Influence of Mesoporous Inorganic Al–B–P Amphiprotic Surfactant Material Resistances of Wood against Brown and White-Rot Fungi (Part 1)
Abstract
1. Introduction
2. Materials and Methods
2.1. Wood Materials
2.2. Chemical Products and Fungi
2.3. Preparation of Al–B–P Amphiprotic Surfactant Solution
2.4. Field Testing Methods
2.5. Preparation Method of Culture of Fungi
2.6. Treatment Procedure of Wood Sample
2.7. Retention Rate of Chemical Effectiveness
2.8. Evaluation of Fungicide and Testing Procedure
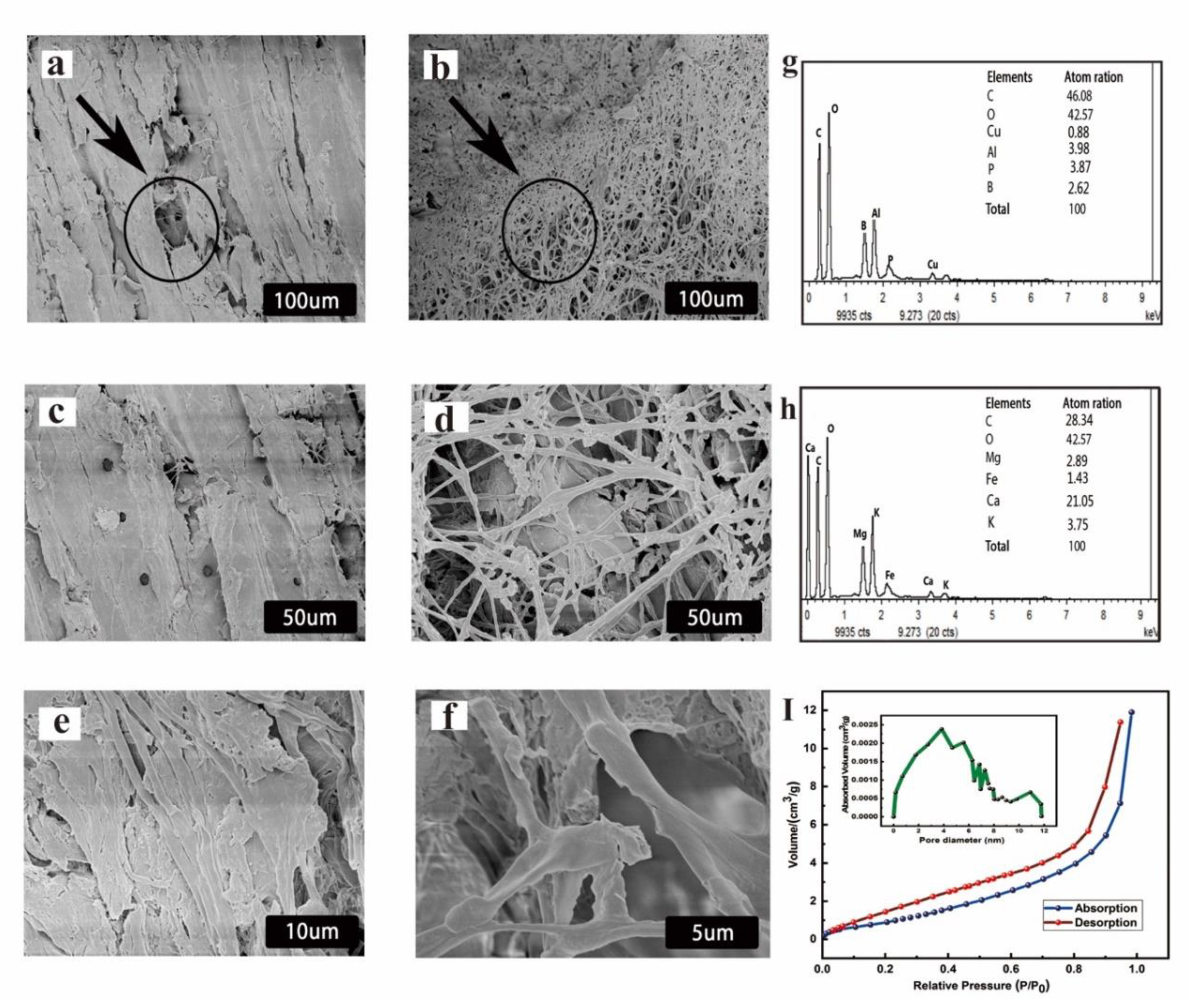
2.9. Materials Characterization
3. Results and Discussion
3.1. Inhibition Efficiency
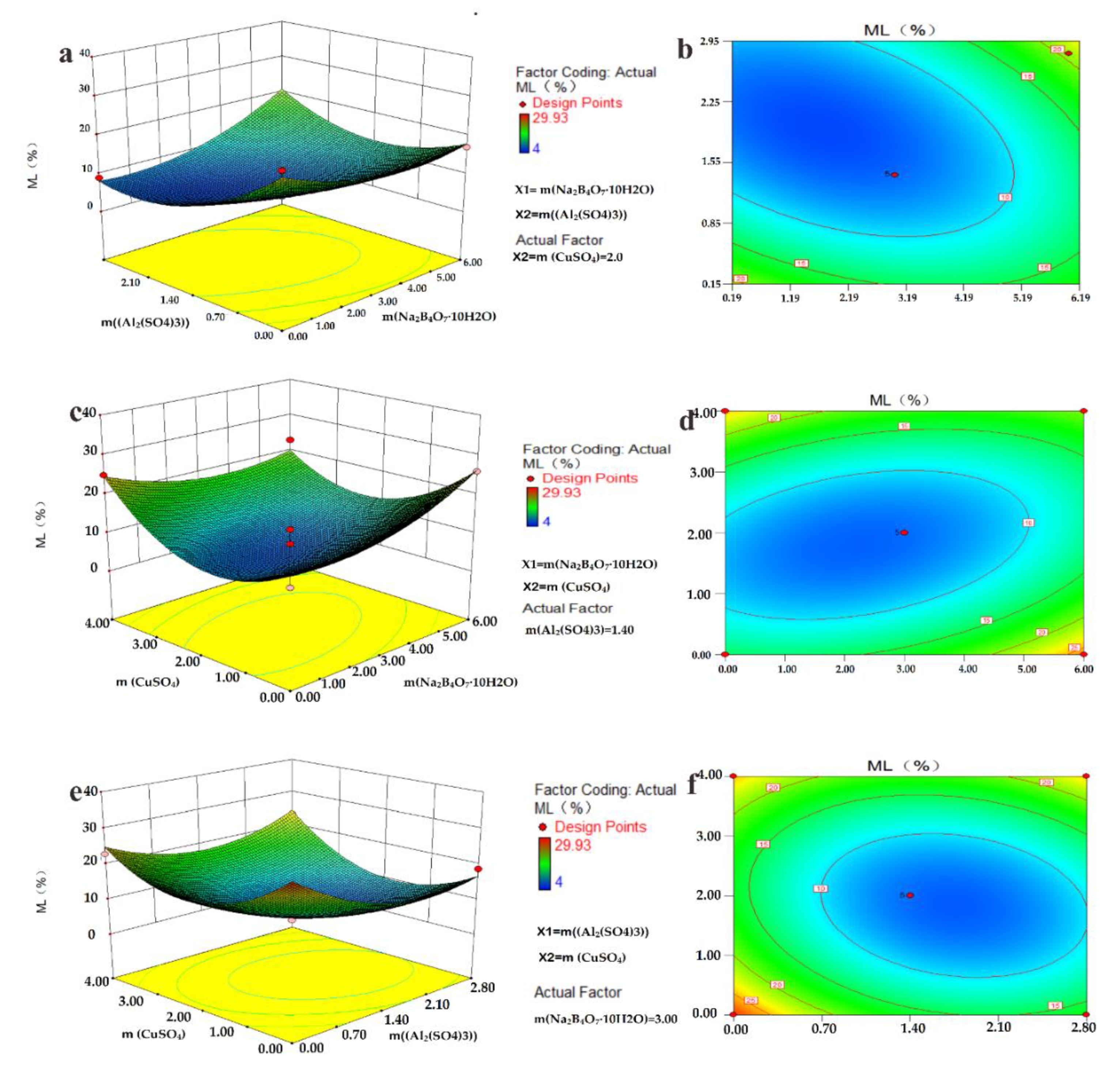
3.2. Optimize Preparation Parameter of Wood
3.3. Microstructure Analysis of the Inoculated Wood
3.4. Reaction Mechanism Analysis
3.5. Mechanical and Physical Properties Analysis
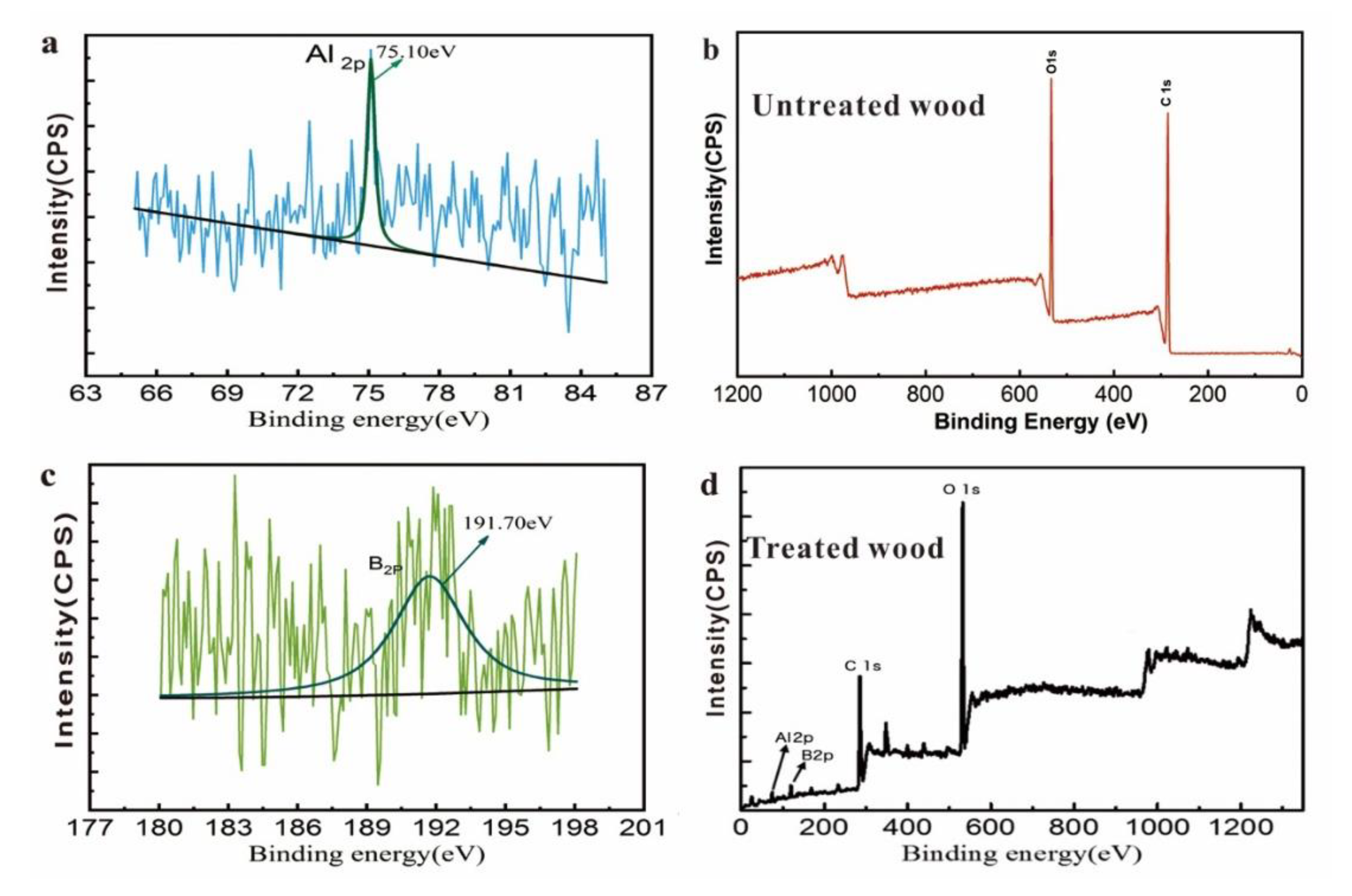
3.6. XPS Surface Chemical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| RSM | response surface methodology |
| Al2(SO4)3 | aluminum sulphate |
| CuSO4 | copper sulphate |
| Na2B4O7 | sodium tetraborate |
| H3PO4 | phosphoric acid |
| H3BO3 | boric acid |
| CAA | copper azole alkaline |
| CQC | copper quaternary creosote |
| ACQ | alkaline copper quaternary |
| C5H11NO2HCl | amphiprotic surfactant |
| FTIR | Fourier Transform infrared spectroscopy |
| XRD | X-ray diffraction |
| MOE | modulus of elasticity |
| MOR | modulus of rupture |
| SEM | scanning electron microscopy |
| EDS | energy-dispersive spectroscopy |
| TGA | Thermogravimetric analysis |
| DTG | differential thermogravimetric |
| XPS | X-ray photoelectron spectroscopy |
| NMR | nuclear magnetic resonance |
| BET | Brunauer−Emmett−Teller equation |
| ANOVA | analysis of variance |
| MLR | Loss mass rate |
References
- Bari, E.; Nazarnezhad, N.; Kazemi, S.M.; Ghanbary, M.A.T.; Mohebby, B.; Schmidt, O.; Clausen, C.A. Comparison between degradation capabilities of the white rot fungi Pleurotus ostreatus and Trametes versicolor in beech wood. Int. Biodeterior. Biodegrad. 2015, 104, 231–237. [Google Scholar] [CrossRef]
- Goodell, B.; Qian, Y.; Jellison, J. Fungal decay of wood: Soft rot—brown rot—white rot. In Development of Commercial Wood Preservatives; American Chemical Society: Washington, DC, USA, 2008; Volume 982, pp. 9–31. [Google Scholar]
- Blanchette, R.A. An unusual decay pattern in brown-rotted wood. Mycologia 1983, 75, 552–556. [Google Scholar] [CrossRef]
- Asif, M.B.; Hai, F.I.; Hou, J.; Price, W.E.; Nghiem, L.D. Impact of wastewater derived dissolved interfering compounds on growth, enzymatic activity and trace organic contaminant removal of white rot fungi—A critical review. J. Environ. Manag. 2017, 201, 89–109. [Google Scholar] [CrossRef] [PubMed]
- Ohkoshi, M.; Kato, A.; Suzuki, K.; Hayashi, N.; Ishihara, M. Characterization of acetylated wood decayed by brown-rot and white-rot fungi. J. Wood Sci. 1999, 45, 69–75. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, T.; Mi, N.; Wang, Y.; Li, G.; Wang, L.; Xie, Y. Antifungal activity of monoterpenes against wood white-rot fungi. Int. Biodeterior. Biodegrad. 2016, 106, 157–160. [Google Scholar] [CrossRef]
- Goodell, B.; Daniel, G.; Jellison, J.; Qian, Y. Iron-reducing capacity of low-molecular-weight compounds produced in wood by fungi. Holzforschung 2006, 60, 630–636. [Google Scholar] [CrossRef]
- Wu, Z.; Huang, D.; Wei, W.; Wang, W.; Wang, X.A.; Wei, Q.; Niu, M.; Lin, M.; Rao, J.; Xie, Y. Mesoporous aluminosilicate improves mildew resistance of bamboo scrimber with CuBP anti-mildew agents. J. Clean. Prod. 2019, 209, 273–282. [Google Scholar] [CrossRef]
- Mercer, T.; Frostick, L. Evaluating the potential for environmental pollution from chromated copper arsenate (CCA)-treated wood waste: A new mass balance approach. J. Hazard. Mater. 2014, 276, 10–18. [Google Scholar] [CrossRef]
- Miyafuji, H.; Saka, S. Na2O-SiO2 wood-inorganic composites prepared by the sol-gel process and their fire-resistant properties. J. Wood Sci. 2001, 47, 483–489. [Google Scholar] [CrossRef]
- Lu, Y.; Feng, M.; Zhan, H. Preparation of SiO2–wood composites by an ultrasonic-assisted sol–gel technique. Cellulose 2014, 21, 4393–4403. [Google Scholar] [CrossRef]
- Yamaguchi, H. Properties of silicic-acid compounds as chemical-agents for impregnation and fixation of wood. Mokuzai Gakkaishi 1994, 40, 830–837. [Google Scholar]
- Campos, K.S.; Lenz e Silva, G.F.L.; Nunes, E.H.; Silva, A.M.; Bestard, G.A.; Vasconcelos, W.L. Surface modification of coked alumina refractories by the deposition of sol-gel derived silica coatings. Ceram. Int. 2019, 45, 8626–8633. [Google Scholar] [CrossRef]
- Hübert, T.; Shabir Mahr, M. Sol-gel wood preservation. Handb. Sol-Gel Sci. Technol. 2016, 1, 1–52. [Google Scholar]
- Hübert, T.; Shabir Mahr, M. Sol-gel wood preservation. In Handbook of Sol-Gel Science and Technology: Processing, Characterization and Applications; Klein, L., Aparicio, M., Jitianu, A., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 2795–2842. [Google Scholar]
- Tsvetkova, I.; Krasil’nikova, L.; Khoroshavina, Y.; Galushko, A.; Yu, V.F.; Kychkin, A.; Shilova, O. Sol-gel preparation of protective and decorative coatings on wood. J. Sol-Gel Sci. Technol. 2019, 92, 474–483. [Google Scholar] [CrossRef]
- Tshabalala, M.A.; Gangstad, J.E. Accelerated weathering of wood surfaces coated with multifunctional alkoxysilanes by sol-gel deposition. J. Coat. Technol. 2003, 75, 37–43. [Google Scholar] [CrossRef]
- Denes, A.R.; Tshabalala, M.A.; Rowell, R.; Denes, F.; Young, R.A. Hexamethyldisiloxane-plasma coating of wood surfaces for creating water repellent characteristics. Holzforschung 1999, 53, 318–326. [Google Scholar] [CrossRef]
- Guo, H.; Bachtiar, E.; Ribera, J.; Heeb, M.; Schwarze, F.; Burgert, I. Non-biocidal preservation of wood against brown-rot fungi with TiO2/Ce Xerogel. Green Chem. 2018, 20, 1375–1382. [Google Scholar] [CrossRef]
- Hill, C.A. Wood modification: An update. BioResources 2011, 6, 918–919. [Google Scholar]
- Humar, M.; Žlindra, D.; Pohleven, F. Improvement of fungicidal properties and copper fixation of copper-ethanolamine wood preservatives usingoctanoic acid and boron compounds. Holz als Roh- und Werkst. 2007, 65, 17–21. [Google Scholar] [CrossRef]
- European Commission. Directive 98/8/EC of the European Parliament and of the Council of 16 February 1998 concerning the placing of biocidal products on the market. Off. J. Eur. Union 1998, 41, 123. [Google Scholar]
- Mourant, D.; Yang, D.-Q.; Lu, X.; Riedl, B.; Roy, C. Copper and boron fixation in wood by pyrolytic resins. Bioresour. Technol. 2009, 100, 1442–1449. [Google Scholar] [CrossRef] [PubMed]
- Råberg, U.; Terziev, N.; Daniel, G. Degradation of Scots pine and beech wood exposed in four test fields used for testing of wood preservatives. Int. Biodeterior. Biodegrad. 2013, 79, 20–27. [Google Scholar] [CrossRef]
- En 252. Field Test Method for Determining the Relative Protective Effectiveness of a Wood Preservative in Ground Contact; British Standards institute: London, UK, 2014. [Google Scholar]
- ASTM D4445. Standard Test Method for Fungicides for Controlling Sapstain and Mold on Unseasoned Lumber (Laboratory Method); ASTM International: West Conshohocken, PA, USA, 2015. [Google Scholar]
- ASTM D2017-05. Standard Test Method of Accelerated Laboratory Test of Natural Decay Resistance of Woods; ASTM International: West Conshohocken, PA, USA, 2017. [Google Scholar]
- Tascioglu, C.; Cooper, P.; Ung, T. Adsorption of ACQ and CuMEA wood preservatives in red pine. Int. Res. Group on Wood Pres. Document No: IRG/WP/05-30374, 2005. [Google Scholar]
- Jiang, X. Fixation Chemistry of Amine-Copper Preservatives. Ph.D. Thesis, University of British Columbia, Vancouver, BC, Canada, November 2000. [Google Scholar]
- En 84. Wood Preservatives–Accelerated Ageing of Treated Wood Prior to Biological Testing–Leaching Procedure; CEN (European Committee for Standardization): Brussels, Belgium, 1997. [Google Scholar]
- Lesar, B.; Podlesnik, B.; Pohleven, F.; Humar, M.; Kralj, P.; Veber, M. Performance of boron-ethanolamine-quaternary ammonium based wood preservatives against leaching, wood decay and blue stain fungi. Wood Res. 2008, 53, 17–26. [Google Scholar]
- Mahr, S.; Hübert, T.; Stephan, I.; Militz, H. Decay protection of wood against brown-rot fungi by titanium alkoxide impregnations. Int. Biodeterior. Biodegrad. 2013, 77, 56–62. [Google Scholar] [CrossRef]
- Usmani, S.M.; Stephan, I.; Hübert, T.; Kemnitz, E. Nano metal fluorides for wood protection against fungi. ACS Appl. Nano Mater. 2018, 1, 1444–1449. [Google Scholar] [CrossRef]
- Dong, Z.; Zhou, Y.; Ren, H. Optimization on ultrasonic-assisted extraction technology of chlorophyll from radish leaf. Trans. Chin. Soc. Agric. Eng. 2011, 27, 288–292. [Google Scholar]
- Hyde, S.M.; Wood, P.M. A mechanism for production of hydroxyl radicals by the brown-rot fungus Coniophora puteana: Fe (III) reduction by cellobiose dehydrogenase and Fe (II) oxidation at a distance from the hyphae. Microbiology 1997, 143, 259–266. [Google Scholar] [CrossRef]
- Takeshi, F.; Kwnta, S.; Tohru, U. Combination of wood and silicate II: Wood-mineral composites using water glass and reactants of barium chloride, boric acid, and borax, and their properties. Mokuzai Gakkaishi 1992, 38, 448–457. [Google Scholar]
- Blanchette, R.A.; Otjen, L.; Effland, M.J.; Eslyn, W.E. Changes in structural and chemical components of wood delignified by fungi. Wood Sci. Technol. 1985, 19, 35–46. [Google Scholar] [CrossRef]
- Chen, T.; Xie, Y.; Cai, L.; Zhuang, B.; Wang, X.A.; Wu, Z.; Niu, M.; Lin, M. Mesoporous aluminosilicate material with hierarchical porosity for ultralow density wood fiber composite (ULD_WFC). ACS Sustain. Chem. Eng. 2016, 4, 3888–3896. [Google Scholar] [CrossRef]
- Traoré, M.; Kaal, J.; Cortizas, A.M. Application of FTIR spectroscopy to the characterization of archeological wood. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 153, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.R.; Hamdan, S. Preparation and characterizations of various clay-and monomers-dispersed wood nanocomposites. In Wood Polymer Nanocomposites; Springer: Cham, Switzerland, 2018; pp. 37–68. [Google Scholar]
- Cesar, T.; Danevčič, T.; Kavkler, K.; Stopar, D. Melamine polymerization in organic solutions and waterlogged archaeological wood studied by FTIR spectroscopy. J. Cult. Herit. 2017, 23, 106–110. [Google Scholar] [CrossRef]
- Özgenç, Ö.; Durmaz, S.; Boyaci, I.H.; Eksi-Kocak, H. Determination of chemical changes in heat-treated wood using ATR-FTIR and FT Raman spectrometry. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 171, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Andersson, S.; Serimaa, R.; Paakkari, T.; Saranpää, P.; Pesonen, E. Crystallinity of wood and the size of cellulose crystallites in Norway spruce (Picea abies). J. Wood Sci. 2003, 49, 531–537. [Google Scholar] [CrossRef]
- Weimer, P.; Hackney, J.; French, A. Effects of chemical treatments and heating on the crystallinity of celluloses and their implications for evaluating the effect of crystallinity on cellulose biodegradation. Biotechnol. Bioeng. 1995, 48, 169–178. [Google Scholar] [CrossRef] [PubMed]
- French, A.D. Idealized powder diffraction patterns for cellulose polymorphs. Cellulose 2014, 21, 885–896. [Google Scholar] [CrossRef]
- Broda, M.; Mazela, B.; Dutkiewicz, A. Organosilicon compounds with various active groups as consolidants for the preservation of waterlogged archaeological wood. J. Cult. Herit. 2019, 35, 123–128. [Google Scholar] [CrossRef]
- Rosu, L.; Varganici, C.-D.; Mustata, F.; Rusu, T.; Rosu, D.; Rosca, I.; Tudorachi, N.; Teacă, C.-A. Enhancing the thermal and fungal resistance of wood treated with natural and synthetic derived epoxy resins. ACS Sustain. Chem. Eng. 2018, 6, 5470–5478. [Google Scholar] [CrossRef]
- Eckert, H. Synthesis of non-siliceous glasses and their structural characterization by solid-state NMR. Handb. Sol-Gel Sci. Technol. Process. Charact. Appl. 2018, 1, 1323–1373. [Google Scholar]
- Dirken, P.J.; Smith, M.E.; Whitfield, H.J. 17O and 29Si solid state NMR study of atomic scale structure in sol-gel-prepared TiO2-SiO2 materials. J. Phys. Chem. 1995, 99, 395–401. [Google Scholar] [CrossRef]
- Wang, X.; Liu, S.; Chang, H.; Liu, J. Sol-gel deposition of TiO2 nanocoatings on wood surfaces with enhanced hydrophobicity and photostability. Wood Fiber Sci 2014, 46, 109–117. [Google Scholar]
- Mattos, B.D.; de Cademartori, P.H.; Lourençon, T.V.; Gatto, D.A.; Magalhães, W.L. Biodeterioration of wood from two fast-growing eucalypts exposed to field test. Int. Biodeterior. Biodegrad. 2014, 93, 210–215. [Google Scholar] [CrossRef]
- Popescu, C.-M.; Lisa, G.; Manoliu, A.; Gradinariu, P.; Vasile, C. Thermogravimetric analysis of fungus-degraded lime wood. Carbohydr. Polym. 2010, 80, 78–83. [Google Scholar] [CrossRef]
- Gašparovič, L.; Koreňová, Z.; Jelemenský, Ľ. Kinetic study of wood chips decomposition by TGA. Chem. Pap. 2010, 64, 174–181. [Google Scholar] [CrossRef]
- Wang, X.H.; Liu, J.L.; Fei, B.H. Effect of vacuum heat treatment temperature on physical and mechanical properties of Eucalyptus pellita wood. Wood Fiber Sci. 2014, 46, 368–375. [Google Scholar]
- Tshabalala, M.A.; Jakes, J.; VanLandingham, M.R.; Wang, S.; Peltonen, J. Surface characterization. In Handbook of wood chemistry and wood composites, 2nd ed.; Tshabalala, M.A.; Jakes, J.; VanLandingham, M.R.; Wang, S.; Peltonen, J. CRC Press, Taylor and Francis group: Boca Raton, FL, USA, 2012; pp. 217–252. [Google Scholar]
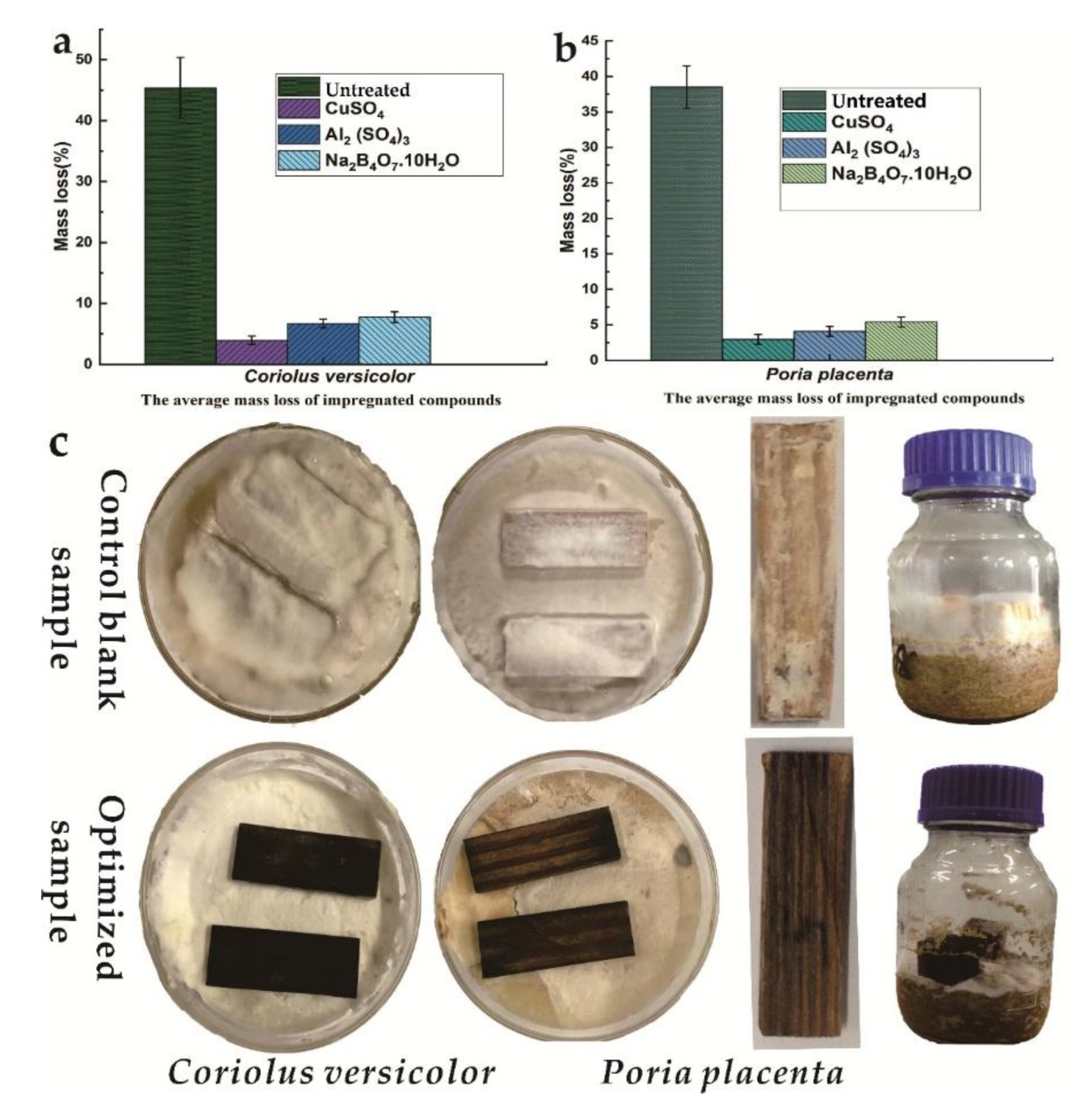


| Factors | Code and Levels * | ||
|---|---|---|---|
| –1 | 0 | 1 | |
| X1 | 0 | 0.7 | 1.4 |
| X2 | 0 | 1 | 2 |
| X3 | 0 | 1.5 | 3 |
| (AMLR%) * of Wood Sample | Level Class Resistance to Test Fungus | Average Residual Weight (%) |
|---|---|---|
| 0%–10% | Strong rot Resistant | 90–100 |
| 11%–24% | rot resistant | 76–89 |
| 25%–44% | slightly rot resistant | 56–75 |
| <45% | not resistant to rot | <55 |
| Test | X1% | X2% | X3% | * Mass Loss% |
|---|---|---|---|---|
| 1 | 3.00 | 2.80 | 0.00 | 18.8 |
| 2 | 3.00 | 1.40 | 2.00 | 11 |
| 3 | 6.00 | 1.40 | 0.00 | 26 |
| 4 | 3.00 | 0.00 | 4.00 | 23 |
| 5 | 3.00 | 1.40 | 2.00 | 5 |
| 6 | 3.00 | 2.80 | 4.00 | 23.3 |
| 7 | 3.00 | 1.40 | 2.00 | 4 |
| 8 | 3.00 | 0.00 | 0.00 | 29.93 |
| 9 | 0.00 | 0.00 | 2.00 | 25.1 |
| 10 | 6.00 | 2.80 | 2.00 | 19.26 |
| 11 | 0.00 | 1.40 | 4.00 | 25 |
| 12 | 6.00 | 0.00 | 2.00 | 17.08 |
| 13 | 3.00 | 1.40 | 2.00 | 7.2 |
| 14 | 0.00 | 2.80 | 2.00 | 9.02 |
| 15 | 3.00 | 1.40 | 2.00 | 6.58 |
| 16 | 6.00 | 1.40 | 4.00 | 23.04 |
| 17 | 0.00 | 1.40 | 0.00 | 11.63 |
| Source | Squares | df | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 1079.80 | 9 | 119.98 | 13.19 | 0.0013 |
| X1 (Na2B4O7) | 26.75 | 1 | 26.75 | 2.94 | 0.1301 |
| X2 Al2(SO4)3 | 76.45 | 1 | 76.45 | 8.40 | 0.0230 |
| X3 (CuSO4) | 7.96 | 1 | 7.96 | 0.87 | 0.3807 |
| X1X2 | 83.36 | 1 | 83.36 | 9.16 | 0.0192 |
| X1X3 | 66.67 | 1 | 66.67 | 7.33 | 0.0303 |
| X2X3 | 32.66 | 1 | 32.66 | 3.59 | 0.1000 |
| X12 | 76.39 | 1 | 76.39 | 8.40 | 0.0231 |
| X22 | 183.38 | 1 | 183.38 | 20.16 | 0.0028 |
| X32 | 455.59 | 1 | 455.59 | 50.08 | 0.0002 |
| Residual | 63.68 | 7 | 9.10 | – | – |
| Lack of Fit | 34.77 | 3 | 11.59 | 1.60 | 0.3219 |
| Pure Error | 28.92 | 4 | 7.23 | – | – |
| Cor Total | 1143.48 | 16 | – | – | – |
| Model | 1079.80 | 9 | 119.98 | 13.19 | 0.0013 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yves Beaudelaire, K.G.; Zhuang, B.; Aladejana, J.T.; Li, D.; Hou, X.; Xie, Y. Influence of Mesoporous Inorganic Al–B–P Amphiprotic Surfactant Material Resistances of Wood against Brown and White-Rot Fungi (Part 1). Coatings 2020, 10, 108. https://doi.org/10.3390/coatings10020108
Yves Beaudelaire KG, Zhuang B, Aladejana JT, Li D, Hou X, Xie Y. Influence of Mesoporous Inorganic Al–B–P Amphiprotic Surfactant Material Resistances of Wood against Brown and White-Rot Fungi (Part 1). Coatings. 2020; 10(2):108. https://doi.org/10.3390/coatings10020108
Chicago/Turabian StyleYves Beaudelaire, Kouomo Guelifack, Biaorong Zhuang, John Tosin Aladejana, Dehong Li, Xinjun Hou, and Yongqun Xie. 2020. "Influence of Mesoporous Inorganic Al–B–P Amphiprotic Surfactant Material Resistances of Wood against Brown and White-Rot Fungi (Part 1)" Coatings 10, no. 2: 108. https://doi.org/10.3390/coatings10020108
APA StyleYves Beaudelaire, K. G., Zhuang, B., Aladejana, J. T., Li, D., Hou, X., & Xie, Y. (2020). Influence of Mesoporous Inorganic Al–B–P Amphiprotic Surfactant Material Resistances of Wood against Brown and White-Rot Fungi (Part 1). Coatings, 10(2), 108. https://doi.org/10.3390/coatings10020108







