WC-Co and WC-Co-Cr Coatings for the Protection of API Pipeline Steel from Corrosion in 4% NaCl Solution
Abstract
:1. Introduction
2. Experimental Procedures
2.1. Materials and Chemicals
2.2. Electrochemical Cell
2.3. Electrochemical Measurements
3. Results and Discussion
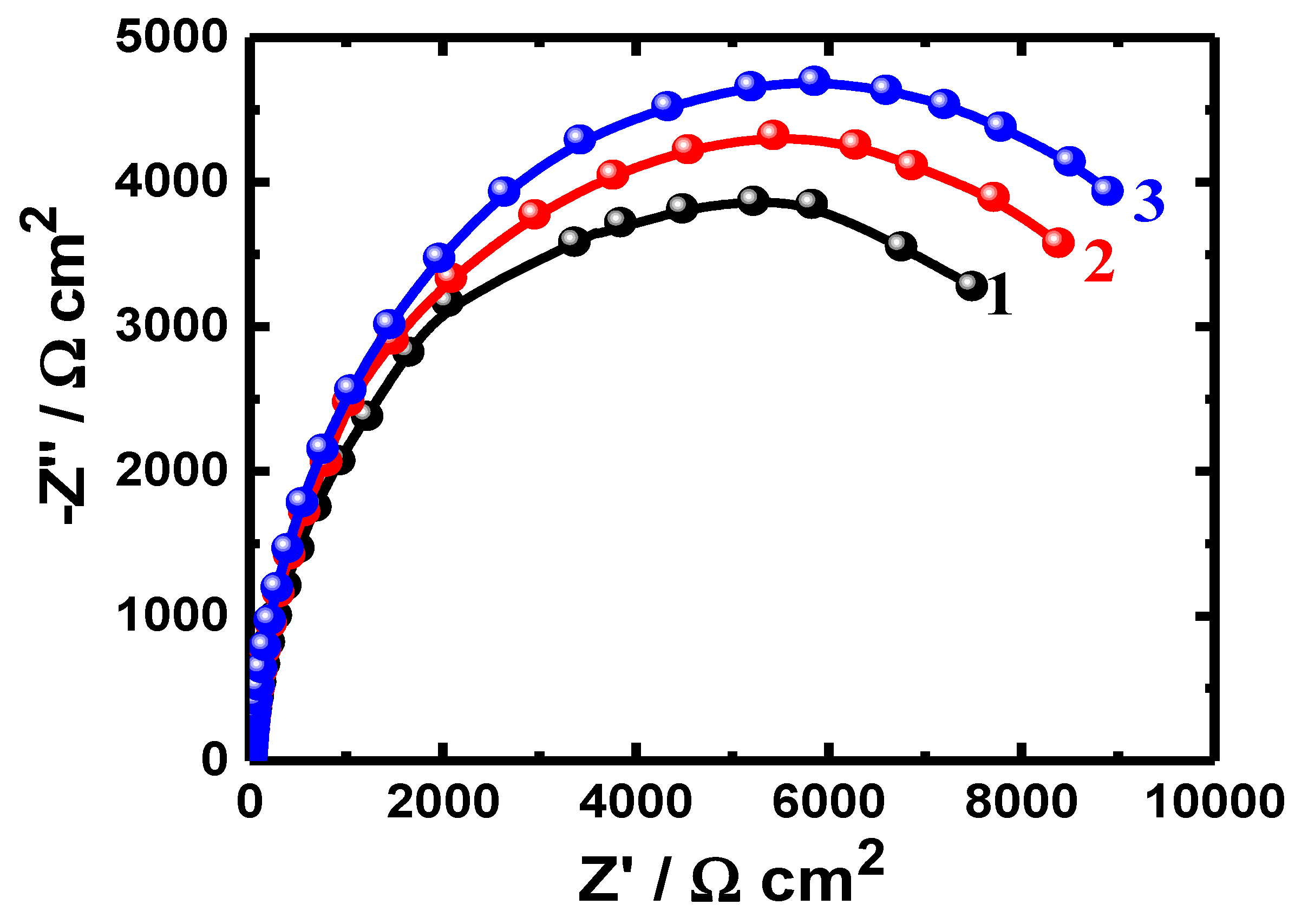
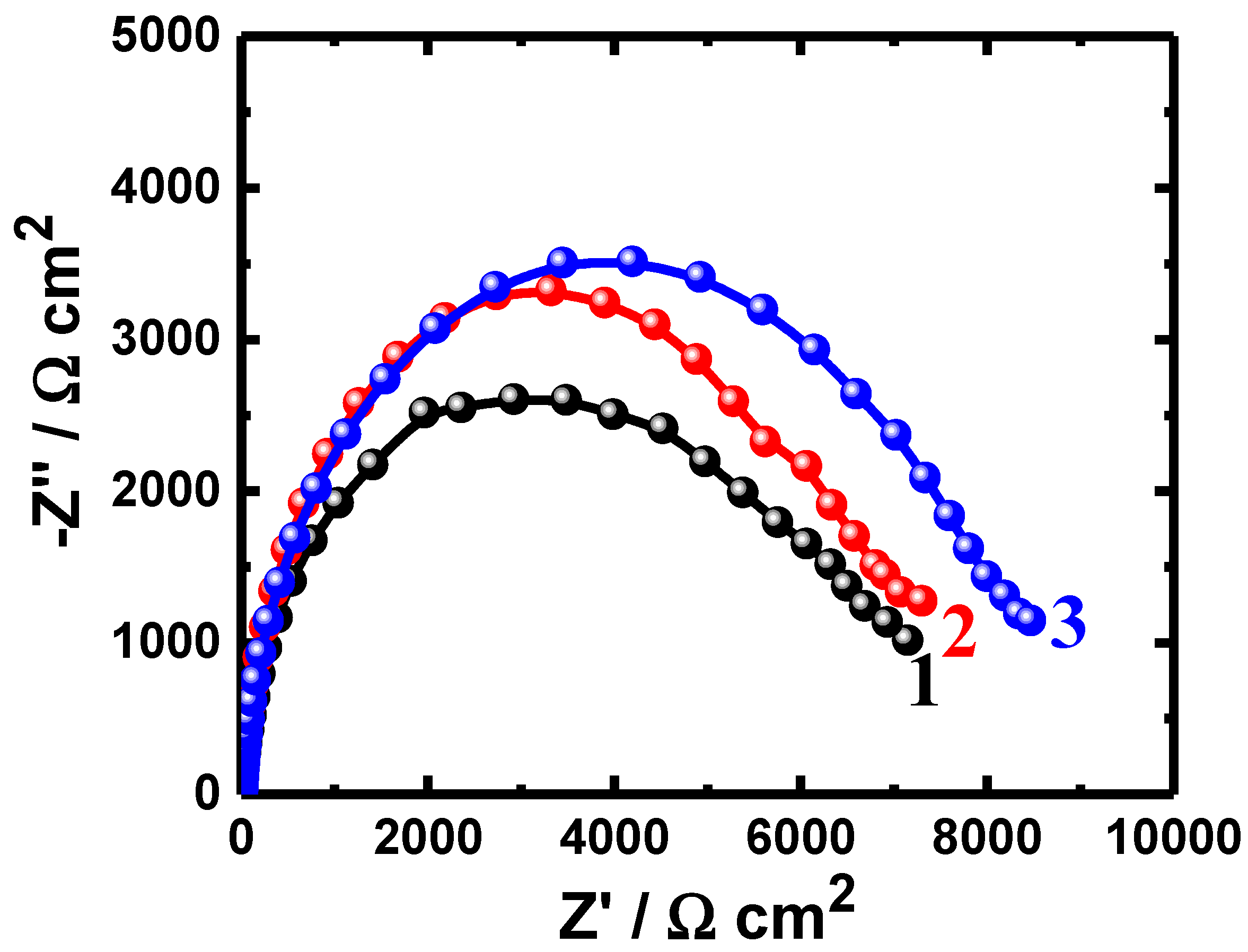
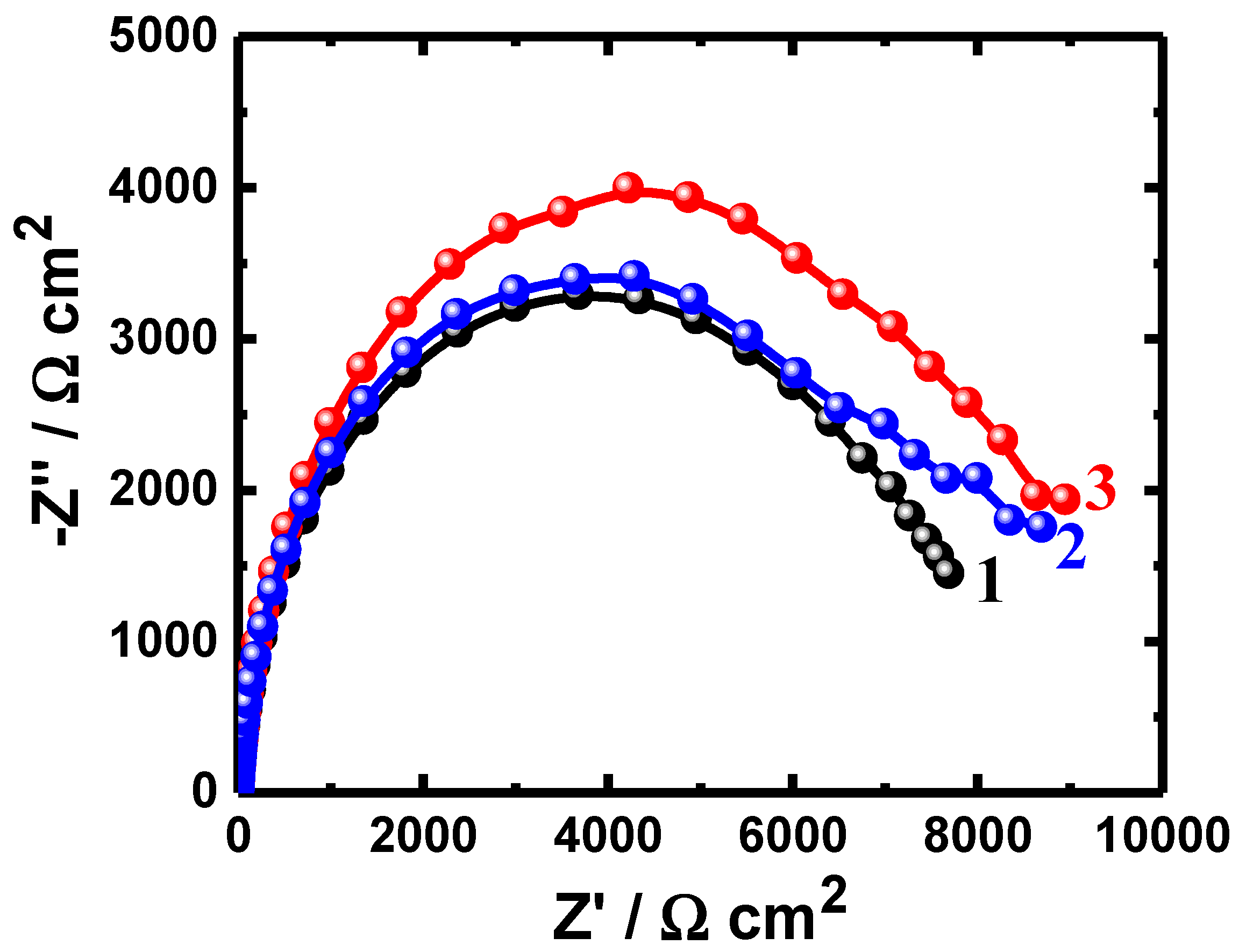
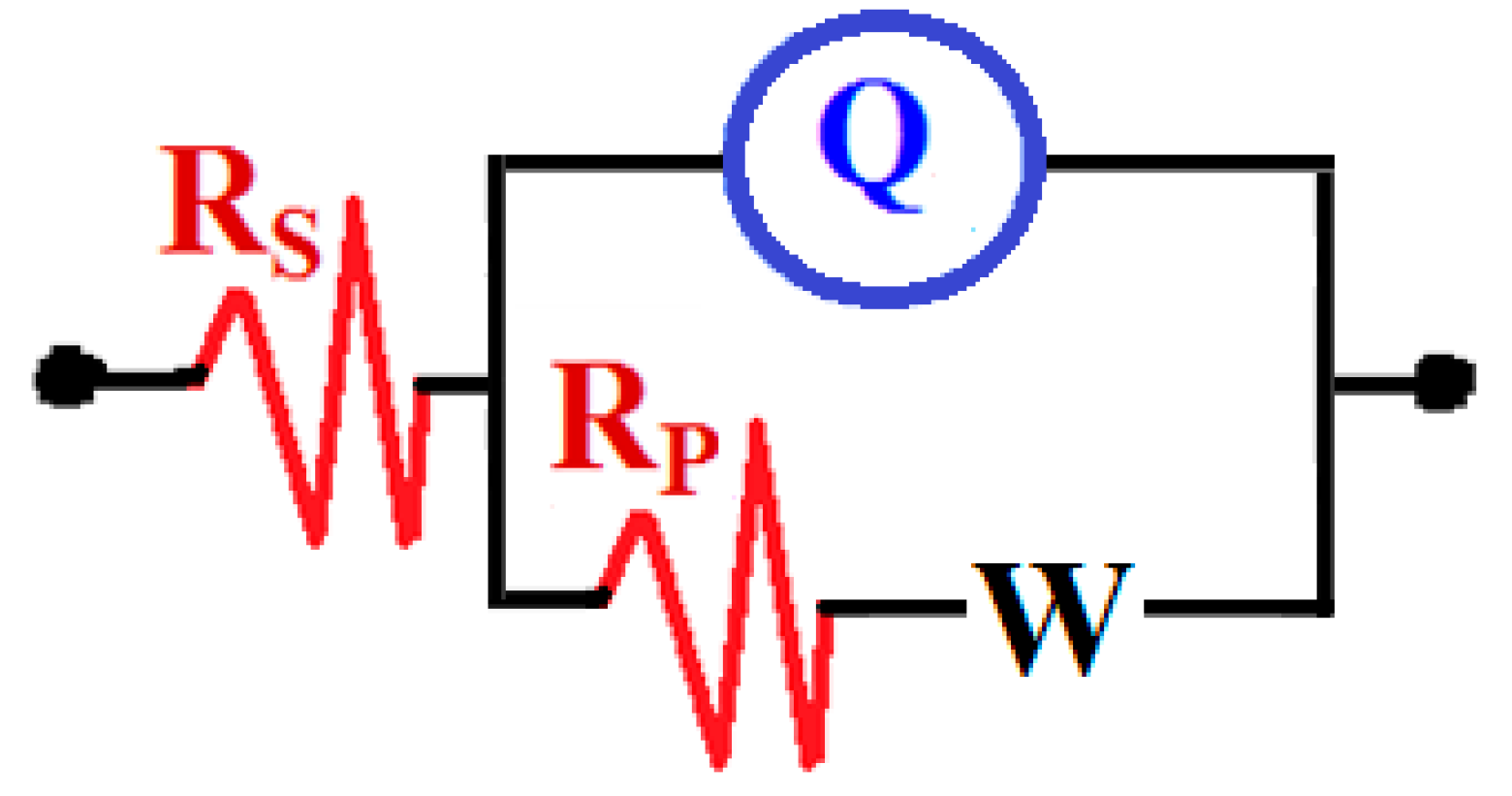
3.1. EIS Data
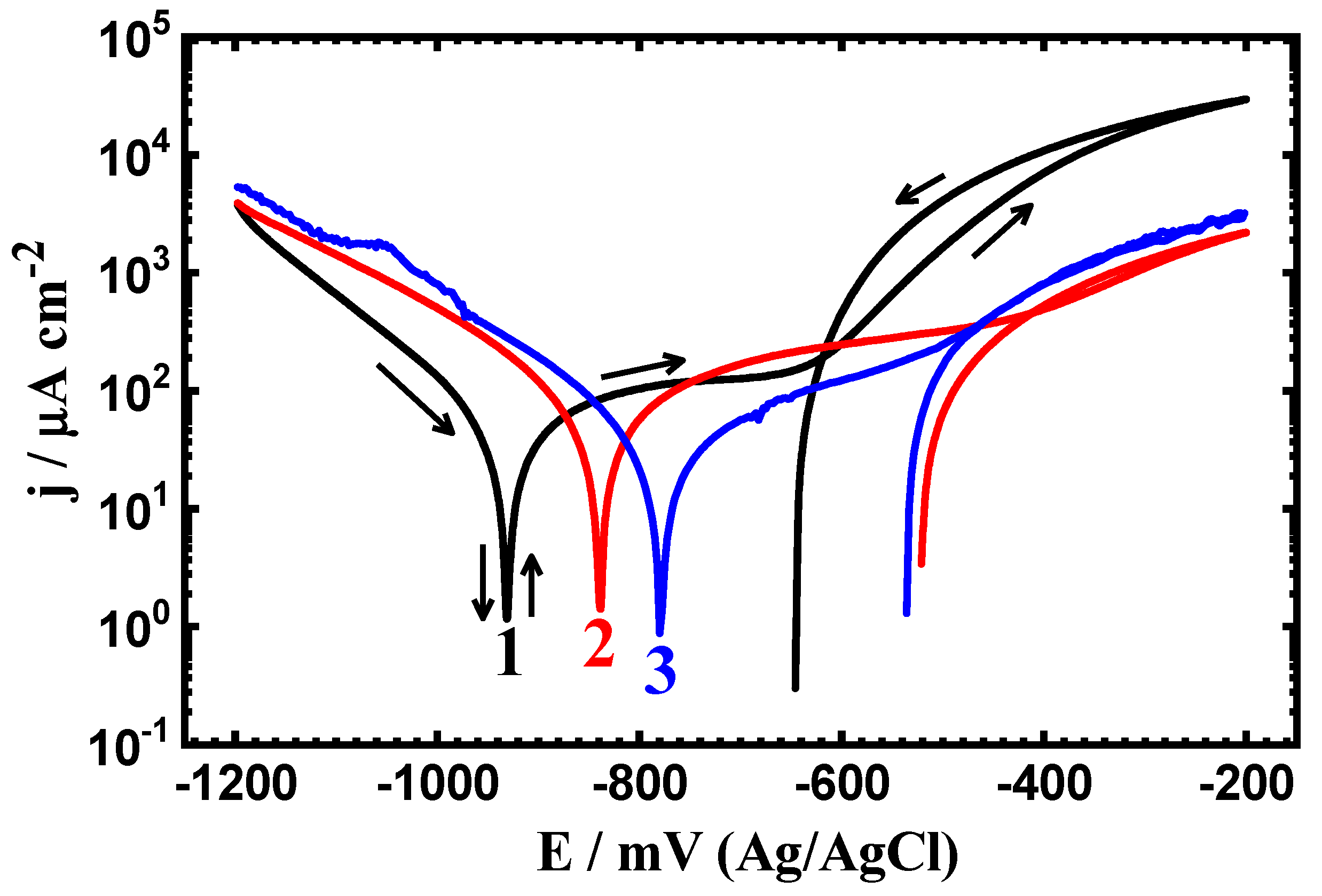
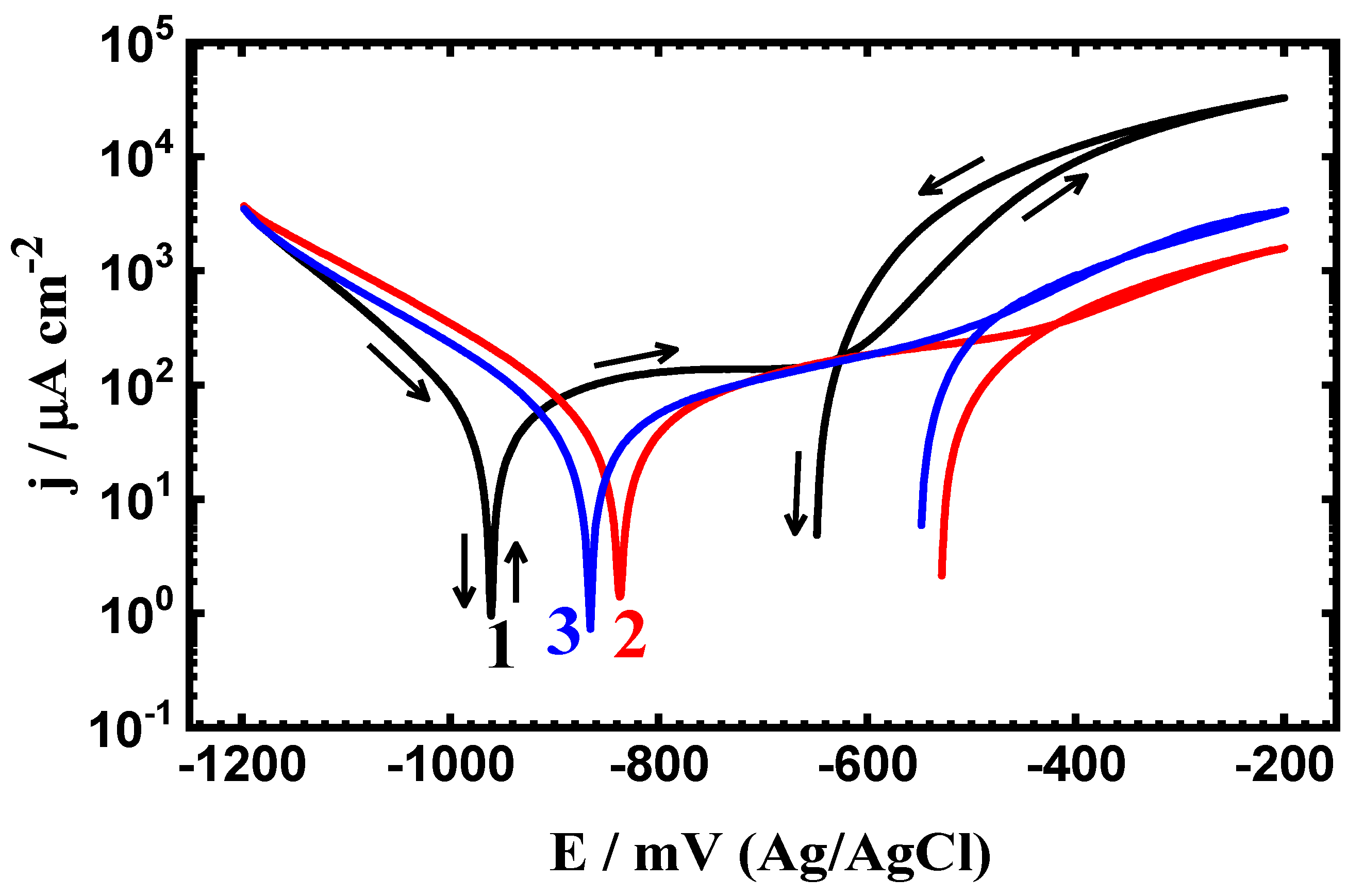
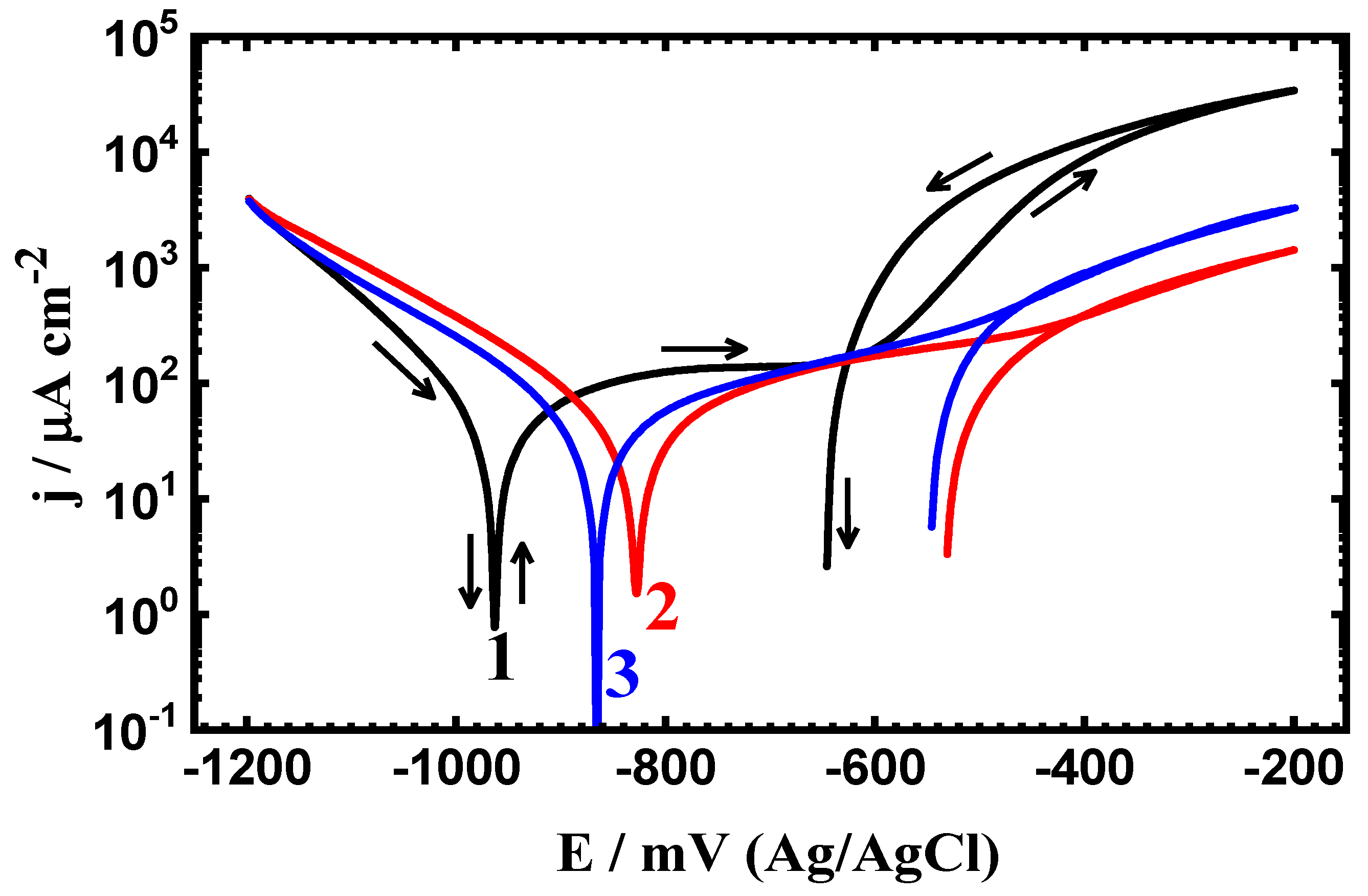
3.2. CPP Measurements
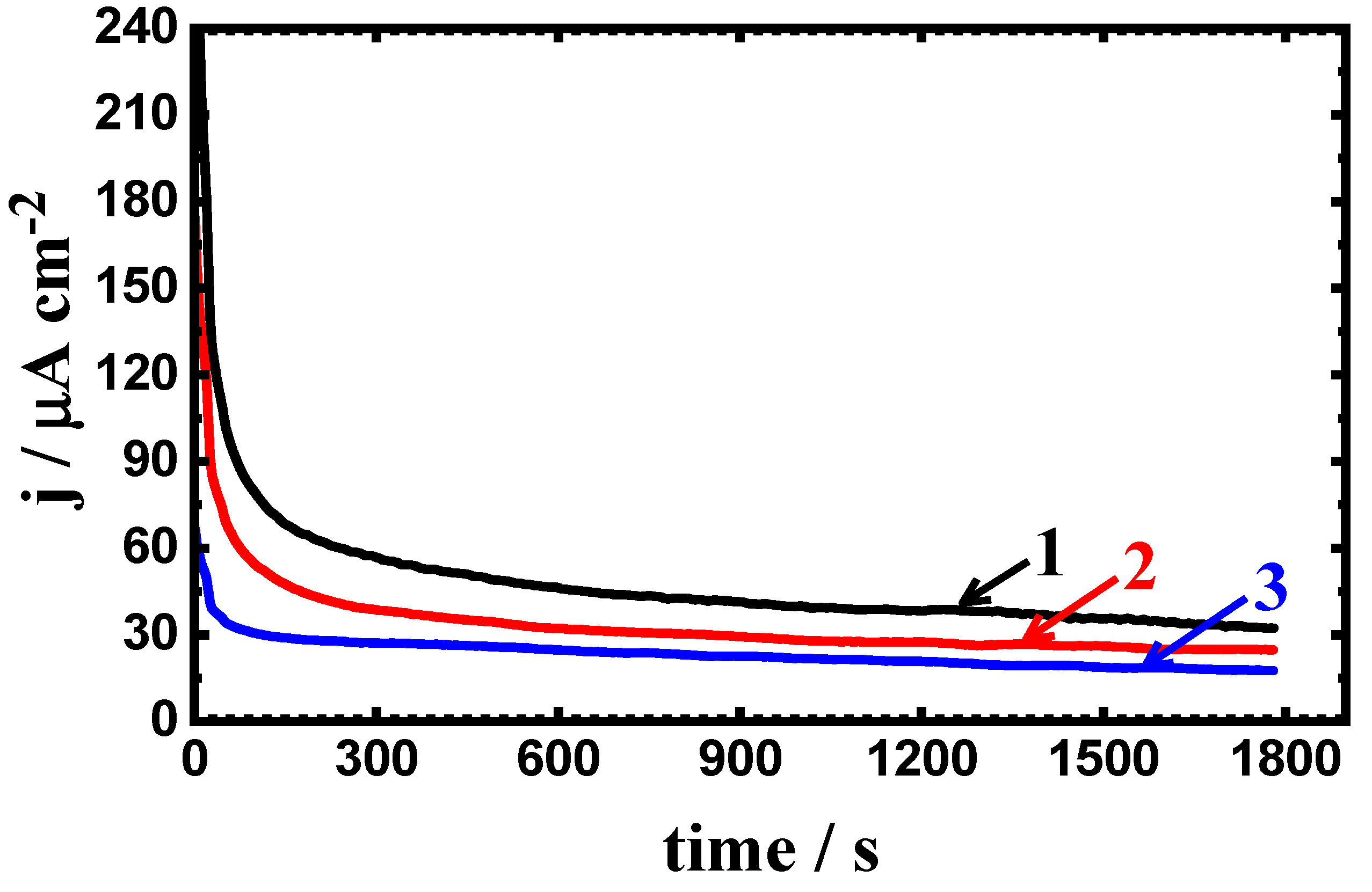
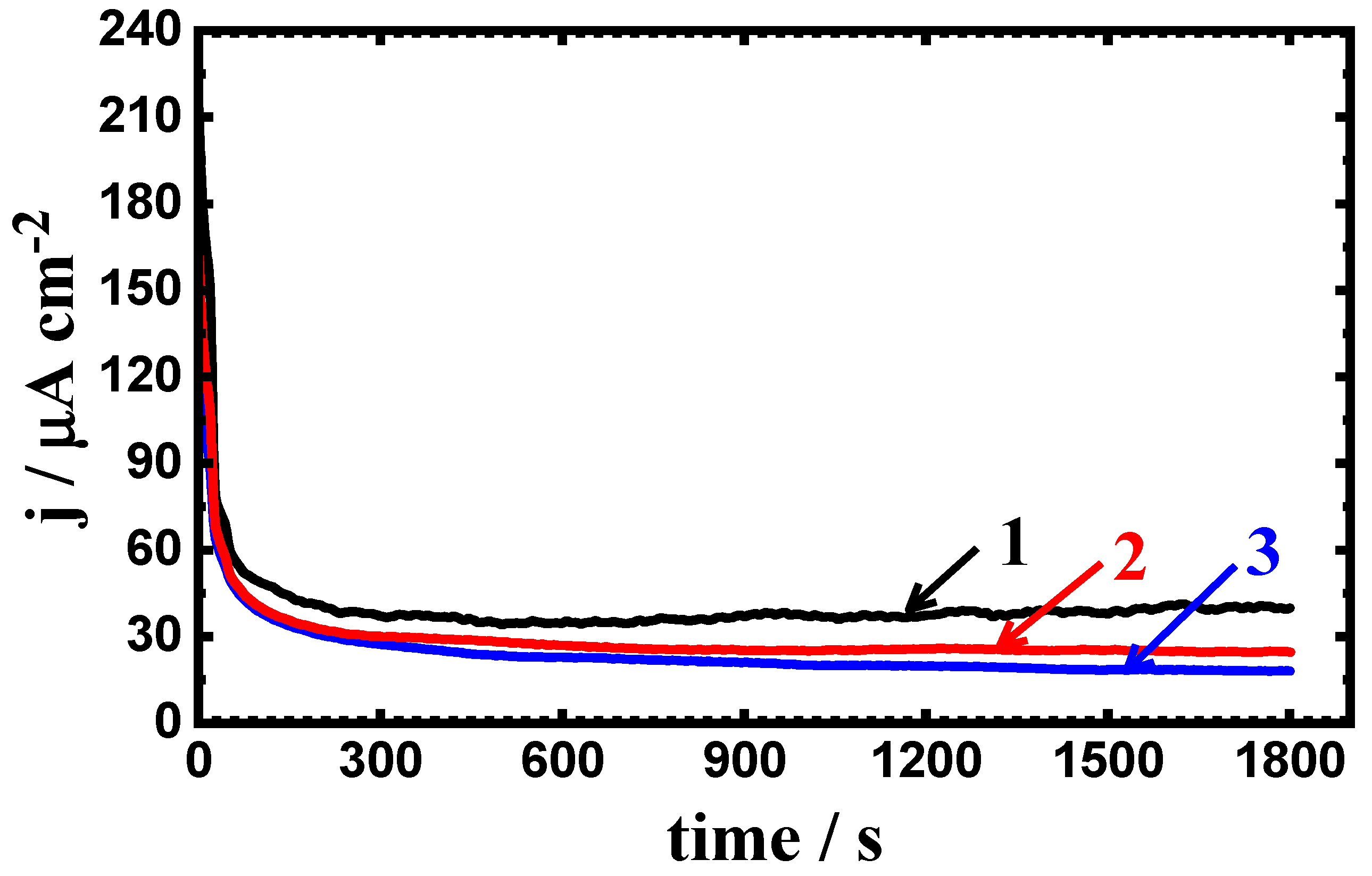
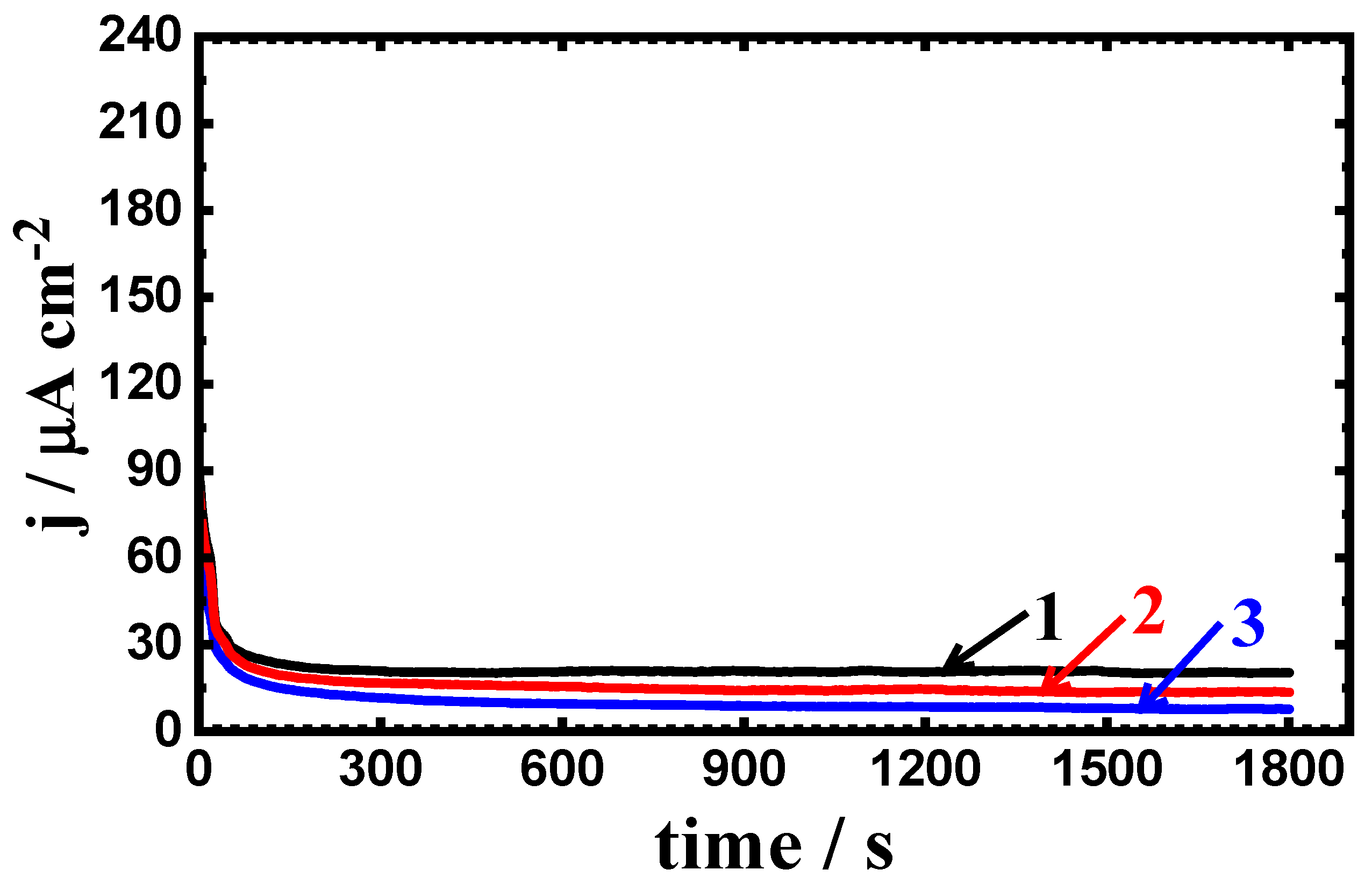
3.3. Potentiostatic Current–Time Experiments
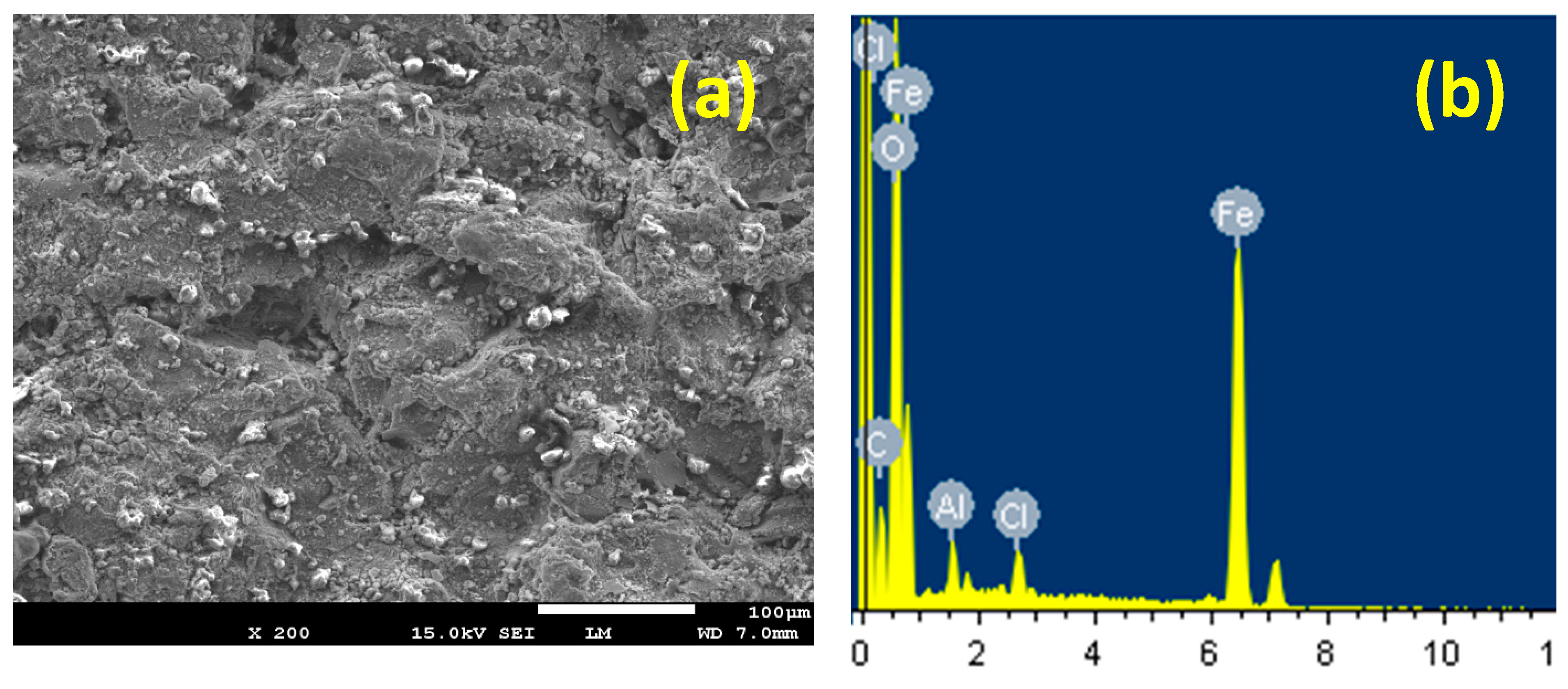
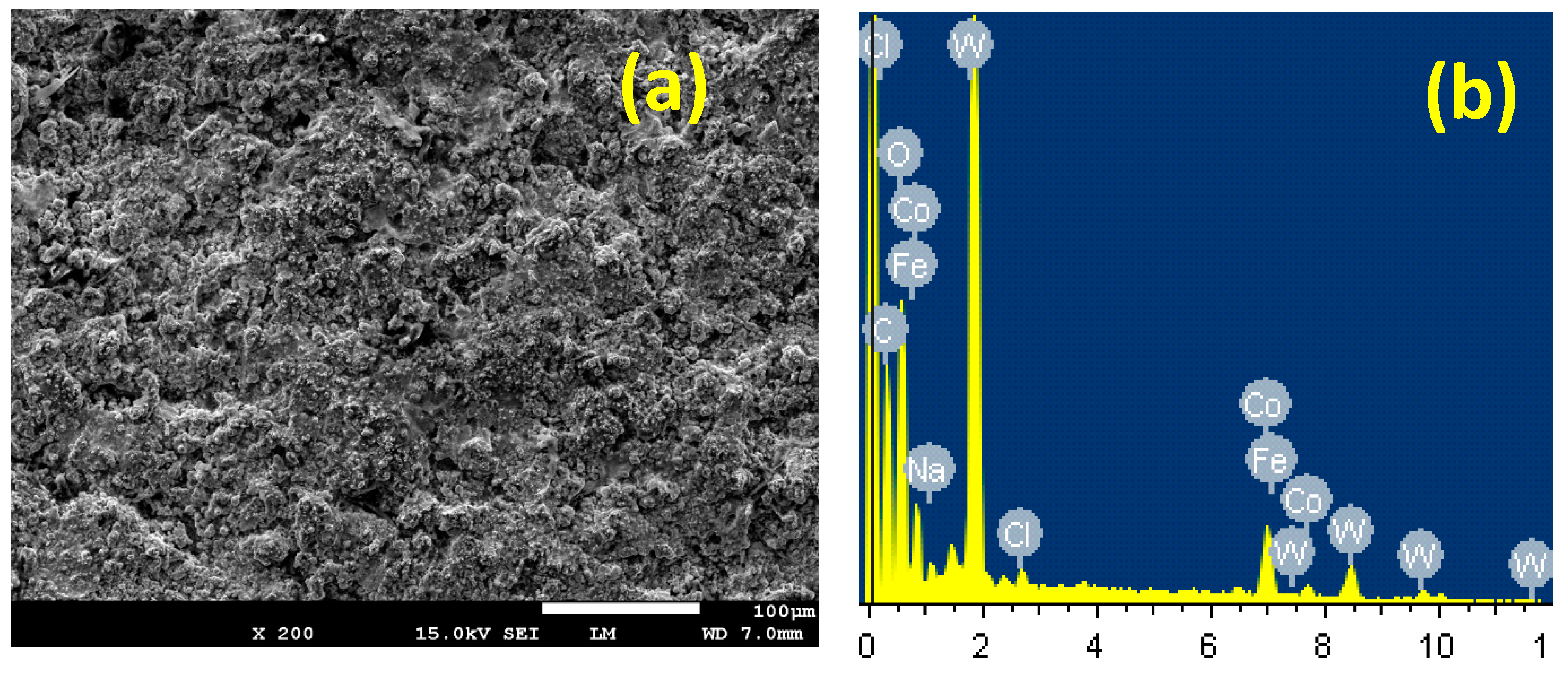
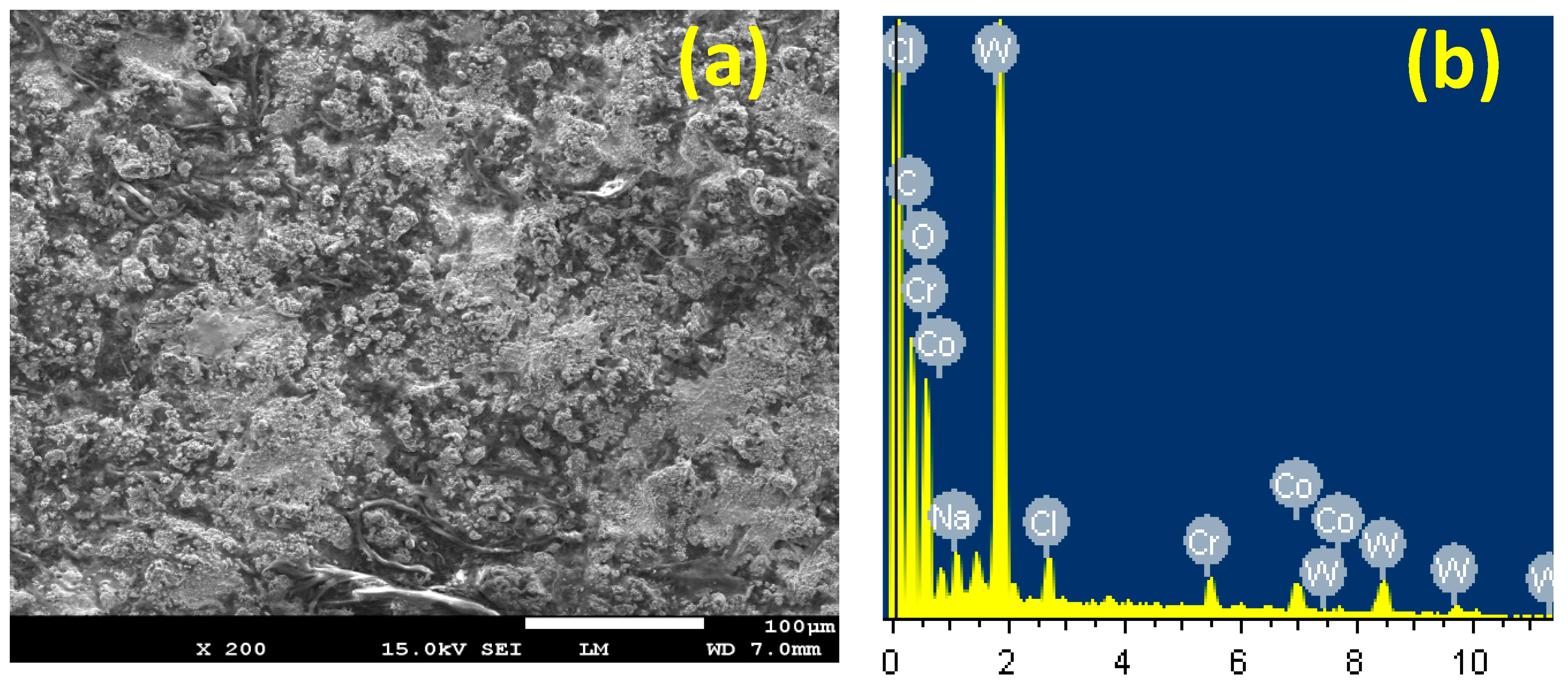
3.4. SEM and EDX Investigations
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sherif, E.S.M.; Almajid, A.A. Electrochemical corrosion behavior of API X-70 5L grade steel in 4.0 wt. % sodium chloride solutions after different immersion periods of time. Int. J. Electrochem. Sci. 2015, 10, 34–45. [Google Scholar]
- Sherif, E.S.M.; Almajid, A.A. Anodic dissolution of API X70 pipeline steel in Arabian Gulf seawater after different exposure intervals. J. Chem. 2014, 2014. [Google Scholar] [CrossRef]
- Guenbour, A.; Hajji, M.A.; Jallouli, E.M.; Bachir, A. Ben Study of corrosion-erosion behaviour of stainless alloys in industrial phosphoric acid medium. Appl. Surf. Sci. 2006, 253, 2362–2366. [Google Scholar] [CrossRef]
- Hegazy, M.A.; Ahmed, H.M.; El-Tabei, A.S. Investigation of the inhibitive effect of p-substituted 4-(N, N, N-dimethyldodecylammonium bromide)benzylidene-benzene-2-yl-amine on corrosion of carbon steel pipelines in acidic medium. Corros. Sci. 2011, 53, 671–678. [Google Scholar] [CrossRef]
- Sherif, E.S.; Almajid, A.A.; Khalil, K.A.; Junaedi, H.; Latief, F.H. Electrochemical studies on the corrosion behavior of API X65 pipeline steel in chloride solutions. Int. J. Electrochem. Sci. 2013, 8, 9360–9370. [Google Scholar]
- Zhao, M.C.; Yang, K.; Shan, Y.Y. Comparison on strength and toughness behaviors of microalloyed pipeline steels with acicular ferrite and ultrafine ferrite. Mater. Lett. 2003, 57, 1496–1500. [Google Scholar] [CrossRef]
- Yakubtsov, I.A.; Poruks, P.; Boyd, J.D. Microstructure and mechanical properties of bainitic low carbon high strength plate steels. Mater. Sci. Eng. A 2008, 480, 109–116. [Google Scholar] [CrossRef]
- Hernández-Espejel, A.; Domínguez-Crespo, M.A.; Cabrera-Sierra, R.; Rodríguez-Meneses, C.; Arce-Estrada, E.M. Investigations of corrosion films formed on API-X52 pipeline steel in acid sour media. Corros. Sci. 2010, 52, 2258–2267. [Google Scholar] [CrossRef]
- Alizadeh, M.; Bordbar, S. The influence of microstructure on the protective properties of the corrosion product layer generated on the welded API X70 steel in chloride solution. Corros. Sci. 2013, 70, 170–179. [Google Scholar] [CrossRef]
- Sherif, E.S.M.; Seikh, A.H. Anodic dissolution in sulfuric acid pickling solutions of the API pipeline X70 grade steel. Int. J. Electrochem. Sci. 2015, 10, 209–222. [Google Scholar]
- Bellaouchou, A.; Kabkab, B.; Guenbour, A.; Ben Bachir, A. Corrosion inhibition under heat transfer of 904L stainless steel in phosphoric acid by benzotriazole. Prog. Org. Coat. 2001, 41, 121–127. [Google Scholar] [CrossRef]
- Hemmingsen, T.; Hovdan, H.; Sanni, P.; Aagotnes, N.O. The influence of electrolyte reduction potential on weld corrosion. Electrochim. Acta 2002, 47, 3949–3955. [Google Scholar] [CrossRef]
- Ma, F.Y.; Wang, W.H. Fatigue crack propagation estimation of SUS 630 shaft based on fracture surface analysis under pitting corrosion condition. Mater. Sci. Eng. A 2006, 430, 1–8. [Google Scholar] [CrossRef]
- Jiang, X.; Zheng, Y.G.; Ke, W. Effect of flow velocity and entrained sand on inhibition performances of two inhibitors for CO2 corrosion of N80 steel in 3% NaCl solution. Corros. Sci. 2005, 47, 2636–2658. [Google Scholar] [CrossRef]
- Hardie, D.; Charles, E.A.; Lopez, A.H. Hydrogen embrittlement of high strength pipeline steels. Corros. Sci. 2006, 48, 4378–4385. [Google Scholar] [CrossRef]
- Kwok, C.T.; Fong, S.L.; Cheng, F.T.; Man, H.C. Pitting and galvanic corrosion behavior of laser-welded stainless steels. J. Mater. Process. Technol. 2006, 176, 168–178. [Google Scholar] [CrossRef]
- Sherif, E.S.M.; El Rayes, M.M. Corrosion behavior of API 2H and API 4F steels in freely aerated 4.0 % sodium chloride solutions. Int. J. Electrochem. Sci. 2015, 10, 7493–7504. [Google Scholar]
- Poursaee, A. Determining the appropriate scan rate to perform cyclic polarization test on the steel bars in concrete. Electrochim. Acta 2010, 55, 1200–1206. [Google Scholar] [CrossRef]
- Park, S.M.; Yoo, J.S. Electrochemical impedance spectroscopy for better electrochemical measurements. Anal. Chem. 2003, 75, 455A–461A. [Google Scholar] [CrossRef] [Green Version]
- Encinas-Sánchez, V.; de Miguel, M.T.; Lasanta, M.I.; García-Martín, G.; Pérez, F.J. Electrochemical impedance spectroscopy (EIS): An efficient technique for monitoring corrosion processes in molten salt environments in CSP applications. Sol. Energy Mater. Sol. Cells 2016, 144, 109–116. [Google Scholar] [CrossRef]
- Zhao, X.; Qi, Y.; Zhang, Z.; Li, K.; Li, Z. Electrochemical Impedance Spectroscopy Investigation on the Corrosive Behaviour of Waterborne Silicate Micaceous Iron Oxide Coatings in Seawater. Coatings 2019, 9, 415. [Google Scholar] [CrossRef] [Green Version]
- Barsoukov, E.; Macdonald, J.R. Impedance Spectroscopy: Theory, Experiment, and Applications; Wiley & Sons: New York, NY, USA, 1987; ISBN 0471647497. [Google Scholar]
- Pan, T.J.; Lu, W.M.; Ren, Y.J.; Wu, W.T.; Zeng, C.L. Electrochemical-impedance-spectroscopy (EIS) study of corrosion of steels 12CrMoV and SS304 beneath a molten ZnCl 2-KCl film at 400 °C in Air. Oxid. Met. 2009, 72, 179–190. [Google Scholar] [CrossRef]
- Sherif, E.S.M.; Abdo, H.S.; Khalil, K.A.; Nabawy, A.M. Corrosion properties in sodium chloride solutions of Al–TiC composites in situ synthesized by HFIHF. Metals 2015, 5, 1799–1811. [Google Scholar] [CrossRef] [Green Version]
- Jones, D.A. Principles and Prevention of Corrosion, 2nd ed.; Prentice Hall, Inc.: Upper Saddle River, NJ, USA, 1996. [Google Scholar]
- Wang, L.; Cheng, L.; Li, J.; Zhu, Z.; Bai, S.; Cui, Z. Combined Effect of Alternating Current Interference and Cathodic Protection on Pitting Corrosion and Stress Corrosion Cracking Behavior of X70 Pipeline Steel in Near-Neutral pH Environment. Materials 2018, 11, 465. [Google Scholar] [CrossRef] [Green Version]
- Galván-Martínez, R.; Orozco-Cruz, R.; Carmona-Hernández, A.; Mejía-Sánchez, E.; Morales-Cabrera, M.A.; Contreras, A. Corrosion Study of Pipeline Steel under Stress at Different Cathodic Potentials by EIS. Metals 2019, 9, 1353. [Google Scholar] [CrossRef] [Green Version]
- Sherif, E.-S.M. A Comparative Study on the Electrochemical Corrosion Behavior of Iron and X-65 Steel in 4.0 wt % Sodium Chloride Solution after Different Exposure Intervals. Molecules 2014, 19, 9962–9974. [Google Scholar] [CrossRef] [Green Version]
| Element | C | Mn | P | S | Si | Cb | Ti | Fe |
|---|---|---|---|---|---|---|---|---|
| Wt % | 0.14 | 1.5 | 0.03 | 0.015 | 0.2 | 0.04 | 0.02 | Balance |
| Powder Types | WC (wt %) | Co (wt %) | Cr (wt %) |
|---|---|---|---|
| PSC1 coating | 88 | 12 | 0.0 |
| PSC2 coating | 86 | 10 | 4.0 |
| Sample/Time | RS/ (Ω·cm2) | Q | RP/ (Ω·cm2) | W/ | |
|---|---|---|---|---|---|
| YQ (YQ/S*s^n) | n | ||||
| Uncoated PS (1.0 h) | 4.41 | 0.000214 | 0.89 | 3946 | 0.01867 |
| Coated PSC1 (1.0 h) | 3.95 | 0.000211 | 0.90 | 6367 | 0.01855 |
| Coated PSC2 (1.0 h) | 4.41 | 0.000205 | 0.90 | 7091 | 0.01187 |
| Uncoated PS (24 h) | 4.68 | 0.000188 | 0.90 | 4886 | 0.01098 |
| Coated PSC1 (24 h) | 4.76 | 0.000191 | 0.91 | 5647 | 0.01087 |
| Coated PSC2 (24 h) | 5.28 | 0.000183 | 0.91 | 5739 | 0.01079 |
| Uncoated PS (48 h) | 4.75 | 0.000184 | 0.91 | 6634 | 0.01047 |
| Coated PSC1 (48 h) | 4.95 | 0.000192 | 0.90 | 7840 | 0.00570 |
| Coated PSC2 (48 h) | 4.64 | 0.000181 | 0.91 | 7908 | 0.00635 |
| Steel/Time | βc (mV·dec−1) | ECorr (mV) | βa (mV·dec−1) | jCorr (µA·cm−2) | Rp (Ω·cm2) | RCorr (mmpy) |
|---|---|---|---|---|---|---|
| Uncoated PS (1.0 h) | 105 | -928 | 150 | 47 | 531.37 | 0.545 |
| Coated PSC1 (1.0 h) | 100 | -840 | 140 | 38 | 667.42 | 0.441 |
| Coated PSC2 (1.0 h) | 95 | -780 | 137 | 29 | 841.11 | 0.336 |
| Uncoated PS (24 h) | 125 | -958 | 148 | 42 | 718.90 | 0.371 |
| Coated PSC1 (24 h) | 122 | -832 | 142 | 34 | 839.14 | 0.344 |
| Coated PSC2 (24 h) | 118 | -865 | 140 | 28 | 959.72 | 0.302 |
| Uncoated PS (48 h) | 98 | -955 | 115 | 32 | 831.85 | 0.359 |
| Coated PSC1 (48 h) | 110 | -825 | 120 | 26 | 994.27 | 0.325 |
| Coated PSC2 (48 h) | 115 | -868 | 125 | 20 | 1302.1 | 0.232 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sherif, E.-S.M.; El Rayes, M.M.; Abdo, H.S. WC-Co and WC-Co-Cr Coatings for the Protection of API Pipeline Steel from Corrosion in 4% NaCl Solution. Coatings 2020, 10, 275. https://doi.org/10.3390/coatings10030275
Sherif E-SM, El Rayes MM, Abdo HS. WC-Co and WC-Co-Cr Coatings for the Protection of API Pipeline Steel from Corrosion in 4% NaCl Solution. Coatings. 2020; 10(3):275. https://doi.org/10.3390/coatings10030275
Chicago/Turabian StyleSherif, El-Sayed M., Magdy M. El Rayes, and Hany S. Abdo. 2020. "WC-Co and WC-Co-Cr Coatings for the Protection of API Pipeline Steel from Corrosion in 4% NaCl Solution" Coatings 10, no. 3: 275. https://doi.org/10.3390/coatings10030275
APA StyleSherif, E.-S. M., El Rayes, M. M., & Abdo, H. S. (2020). WC-Co and WC-Co-Cr Coatings for the Protection of API Pipeline Steel from Corrosion in 4% NaCl Solution. Coatings, 10(3), 275. https://doi.org/10.3390/coatings10030275







