SiCp/Al5056 Composite Coatings Applied to A Magnesium Substrate by Cold Gas Dynamic Spray Method for Corrosion Protection
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cold Spraying Procedure
2.3. Coating Characterization
3. Results
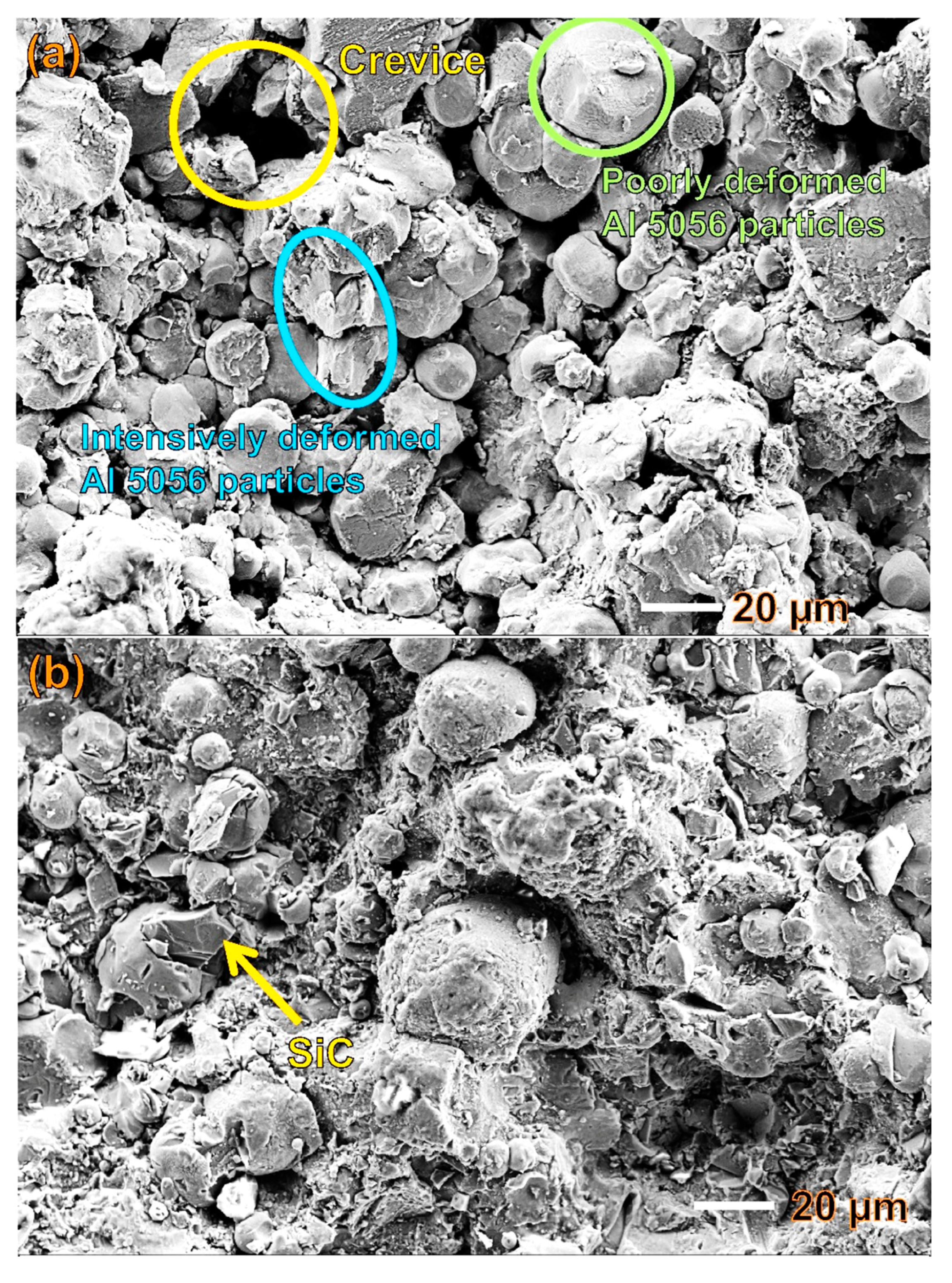
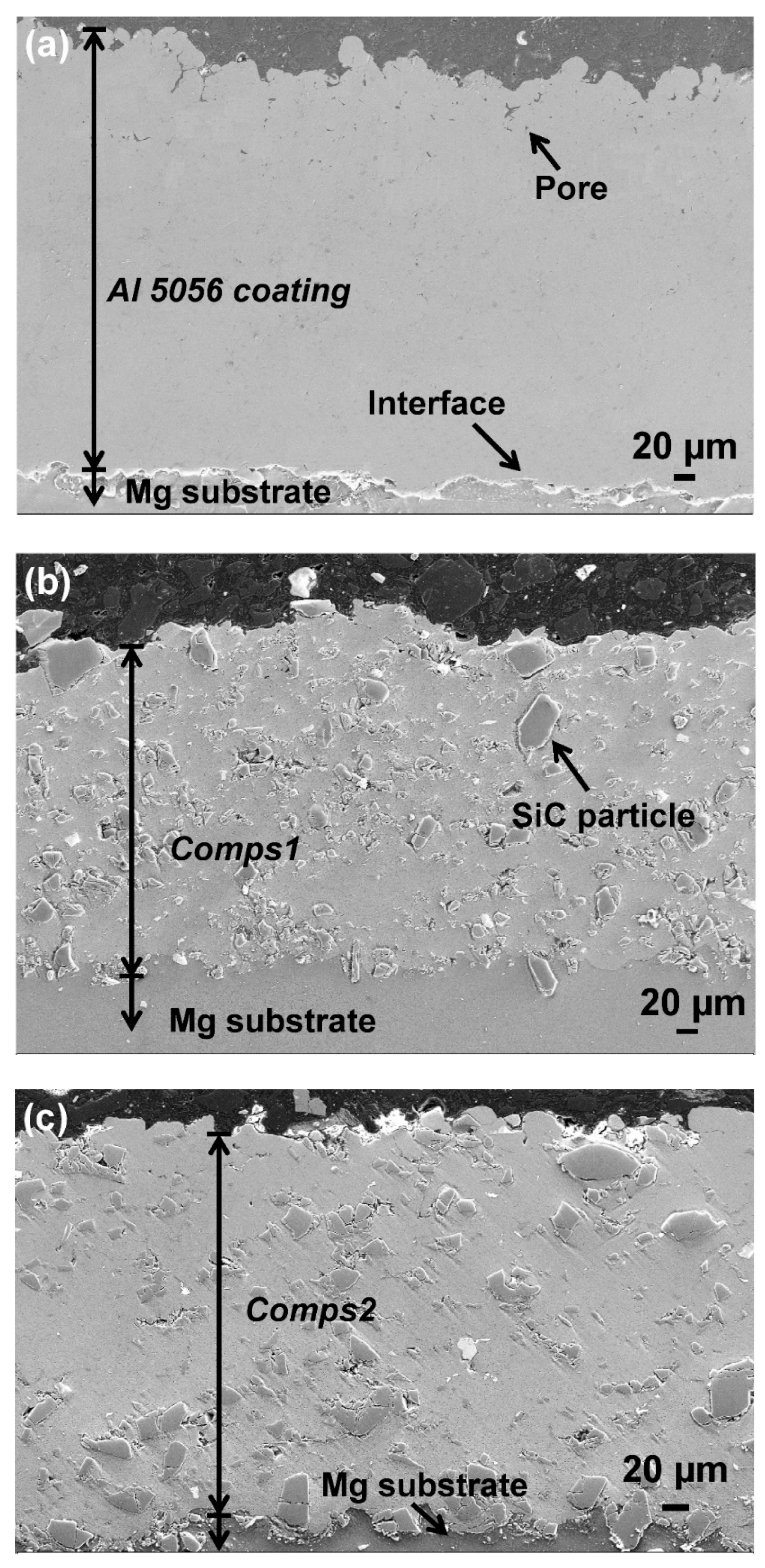
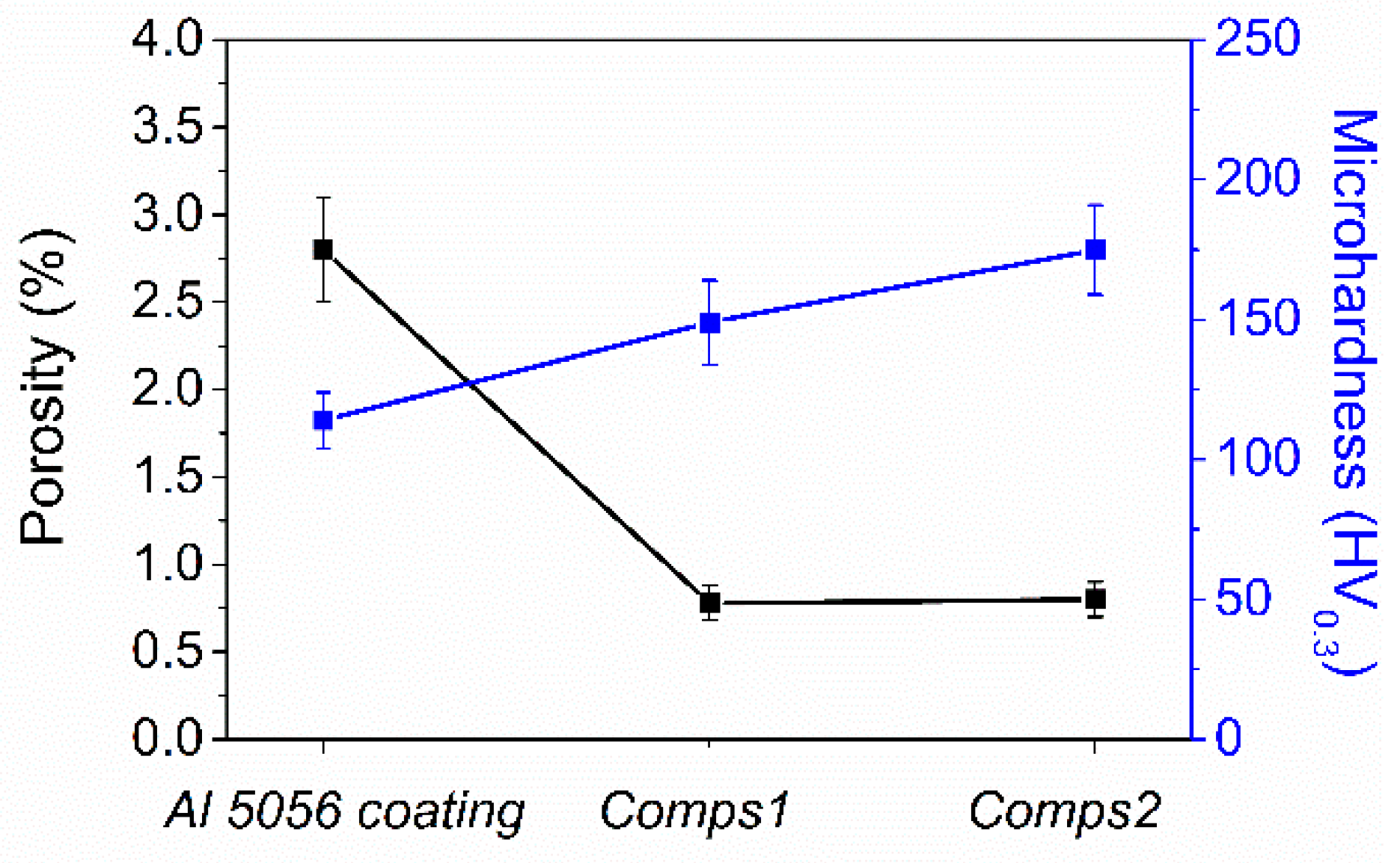
3.1. Microstructure
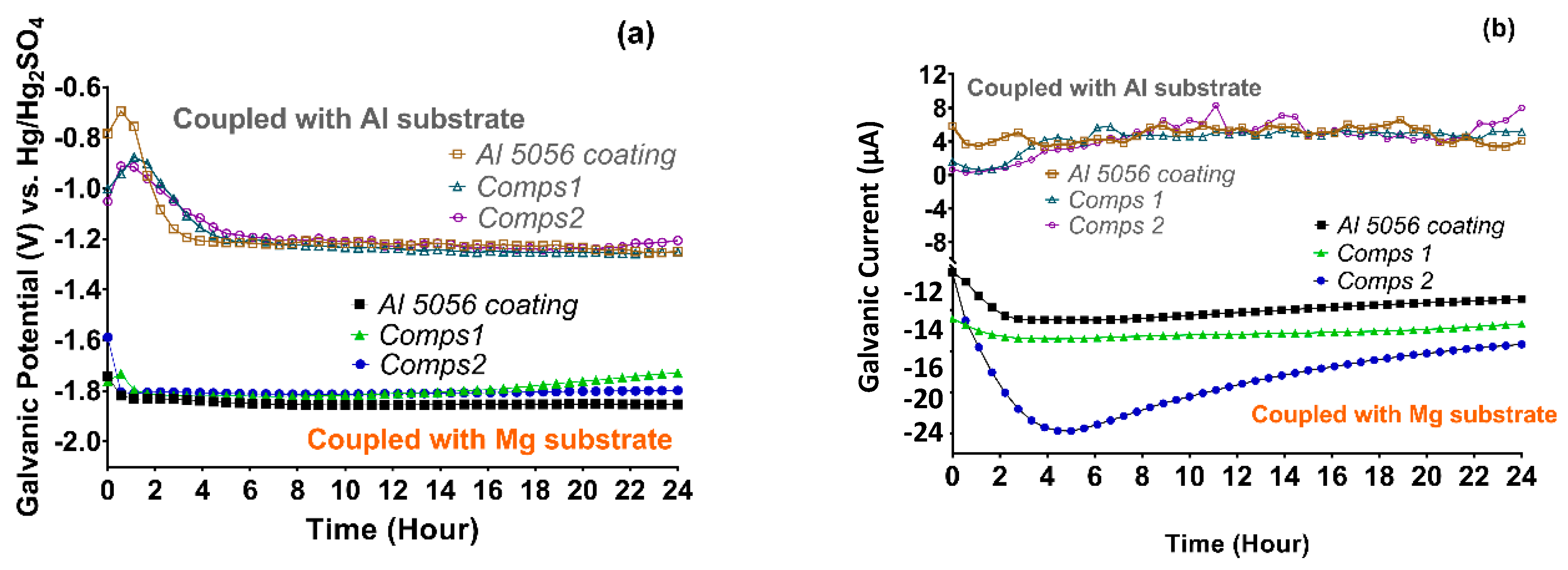
3.2. Corrosion Behaviour
3.3. Thermal Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Luo, A.A. Applications: Aerospace, Automotive and other Structural Applications of Magnesium. In Fundamentals of Magnesium Alloy Metallurgy; Pekguleryuz, M.O., Kainer, K.U., Kaya, A.A., Eds.; Woodhead Publishing: Cambridge, UK, 2013; pp. 266–316. [Google Scholar]
- Westengen, H.; Rashed, H.M.M.A. Magnesium Alloys: Properties and Applications. In Reference Module in Materials Science and Materials Engineering; Hashmi, S., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 1–9. [Google Scholar]
- Westengen, W. Magnesium: Alloying. In Encyclopedia of Materials: Science and Technology; Buschow, K.H.J., Cahn, R.W., Fleming, M., Ilschner, B., Kramer, E., Mahajan, S., Veyssière, P., Eds.; Elsevier: Amsterdam, NY, USA, 2008; pp. 4739–4743. [Google Scholar]
- Cole, G.S. Magnesium (Mg) Corrosion Protection Techniques in the Automotive Industry. In Corrosion Prevention of Magnesium Alloys; Song, G.-L., Ed.; Woodhead Publishing: Cambridge, UK, 2013; pp. 489–508. [Google Scholar]
- García-Rodríguez, S.; López, A.J.; Torres, B.; Rams, J. 316L stainless steel coatings on ZE41 magnesium alloy using HVOF thermal spray for corrosion protection. Surf. Coat. Technol. 2016, 287, 9–19. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Hamzah, E.; Bagheriyan, S.; Daroonparvar, M.; Kasiri-Asgarani, M.; Shah, A.M.; Medraj, M. Preparation and performance of plasma/polymer composite coatings on magnesium alloy. J. Mater. Eng. Perform. 2016, 25, 3948–3959. [Google Scholar] [CrossRef]
- Narayanan, T.S.S.; Lee, M.H. Incorporation of ZrO2 particles in the oxide layer formed on Mg by anodizing: Influence of electrolyte concentration and current modes. J. Colloid Interface Sci. 2016, 464, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Si, Y.; Xiong, Z.; Zheng, X.; Li, M.; Yang, Q. Improving the anti-corrosion ability of anodization film of AZ31B magnesium alloy by addition of NH4VO3 in the electrolyte. Int. J. Electrochem. Sci. 2016, 11, 3261–3268. [Google Scholar] [CrossRef]
- Daroonparvar, M.; Yajid, M.A.M.; Yusof, N.M.; Bakhsheshi-Rad, H.R. Preparation and corrosion resistance of a nanocomposite plasma electrolytic oxidation coating on Mg-1%Ca alloy formed in aluminate electrolyte containing titania nano-additives. J. Alloys Compd. 2016, 688, 841–857. [Google Scholar] [CrossRef]
- Vatan, H.N.; Ebrahimi-Kahrizsangi, R.; Kasiri-Asgarani, M. Structural, tribological and electrochemical behavior of SiC nanocomposite oxide coatings fabricated by plasma electrolytic oxidation (PEO) on AZ31 magnesium alloy. J. Alloys Compd. 2016, 683, 241–255. [Google Scholar] [CrossRef]
- Fan, L.; Xu, C.; Yuekun, G.; Chao, M.; Xiaopeng, H.; Yida, D.; Wenbin, H.; Zhong, C. Effect of pretreatment and annealing on aluminum coating prepared by physical vapor deposition on AZ91D magnesium alloys. Int. J. Electrochem. Sci. 2016, 11, 5655–5668. [Google Scholar] [CrossRef]
- Śliwa, A.; Mikuła, J.; Gołombek, K.; Tański, T.; Kwaśny, W.; Bonek, M.; Brytan, Z. Prediction of the properties of PVD/CVD coatings with the use of FEM analysis. Appl. Surf. Sci. 2016, 388, 281–287. [Google Scholar] [CrossRef]
- Xu, R.; Yang, X.; Zhang, X.; Wang, M.; Li, P.; Zhao, Y.; Wu, G.; Chu, P.K. Effects of carbon dioxide plasma immersion ion implantation on the electrochemical properties of AZ31 magnesium alloy in physiological environment. Appl. Surf. Sci. 2013, 286, 257–260. [Google Scholar] [CrossRef]
- Jamesh, M.I.; Wu, G.; Zhao, Y.; McKenzie, D.R.; Bilek, M.M.M.; Chu, P.K. Effects of zirconium and oxygen plasma ion implantation on the corrosion behavior of ZK60 Mg alloy in simulated body fluids. Corros. Sci. 2014, 82, 7–26. [Google Scholar] [CrossRef]
- Melia, M.A.; Steiner, P.; Birbilis, N.; Fitz-Gerald, J.M.; Scully, J.R. Excimer laser surface modification of AZ31B-H24 for improved corrosion resistance. Corrosion 2015, 72, 95–109. [Google Scholar] [CrossRef]
- Liu, F.; Ji, Y.; Meng, Q.; Li, Z. Microstructure and corrosion resistance of laser cladding and friction stir processing hybrid modification Al-Si coatings on AZ31B. Vacuum 2016, 133, 31–37. [Google Scholar] [CrossRef]
- Li, W.; Liao, H.; Wang, H. Cold Spraying of Light Alloys. In Surface Engineering of Light Alloys; Dong, H., Ed.; Woodhead Publishing: Cambridge, UK, 2010; pp. 242–293. [Google Scholar]
- Diab, M.; Pang, X.; Jahed, H. The effect of pure aluminum cold spray coating on corrosion and corrosion fatigue of magnesium (3% Al-1% Zn) extrusion. Surf. Coat. Technol. 2017, 309, 423–435. [Google Scholar] [CrossRef]
- Tao, Y.; Xiong, T.; Sun, C.; Jin, H.; Du, H.; Li, T. Effect of α-Al2O3 on the properties of cold sprayed Al/α-Al2O3 composite coatings on AZ91D magnesium alloy. Appl. Surf. Sci. 2009, 256, 261–266. [Google Scholar] [CrossRef]
- Shayegan, G.; Mahmoudi, H.; Ghelichi, R.; Villafuerte, J.; Wang, J.; Guagliano, M.; Jahed, H. Residual stress induced by cold spray coating of magnesium AZ31B extrusion. Mater. Des. 2014, 60, 72–84. [Google Scholar] [CrossRef]
- Ziemian, C.W.; Sharma, M.M.; Bouffard, B.D.; Nissley, T.; Eden, T.J. Effect of substrate surface roughening and cold spray coating on the fatigue life of AA2024 specimens. Mater. Des. 2014, 54, 212–221. [Google Scholar] [CrossRef]
- Jones, R.; Matthews, N.; Rodopoulos, C.A.; Cairns, K.; Pitt, S. On the use of supersonic particle deposition to restore the structural integrity of damaged aircraft structures. Int. J. Fatigue 2011, 33, 1257–1267. [Google Scholar] [CrossRef]
- Wu, J.; Fang, H.; Yoon, S.; Kim, H.; Lee, C. The rebound phenomenon in kinetic spraying deposition. Scr. Mater. 2006, 54, 665–669. [Google Scholar] [CrossRef]
- Papyrin, A. Cold spray technology. Adv. Mater Proc. 2001, 159, 49–51. [Google Scholar]
- Bala, N.; Singh, H.; Prakash, S. Accelerated hot corrosion studies of cold spray Ni–50Cr coating on boiler steels. Mater. Des. 2010, 31, 244–253. [Google Scholar] [CrossRef]
- Yao, J.; Yang, L.; Li, B.; Li, Z. Characteristics and performance of hard Ni60 alloy coating produced with supersonic laser deposition technique. Mater. Des. 2015, 83, 26–35. [Google Scholar] [CrossRef]
- Wang, Y.; Normand, B.; Mary, N.; Yu, M.; Yao, H. Contribution à l’optimisation de la résistance à la corrosion de revêtements d’alliage d’aluminium élaborés par projection dynamique à froid (cold spray). Ann. Chim. Sci. Matér. 2013, 37, 1–12. [Google Scholar] [CrossRef]
- Wang, Y.; Normand, B.; Mary, N.; Yu, M.; Liao, H. Microstructure and corrosion behavior of cold sprayed SiCp/Al 5056 composite coatings. Surf. Coat. Technol. 2014, 251, 264–275. [Google Scholar] [CrossRef]
- Yu, M.; Li, W.Y.; Suo, X.K.; Liao, H.L. Effects of gas temperature and ceramic particle content on microstructure and microhardness of cold sprayed SiCp/Al 5056 composite coatings. Surf. Coat. Technol. 2013, 220, 102–106. [Google Scholar] [CrossRef]
- Wang, Y.; Normand, B.; Mary, N.; Yu, M.; Liao, H. Effects of ceramic particle size on microstructure and the corrosion behavior of cold sprayed SiCp/Al 5056 composite coatings. Surf. Coat. Technol. 2017, 315, 314–325. [Google Scholar] [CrossRef]
- Normand, B.; Herbin, W.; Landemarre, O.; Coddet, C.; Pagetti, J. Electrochemical methods to the evaluation of thermal spray coatings corrosion resistance. Mater. Sci. Forum 1998, 289, 607–612. [Google Scholar] [CrossRef]
- Schütze, M. Corrosion and Environmental Degradation; Wiley-VCH: Weinheim, Germany, 2000. [Google Scholar]
- Marcelin, S.; Ter-Ovanessian, B.; Normand, B. Electronic properties of passive films from the multi-frequency mott–schottky and power-law coupled approach. Electrochem. Commun. 2016, 66, 62–65. [Google Scholar] [CrossRef]
- Noorakma, A.C.W.; Zuhailawati, H.; Aishvarya, V.; Dhindaw, B.K. Hydroxyapatite-coated magnesium-based biodegradable alloy: Cold spray deposition and simulated body fluid studies. J. Mater. Eng. Perform. 2013, 22, 2997–3004. [Google Scholar] [CrossRef]
- Chen, J.; Wang, J.; Han, E.; Dong, J.; Ke, W. AC impedance spectroscopy study of the corrosion behavior of an AZ91 magnesium alloy in 0.1M sodium sulfate solution. Electrochim. Acta 2007, 52, 3299–3309. [Google Scholar] [CrossRef]
- Nakatsugawa, I.; Martin, R.; Knystautas, E. Improving corrosion resistance of AZ91D magnesium alloy by nitrogen ion implantation. Corrosion 1996, 52, 921–926. [Google Scholar] [CrossRef]
- Qiao, Z.; Shi, Z.; Hort, N.; Abidin, N.I.Z.; Atrens, A. Corrosion behaviour of a nominally high purity Mg ingot produced by permanent mould direct chill casting. Corros. Sci. 2012, 61, 185–207. [Google Scholar] [CrossRef]
- Funamizu, Y.; Watanabe, K. Interdiffusion in the Al–Mg system. Trans. Jpn. Inst. Met. 1972, 13, 278–283. [Google Scholar] [CrossRef]
- Shaha, S.K.; Dayani, S.B.; Jahed, H. Fatigue Life Enhancement of Cast Mg Alloy by Surface Modification in Cold Spray Process. 12th International Fatigue Congress (Fatigue 2018). MATEC Web Conf. 2018, 165, 03014. [Google Scholar] [CrossRef][Green Version]
- Kanchidurai1, S.; Krishanan, P.A.; Baskar, K.; Saravana, K.R.M. A review of inducing compressive residual stress–shot peening; on structural metal and welded connection. IOP Conf. Ser. Earth Environ. Sci. 2017, 80, 012033. [Google Scholar] [CrossRef]


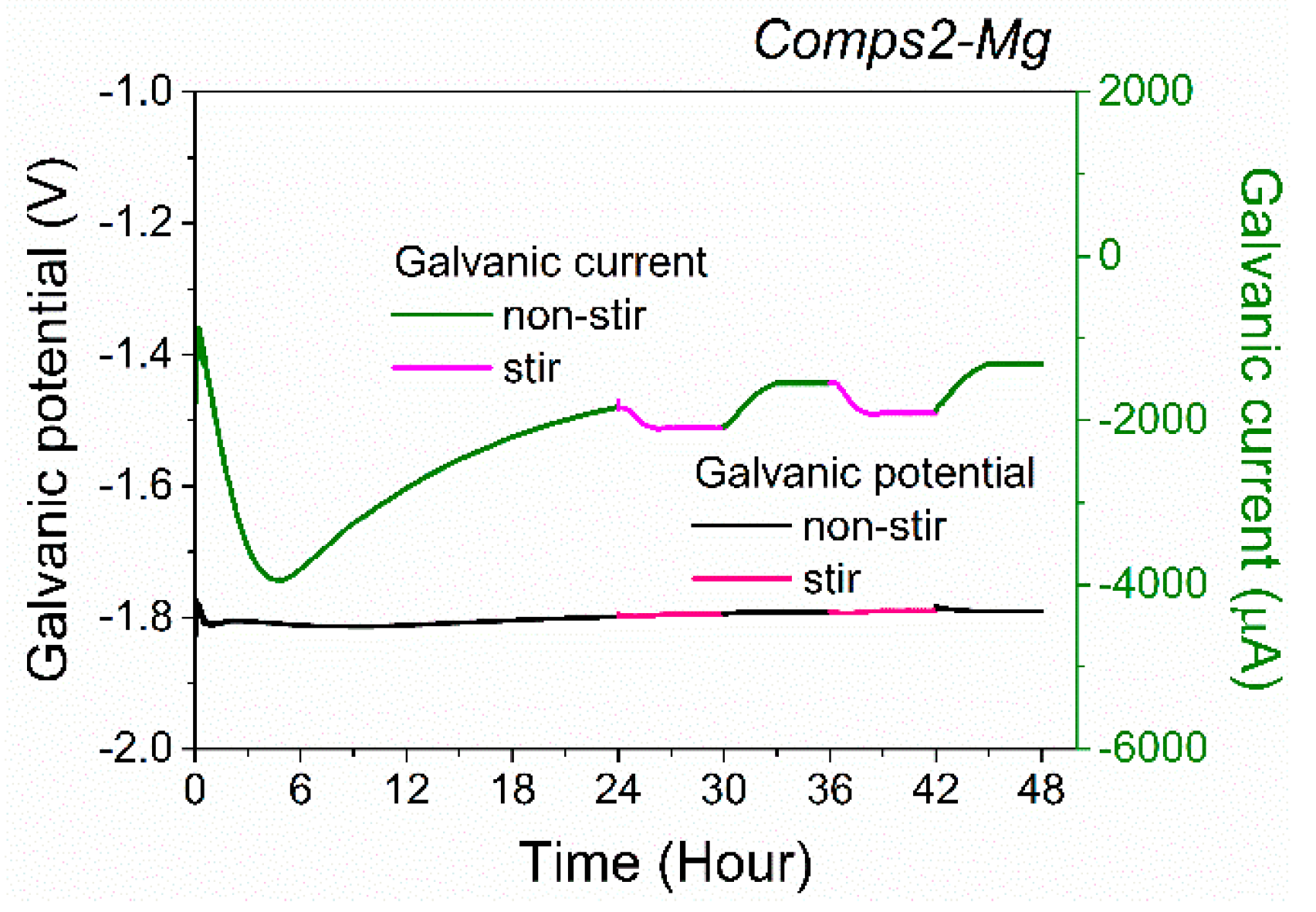
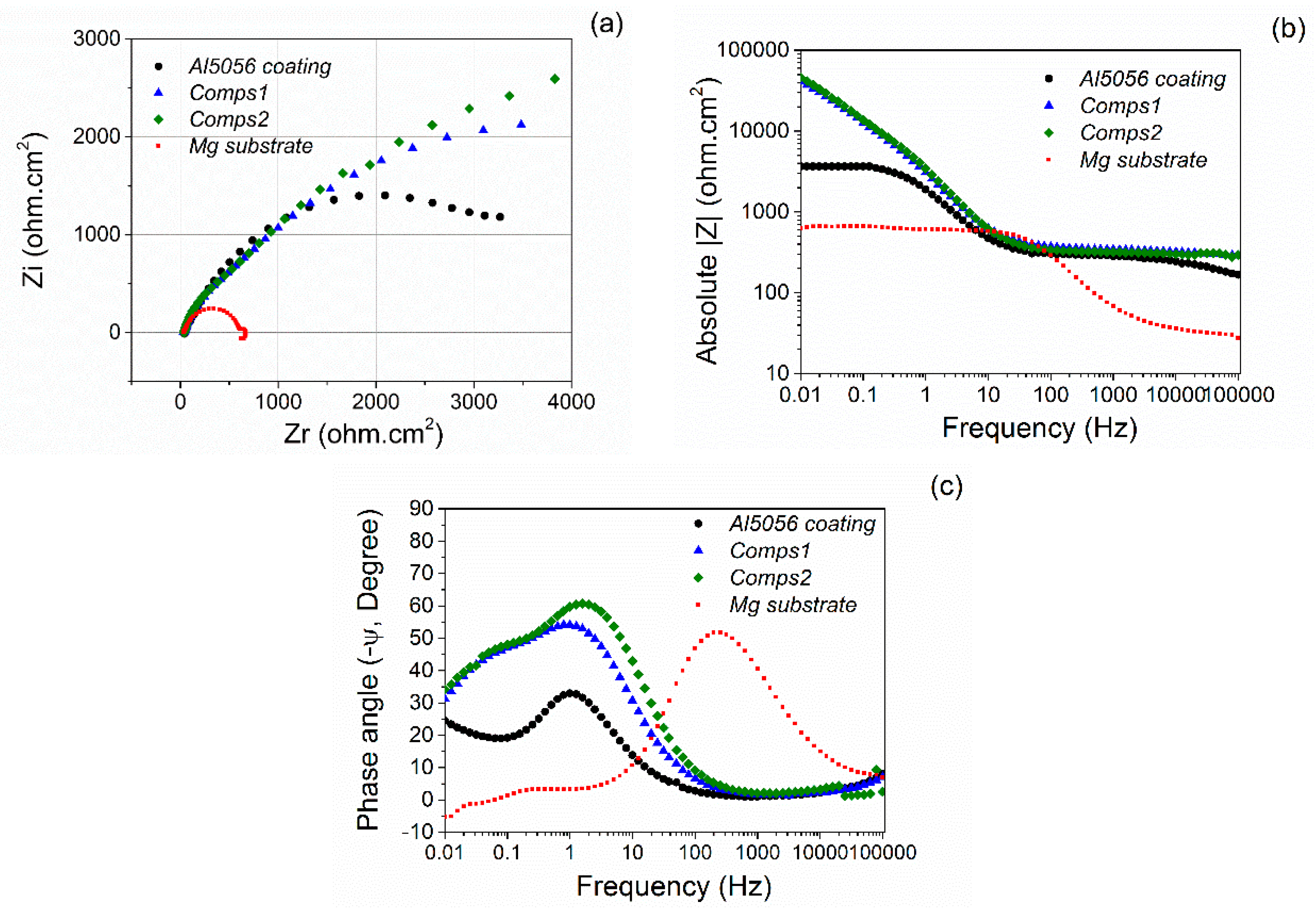
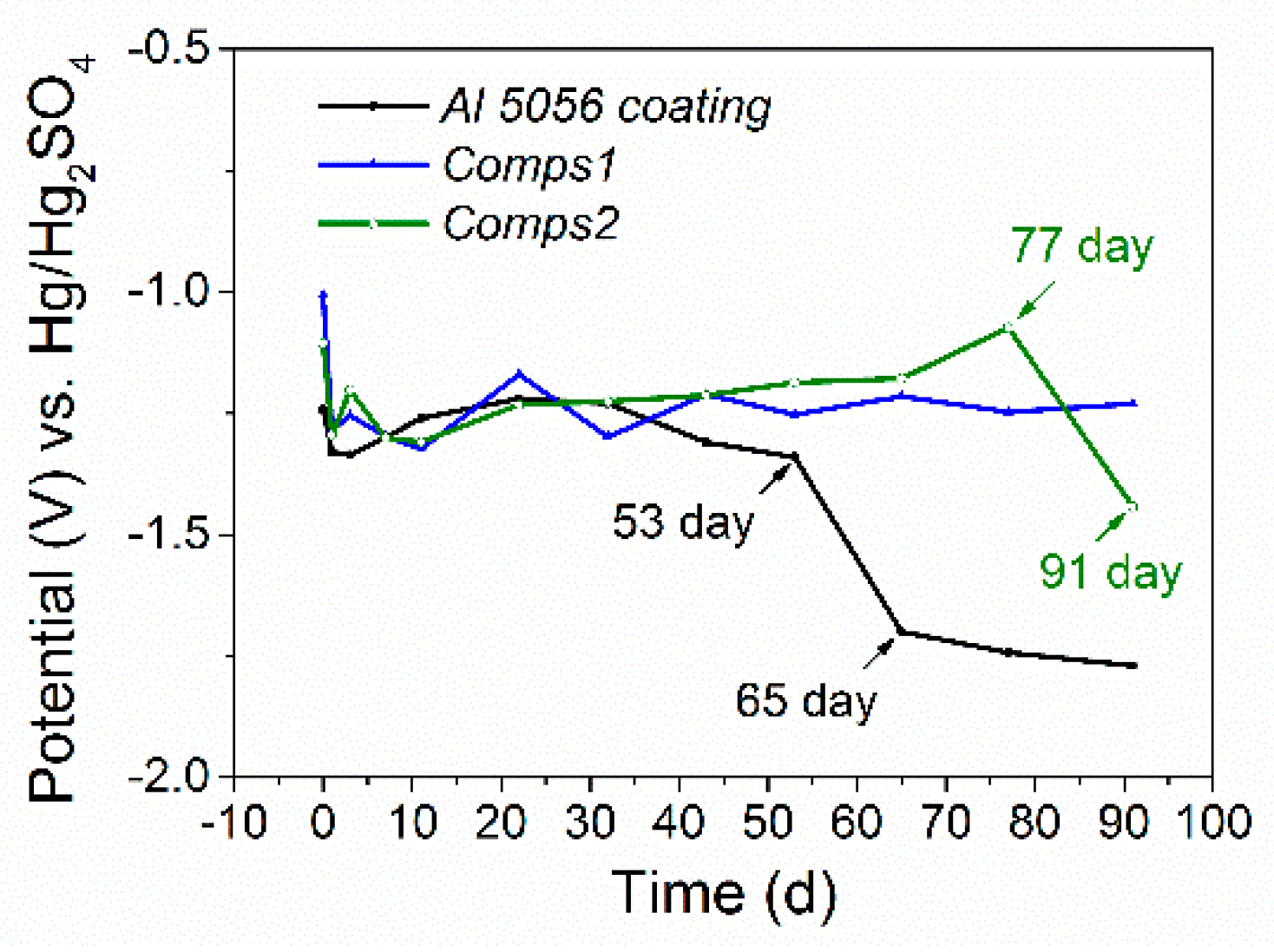
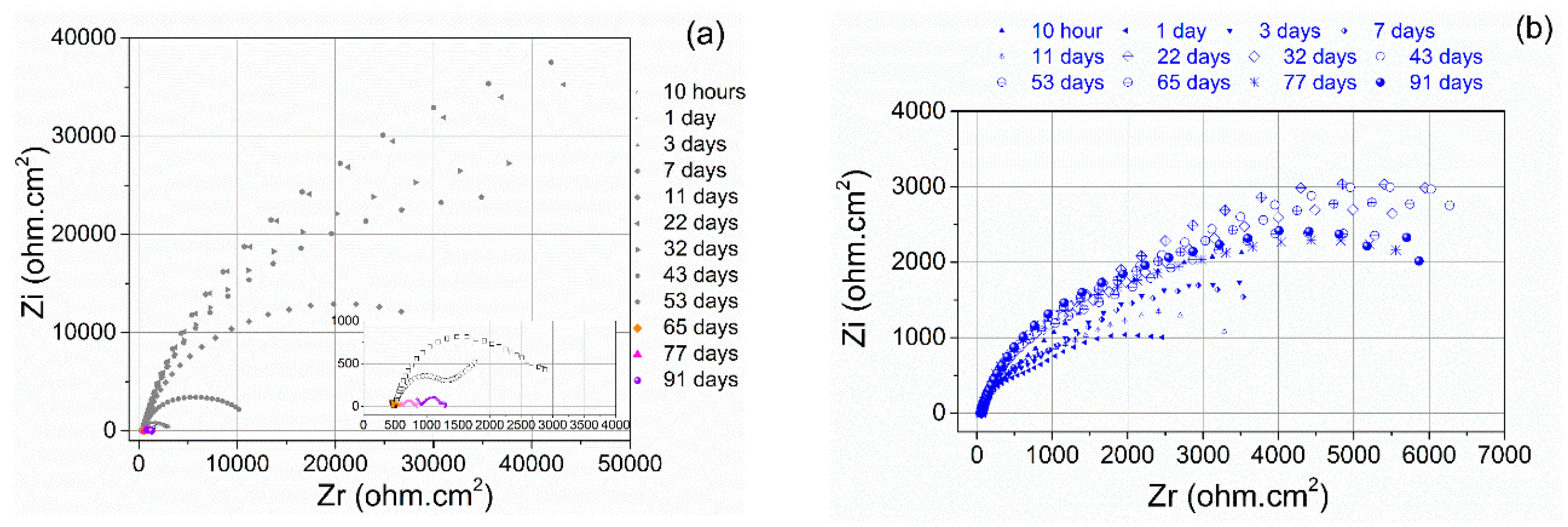
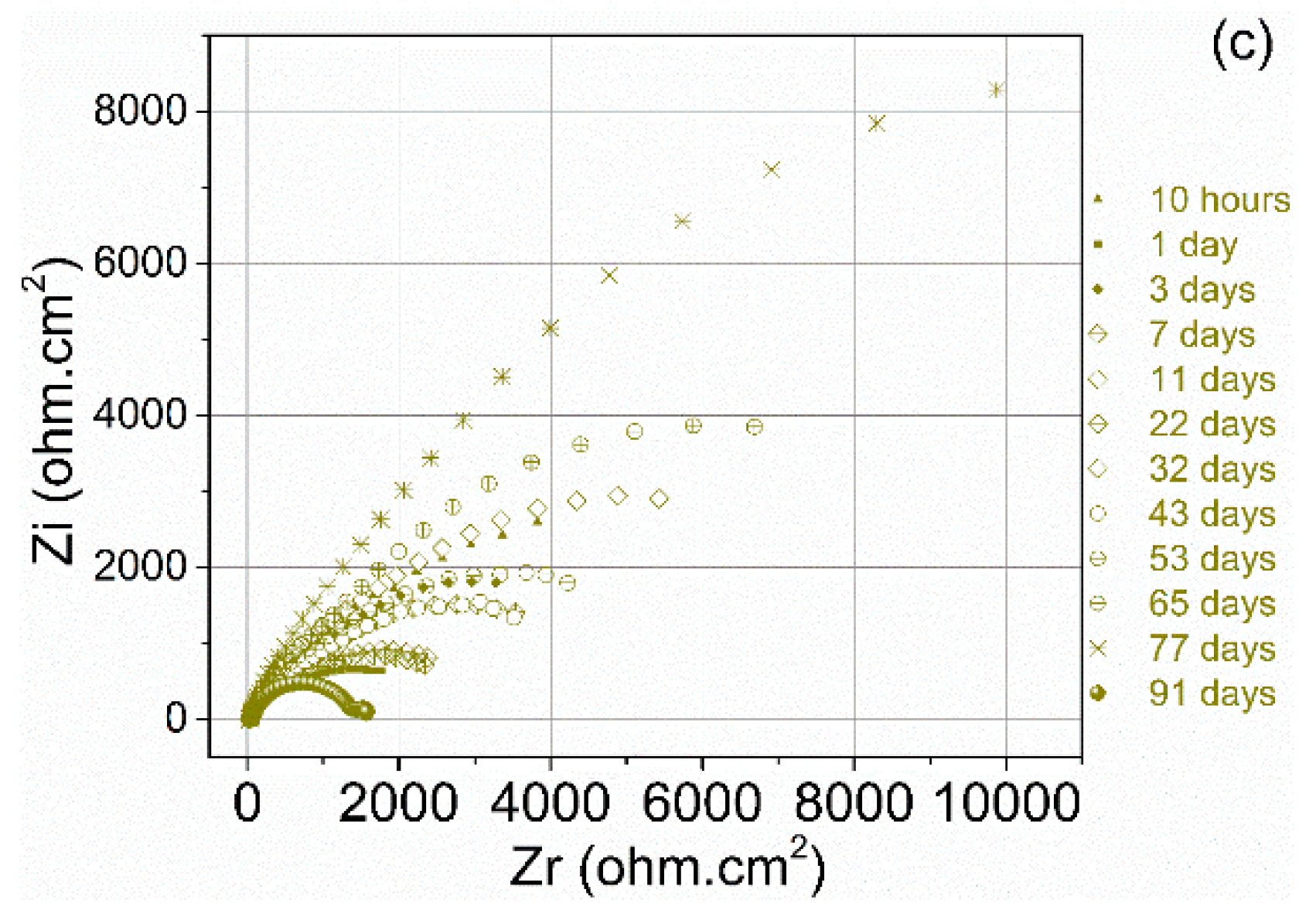
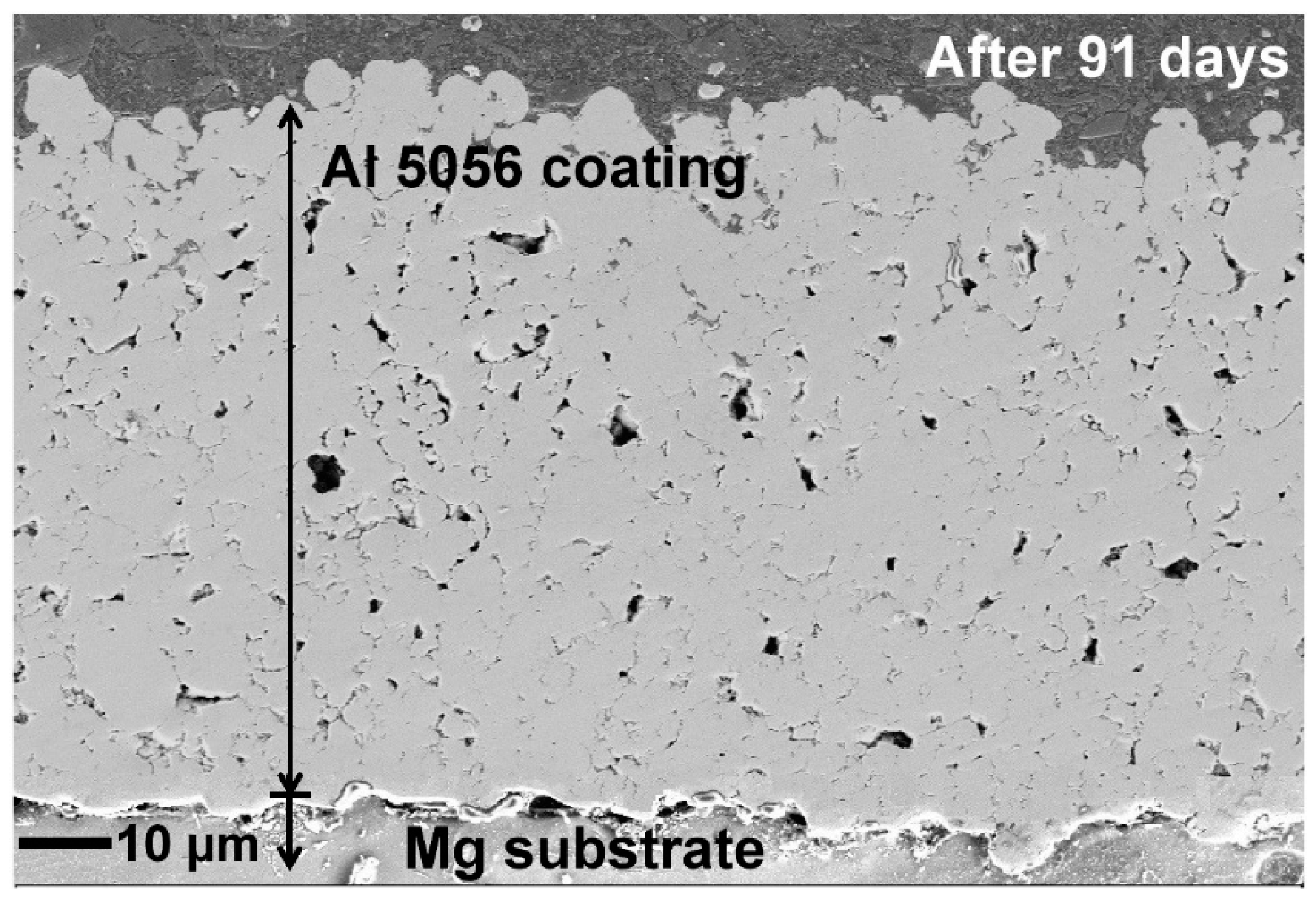
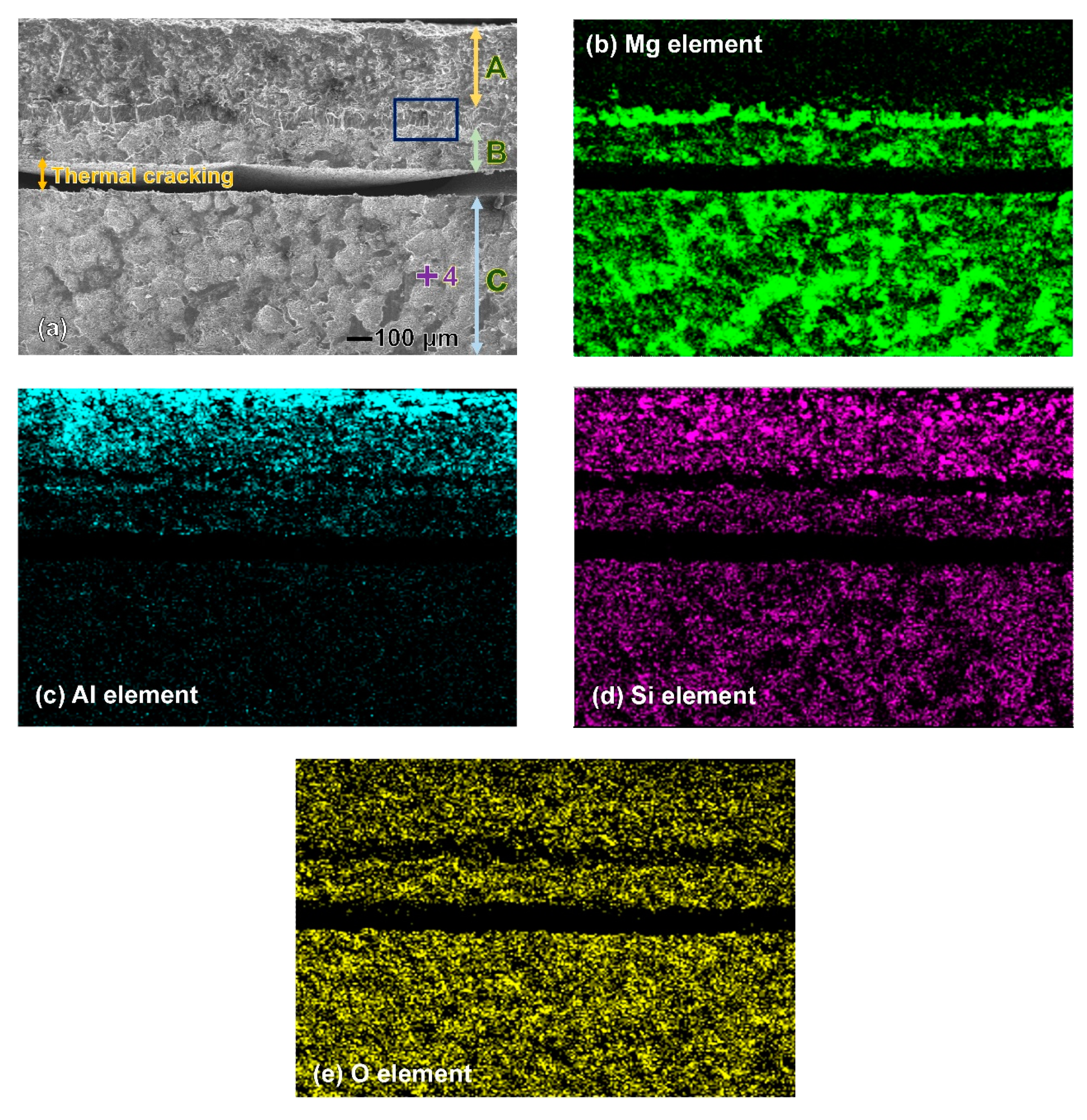



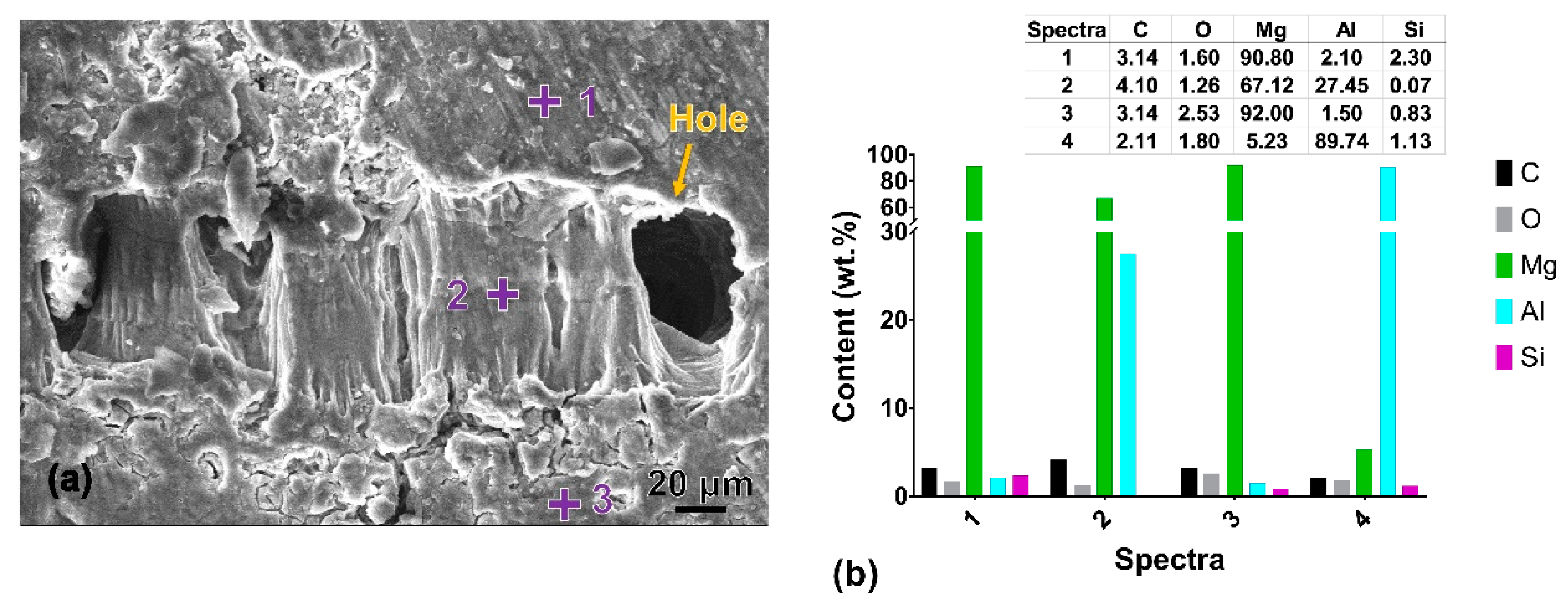
| Specimen | OCP (V) | Ecorr (V) | Egal (V) |
|---|---|---|---|
| Mg substrate | −1.87 | −1.83 | - |
| Al 5056 coating | −1.26 | −1.27 | −1.85 |
| Comps1 | −1.21 | −1.25 | −1.73 |
| Comps2 | −1.19 | −1.21 | −1.80 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Normand, B.; Liao, H.; Zhao, G.; Mary, N.; Tang, J. SiCp/Al5056 Composite Coatings Applied to A Magnesium Substrate by Cold Gas Dynamic Spray Method for Corrosion Protection. Coatings 2020, 10, 325. https://doi.org/10.3390/coatings10040325
Wang Y, Normand B, Liao H, Zhao G, Mary N, Tang J. SiCp/Al5056 Composite Coatings Applied to A Magnesium Substrate by Cold Gas Dynamic Spray Method for Corrosion Protection. Coatings. 2020; 10(4):325. https://doi.org/10.3390/coatings10040325
Chicago/Turabian StyleWang, Yingying, Bernard Normand, Hanlin Liao, Guofeng Zhao, Nicolas Mary, and Junlei Tang. 2020. "SiCp/Al5056 Composite Coatings Applied to A Magnesium Substrate by Cold Gas Dynamic Spray Method for Corrosion Protection" Coatings 10, no. 4: 325. https://doi.org/10.3390/coatings10040325
APA StyleWang, Y., Normand, B., Liao, H., Zhao, G., Mary, N., & Tang, J. (2020). SiCp/Al5056 Composite Coatings Applied to A Magnesium Substrate by Cold Gas Dynamic Spray Method for Corrosion Protection. Coatings, 10(4), 325. https://doi.org/10.3390/coatings10040325







