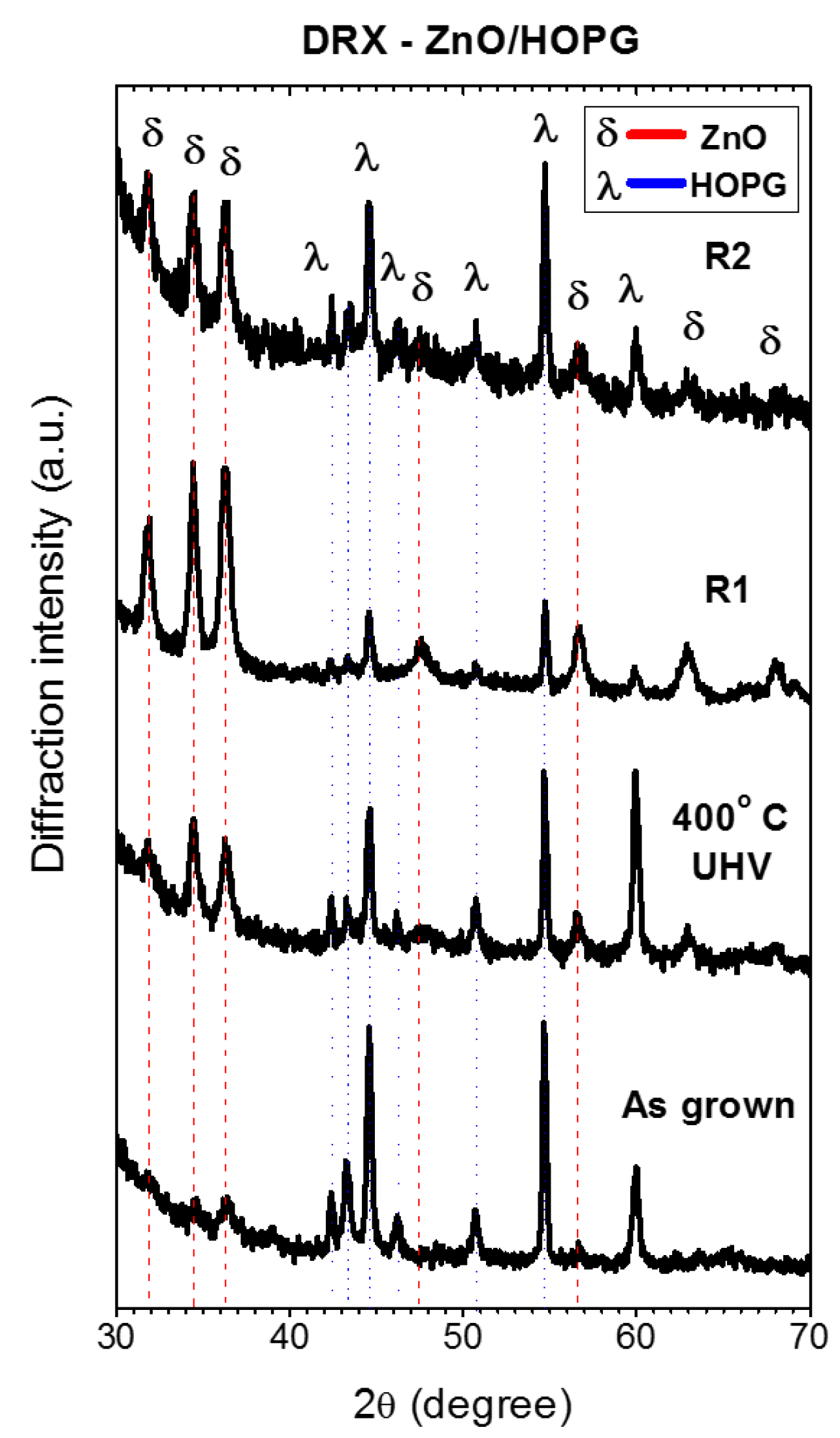
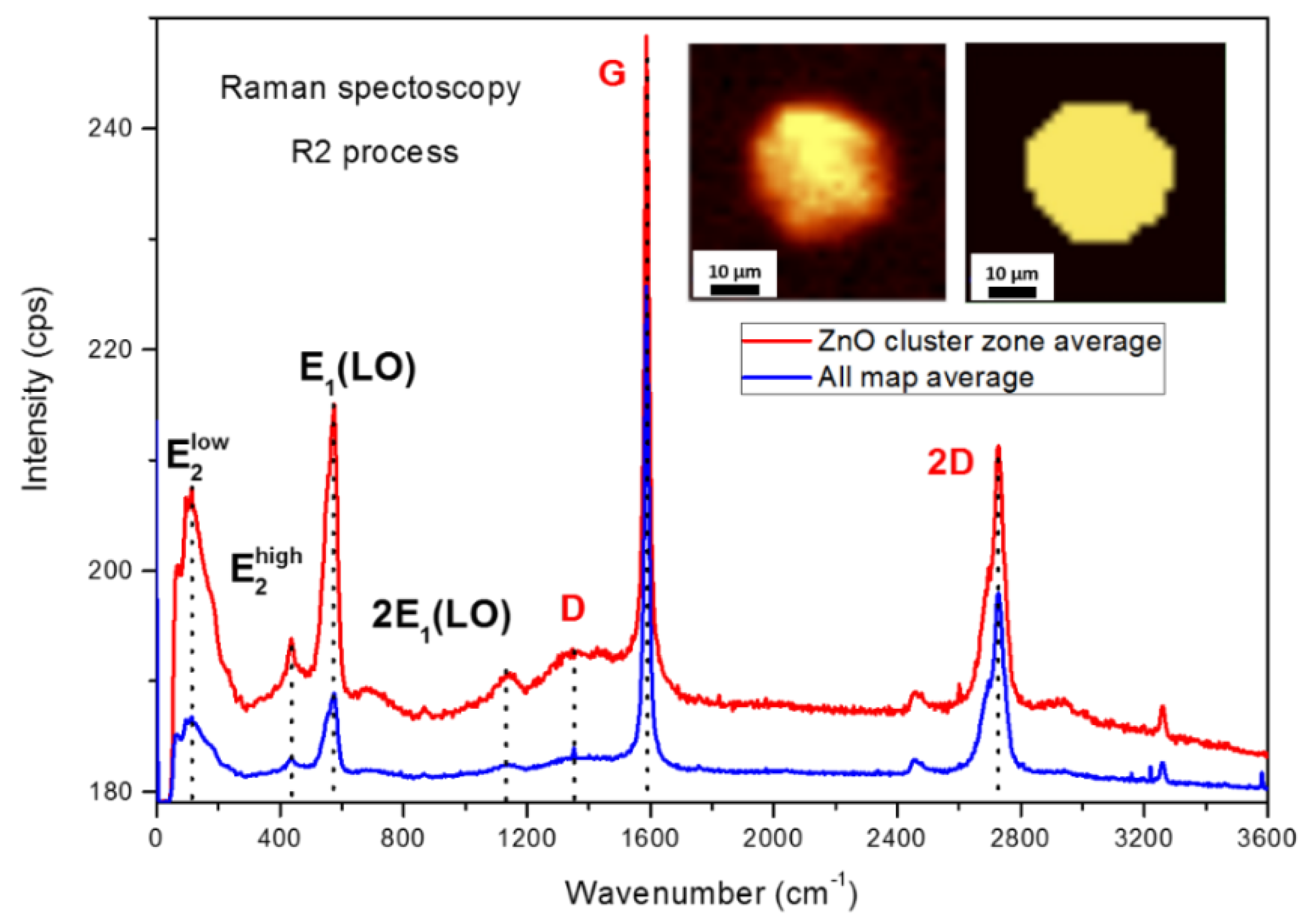
3.2. Thermal Re-Oxidation of ZnO/HOPG
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Özgür, Ü.; Alivov, Y.I.; Liu, C.; Teke, A.; Reshchikov, M.A.; Dogán, S.; Avrutin, V.; Cho, S.-J.; Morkoç, H. A comprehensive review of ZnO materials and devices. J. Appl. Phys. 2005, 98, 041301. [Google Scholar] [CrossRef]
- Ataev, B.M.; Bagamadova, A.M.; Djabrailov, A.M.; Mamedo, V.V.; Rabadanov, R.A. Highly conductive and transparent Ga-doped epitaxial ZnO films on sapphire by CVD. Thin Solid Films 1995, 260, 19–20. [Google Scholar] [CrossRef]
- Kim, H.; Gilmore, C.M.; Horwitz, J.S.; Piqué, A.; Murata, H.; Kushto, G.P.; Schlaf, R.; Kafafi, Z.H. Transparent conducting aluminum-doped zinc oxide thin films for organic light-emitting devices. Appl. Phys. Lett. 2000, 76, 259. [Google Scholar] [CrossRef]
- Zhou, X.H.; Hu, Q.-H.; Fu, Y. First-principles LDA+U studies of the In-doped ZnO transparent conductive oxide. J. Appl. Phys. 2008, 104, 063703. [Google Scholar] [CrossRef]
- Rousset, J.; Saucedo, E.; Lincot, D. Extrinsic doping of electrodeposited zinc oxide films by chlorine for transparent conductive oxide applications. Chem. Mater. 2009, 21, 534–540. [Google Scholar] [CrossRef]
- Klingshirn, C. ZnO: From basics towards applications. Phys. Status Solidi 2007, 244, 3027–3073. [Google Scholar] [CrossRef]
- Upadhyay, R.K.; Soin, N.; Roy, S.S. Role of graphene/metal oxide composites as photocatalysts, adsorbents and disinfectants in water treatment: A review. RSC Adv. 2014, 4, 3823–3851. [Google Scholar] [CrossRef]
- Su, Y.; Li, S.; Wu, D.; Zhang, F.; Liang, H.; Gao, P.; Cheng, C.; Feng, X. Two-Dimensional carbon-coated graphene/metal oxide hybrids for enhanced lithium storage. ACS Nano 2012, 6, 8349–8356. [Google Scholar] [CrossRef] [PubMed]
- Saranya, M.; Ramachandran, R.; Wang, F. Graphene-zinc oxide (G-ZnO) nanocomposite for electrochemical supercapacitor applications. J. Sci. Adv. Mater. Devices 2016, 1, 454–460. [Google Scholar] [CrossRef]
- Raizada, P.; Sudhaik, A.; Singh, P. Photocatalytic water decontamination using graphene and ZnO coupled photocatalysts: A review. Mater. Sci. Energy Technol. 2019, 2, 509–525. [Google Scholar] [CrossRef]
- Lonkar, S.P.; Pillai, V.; Abdala, A. Solvent-free synthesis of ZnO-graphene nanocomposite with superior photocatalytic activity. Appl. Surf. Sci. 2019, 465, 1107–1113. [Google Scholar] [CrossRef]
- Anand, K.; Singh, O.; Singh, M.P.; Kaur, J.; Singh, R.C. Hydrogen sensor based on graphene/ZnO nanocomposite. Sens. Actuators B Chem. 2014, 195, 409–415. [Google Scholar] [CrossRef]
- Tavakoli, M.M.; Tavakoli, R.; Yadav, P.; Kong, J. A graphene/ZnO electron transfer layer together with perovskite passivation enables highly efficient and stable perovskite solar cells. J. Mater. Chem. A 2019, 7, 679–686. [Google Scholar] [CrossRef]
- Morales, C.; Urbanos, F.J.; del Campo, A.; Leinen, D.; Granados, D.; Rodríguez, M.A.; Soriano, L. Electronic decoupling of graphene from copper induced by deposition of ZnO: A complex substrate/graphene/deposit/environment interaction. Adv. Mater. Interfaces 2020, 1902062. [Google Scholar] [CrossRef]
- Morales, C.; Black, A.; Urbanos, F.J.; Granados, D.; Méndez, J.; del Campo, A.; Yubero, F.; Soriano, L. Study of the interface of the early stages of growth under quasi-equilibrium conditions of ZnO on graphene/Cu and graphite. Adv. Mater. Interfaces 2019, 6, 1801689. [Google Scholar] [CrossRef]
- Konishi, S.; Sugimoto, W.; Murakami, Y.; Takasu, Y. Catalytic creation of channels in the surface layers of highly oriented pyrolytic graphite by Cobalt nanoparticles. Carbon 2006, 44, 2338–2340. [Google Scholar] [CrossRef]
- Datta, S.S.; Strachan, D.R.; Khamis, S.M.; Johnson, A.T. Crystallographic etching of few-layer graphene. Nano Lett. 2008, 8, 1912–1917. [Google Scholar] [CrossRef]
- Díaz-Fernández, D.; Méndez, J.; del Campo, A.; Mossanek, R.J.O.; Abbate, M.; Rodríguez, M.A.; Domínguez-Cañizares, G.; Bomatí-Miguel, O.; Gutiérrez, A.; Soriano, L. Nanopatterning on highly oriented pyrolytic graphite surfaces promoted by cobalt oxides. Carbon 2015, 85, 89–98. [Google Scholar] [CrossRef]
- Morales, C.; Díaz-Fernández, D.; Prieto, P.; Lu, Y.H.; Kersell, H.; del Campo, A.; Escudero, C.; Pérez-Dieste, V.; Ashby, P.; Méndez, J.; et al. In situ study of the carbon gasification reaction of highly oriented pyrolytic graphite promoted by cobalt oxides and the novel nanostructures appeared after reaction. Carbon 2020, 158, 588–597. [Google Scholar] [CrossRef]
- Morales, C.; Díaz-Fernández, D.; Mossanek, R.J.O.; Abbate, M.; Méndez, J.; Pérez-Dieste, V.; Escudero, C.; Rubio-Zuazo, J.; Prieto, P.; Soriano, L. Controlled ultra-thin oxidation of Graphite promoted by cobalt oxides: Influence of the initial 2D CoO wetting layer. Appl. Surf. Sci. 2009, 509, 145118. [Google Scholar] [CrossRef]
- Horcas, I.; Fernández, R.; Gómez-Rodríguez, J.M.; Colchero, J.; Gómez Herrero, J.; Baró, A.M. WSXM: A software for scanning probe microscopy and a tool for nanotechnology. Rev. Sci. Instrum. 2007, 78, 013705. [Google Scholar] [CrossRef] [PubMed]
- Deroubaix, G.; Marcus, P. X-ray photoelectron spectroscopy analysis of copper and zinc oxides and sulphides. Surf. Interface Anal. 1992, 18, 39–46. [Google Scholar] [CrossRef]
- Díaz-Fernández, D.; Salas, E.; Méndez, J.; Mossanek, R.J.O.; Abbate, M.; Morales, C.; Domínguez-Cañizares, G.; Castro, G.R.; Gutiérrez, A.; Soriano, L. Ultra-thin CoO films grown on different oxide substrates: Size and support effects and chemical stability. J. Alloys Compd. 2018, 758, 5–13. [Google Scholar] [CrossRef]
- Rodriguez, J.A.; Campbell, R.A.; Goodman, D.W. Interaction of ultrathin films of copper with rhodium (100) and ruthenium(0001): An XPS study. J. Phys. Chem. 1991, 95, 2477–2483. [Google Scholar] [CrossRef]
- Wu, Y.; Garfunkel, E.; Madey, T.E. Initial stages of Cu growth on ordered Al2O3 ultrathin films. J. Vac. Sci. Technol. A Vac. Surf. Films 1996, 14, 1662–1667. [Google Scholar] [CrossRef]
- Kowalczyk, S.P.; Ley, L.; McFeely, F.R.; Shirley, D.A. Multiplet splitting of the manganese 2 p and 3 p levels in MnF2, single crystals. Phys. Rev. B 1975, 11, 1721–1727. [Google Scholar] [CrossRef]
- Dupin, J.-C.; Gonbeau, D.; Vinatier, P.; Levasseur, A. Systematic XPS studies of metal oxides, hydroxides and peroxides. Phys. Chem. Chem. Phys. 2000, 2, 1319–1324. [Google Scholar] [CrossRef]
- Benito, N.; Recio-Sánchez, G.; Escobar-Galindo, R.; Palacio, C. Formation of antireflection Zn/ZnO core-shell nano-pyramidal arrays by O2+ ion bombardment of Zn surfaces. Nanoscale 2017, 9, 14201–14207. [Google Scholar] [CrossRef]
- Kotsis, K.; Staemmler, V. Ab initio calculations of the O 1 s XPS spectra of ZnO and Zn oxo compounds. Phys. Chem. Chem. Phys. 2006, 8, 1490–1498. [Google Scholar] [CrossRef]
- Rousseau, B.; Estrade-Szwarckopf, H.; Thoman, A.-L.; Brault, P. Stable C-atom displacements on HOPG surface under plasma low-energy argon-ion bombardment. Appl. Phys. A 2003, 77, 591–597. [Google Scholar] [CrossRef]
- Yang, D.; Velamakanni, A.; Bozoklu, G.; Park, S.; Stoller, M.; Piner, R.D.; Stankovich, S.; Jung, I.; Field, D.A.; Ventrice, C.A., Jr.; et al. Chemical analysis of graphene oxide films after heat and chemical treatments by X-ray photoelectron and Micro-Raman spectroscopy. Carbon 2009, 47, 145–152. [Google Scholar] [CrossRef]
- Haubner, K.; Murawski, J.; Olk, P.; Eng, L.M.; Ziegler, C.; Adolphi, B.; Jaehne, E. The route to functional graphene oxide. Chem. Phys. Chem. 2010, 11, 2131–2139. [Google Scholar] [CrossRef] [PubMed]
- Webb, M.J.; Palmgren, P.; Pal, P.; Karis, O.; Grennberg, H. A simple method to produce almost perfect graphene on highly oriented pyrolytic graphite. Carbon 2011, 49, 3242–3249. [Google Scholar] [CrossRef]
- Scepanovic, M.; Grujic-Brojcin, M.; Vojisavljevic, K.; Bernikcm, S.; Sreckovic, T. Raman study of structural disorder in ZnO nanopowders. J. Raman Spectrosc. 2010, 41, 914–921. [Google Scholar] [CrossRef]
- Reich, S.; Thomsen, C. Raman spectroscopy of graphite. Philos. Trans. R. Soc. Lond. Ser. A Math. Phys. Eng. Sci. 2004, 362, 2271–2288. [Google Scholar] [CrossRef]
- Kelemen, S.R.; Fang, H.L. Maturity trends in Raman spectra from kerogen and coal. Energy Fuels 2001, 15, 653–658. [Google Scholar] [CrossRef]
- Errico, V.; Arrabito, G.; Plant, S.R.; Medaglia, P.G.; Palmer, R.E.; Falconi, C. Chromium inhibition and size-selected Au nanocluster catalysis for the solution growth of low-density ZnO nanowires. Sci. Rep. 2015, 5, 12336. [Google Scholar] [CrossRef]
- Gayathri, S.; Jayabal, P.M.; Kottaisamy, M.; Ramakrishnan, V. Synthesis of ZnO decorated graphene nanocomposite for enhanced photocatalytic properties. J. Appl. Phys. 2014, 115, 173504. [Google Scholar] [CrossRef]
- Esconjauregui, S.; Whelan, C.M.; Maex, K. The reasons why metals catalyze the nucleation and growth of carbon nanotubes and other carbon nanomorphologies. Carbon 2009, 47, 659–669. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morales, C.; del Campo, A.; Méndez, J.; Prieto, P.; Soriano, L. Re-Oxidation of ZnO Clusters Grown on HOPG. Coatings 2020, 10, 401. https://doi.org/10.3390/coatings10040401
Morales C, del Campo A, Méndez J, Prieto P, Soriano L. Re-Oxidation of ZnO Clusters Grown on HOPG. Coatings. 2020; 10(4):401. https://doi.org/10.3390/coatings10040401
Chicago/Turabian StyleMorales, Carlos, Adolfo del Campo, Javier Méndez, Pilar Prieto, and Leonardo Soriano. 2020. "Re-Oxidation of ZnO Clusters Grown on HOPG" Coatings 10, no. 4: 401. https://doi.org/10.3390/coatings10040401
APA StyleMorales, C., del Campo, A., Méndez, J., Prieto, P., & Soriano, L. (2020). Re-Oxidation of ZnO Clusters Grown on HOPG. Coatings, 10(4), 401. https://doi.org/10.3390/coatings10040401








