Surface Modification of 316L SS Implants by Applying Bioglass/Gelatin/Polycaprolactone Composite Coatings for Biomedical Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Bioglass Nanopowder
2.2. Fabrication of PCL-10 wt.% Gelatin–Bioglass Composite Coatings
2.3. Characterization of the Nanofibrous Composite Coating
2.4. Electrochemical Evaluation
2.5. In Vitro Study
2.6. Cell Culture and Cell Viability Study
2.7. In Vivo Study
3. Result and Discussion
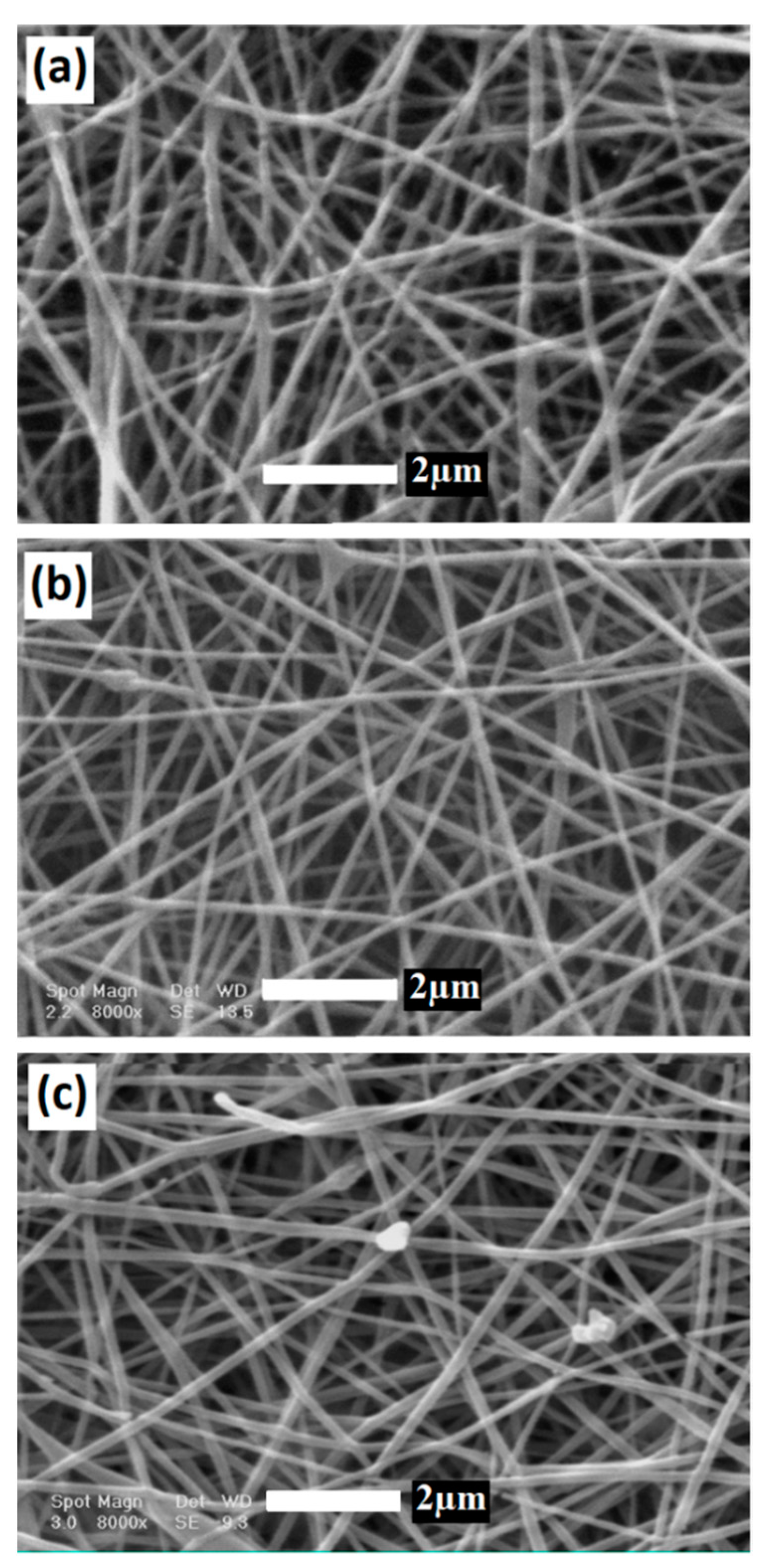
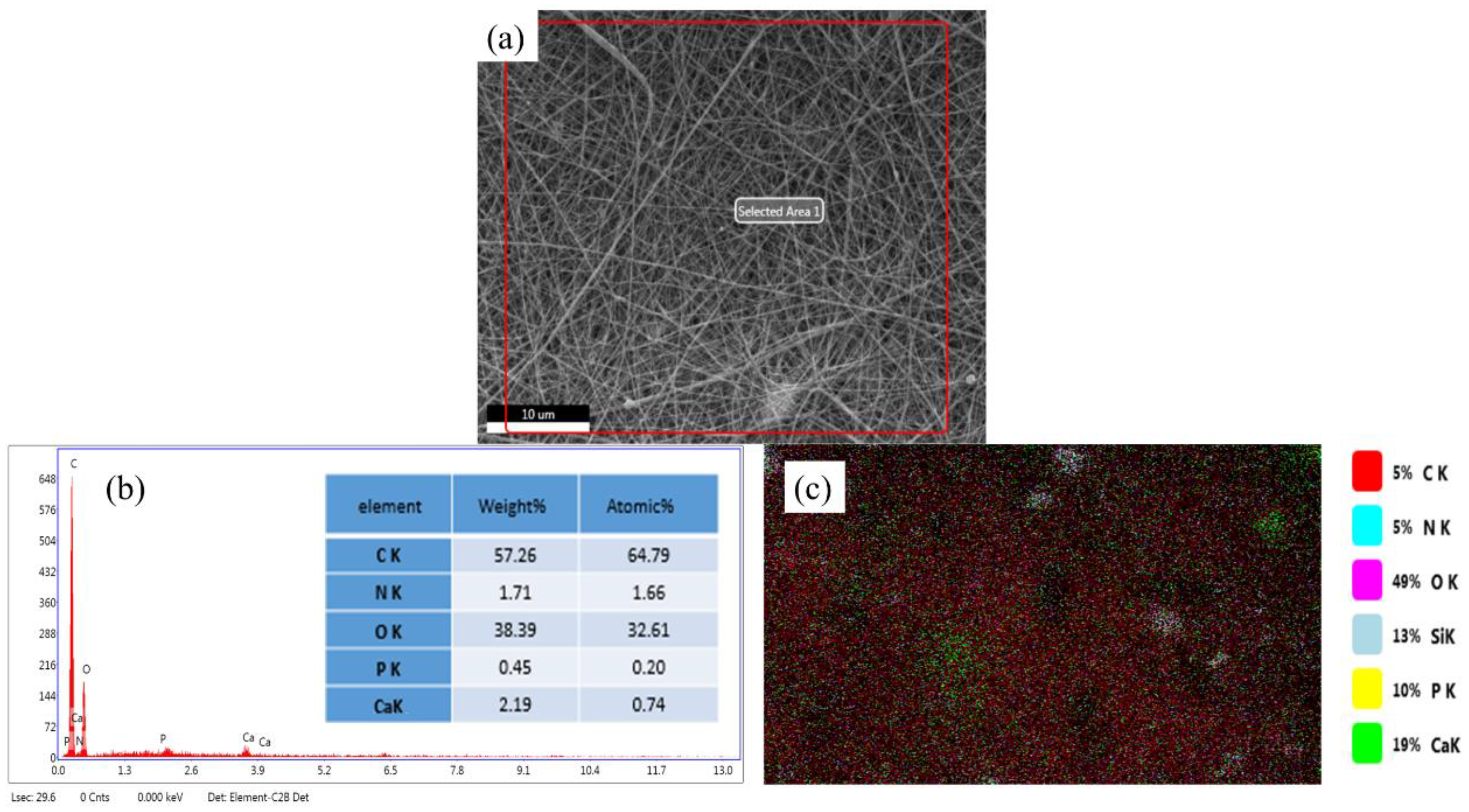
3.1. Surface Morphology and Elemental Analysis of BG/GE/PCL Composites
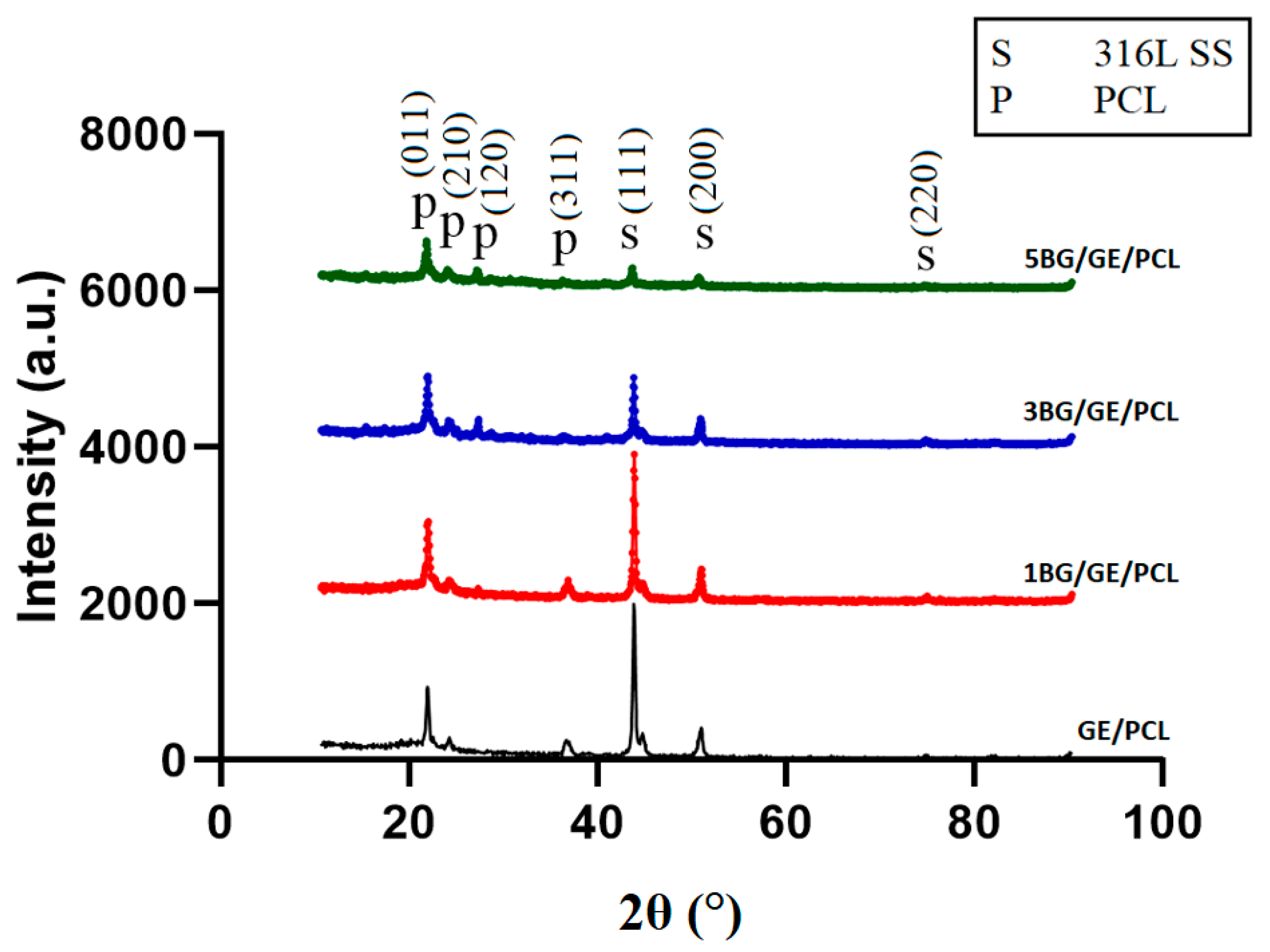
3.2. Phase Analysis
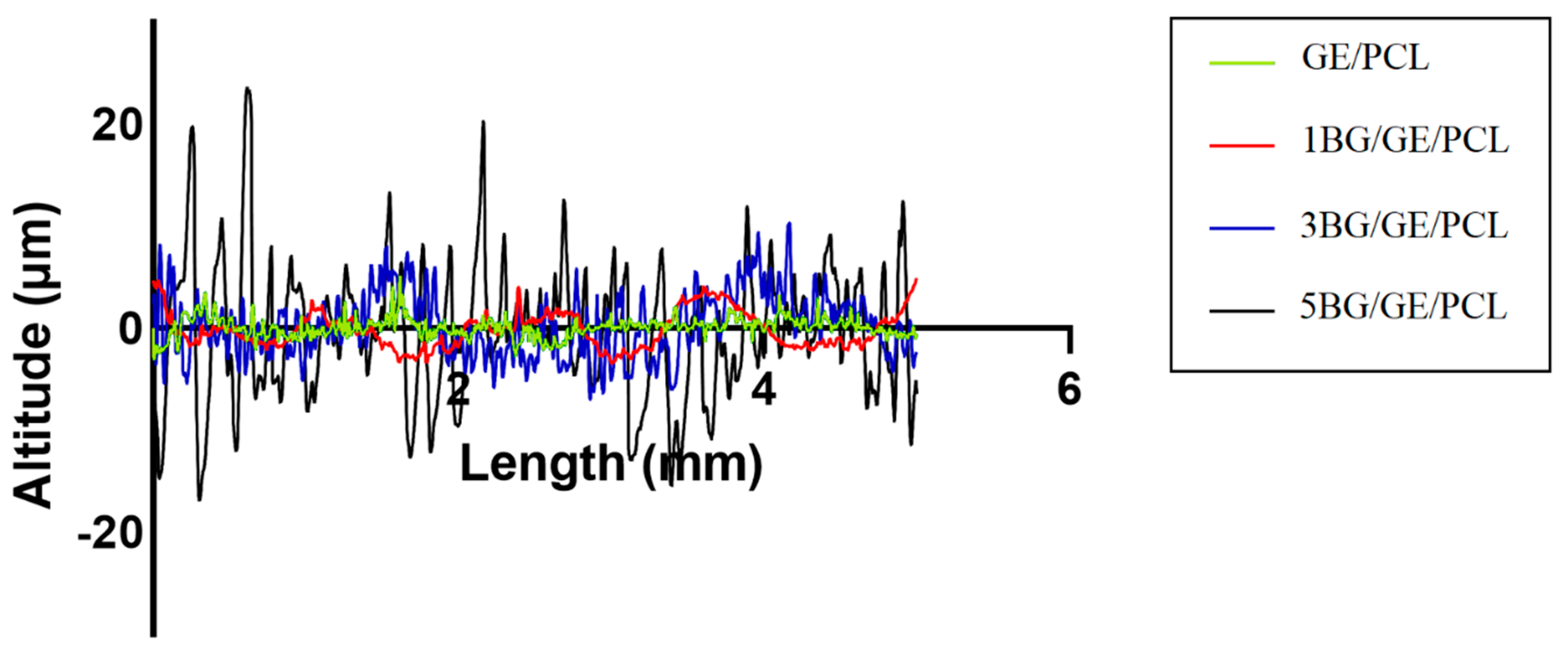
3.3. Surface Roughness and Adhesion Strength of the Coated Samples
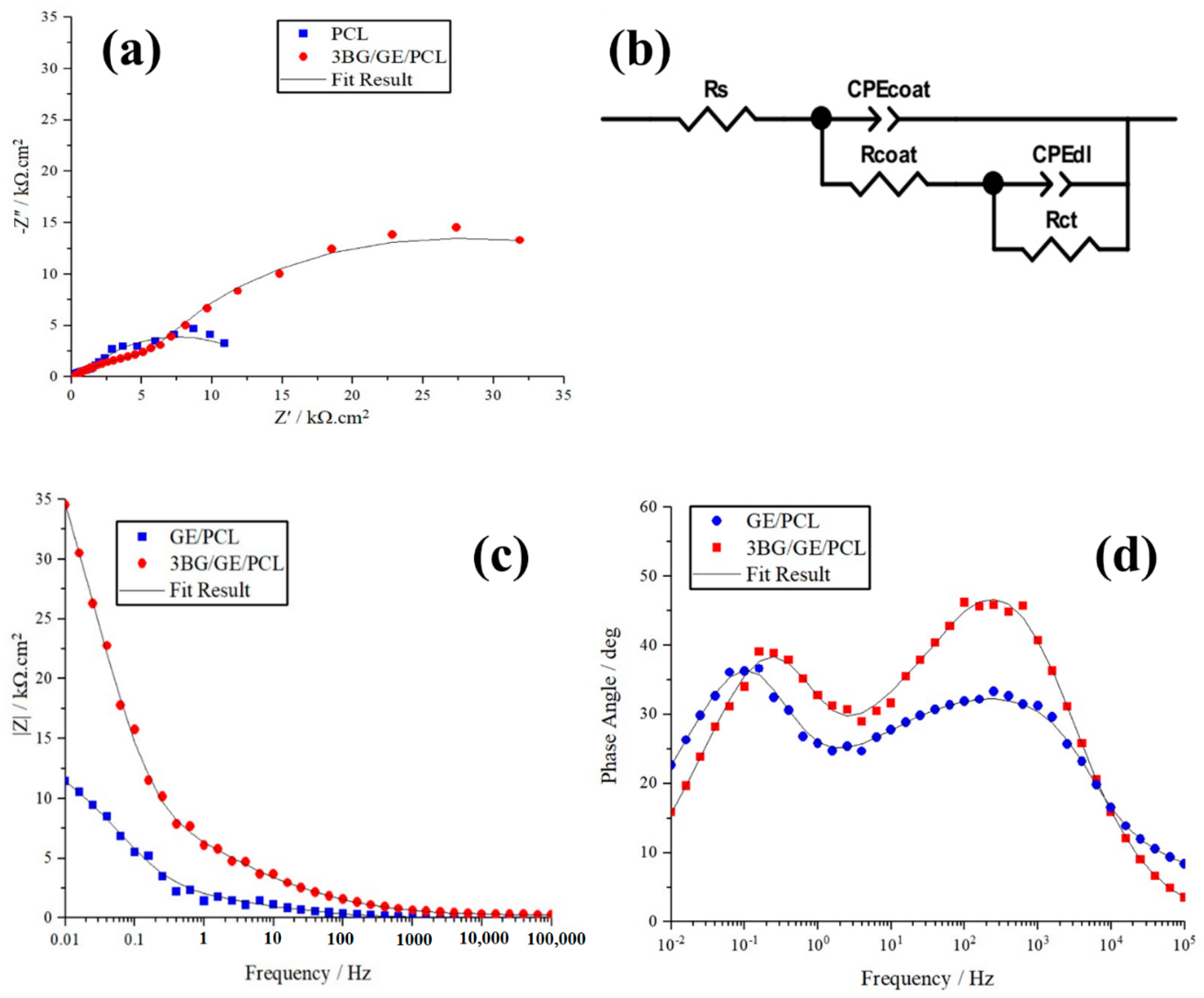
3.4. Electrochemical Behavior of Coated Substrates
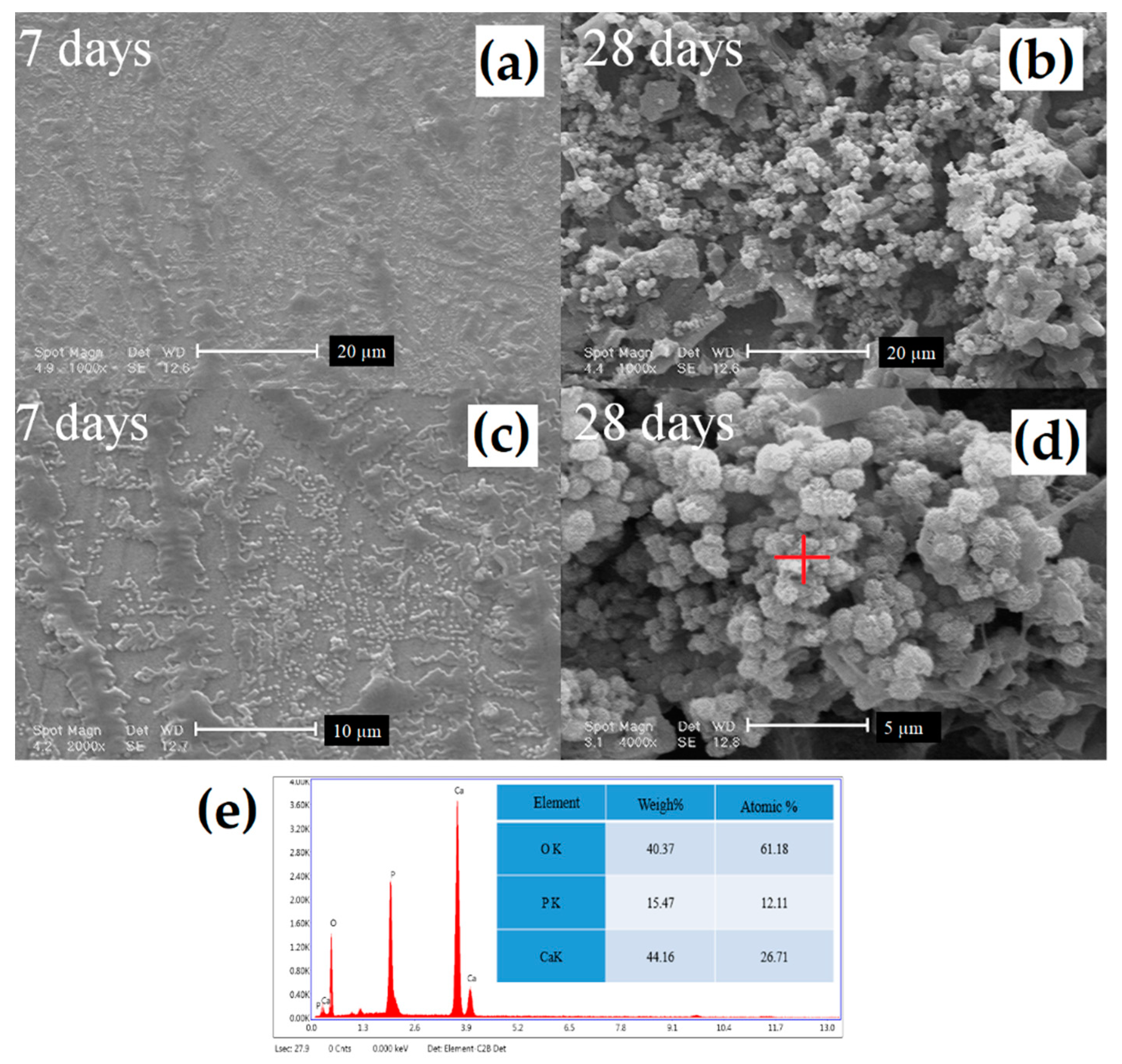
3.5. Bioactivity Evaluation
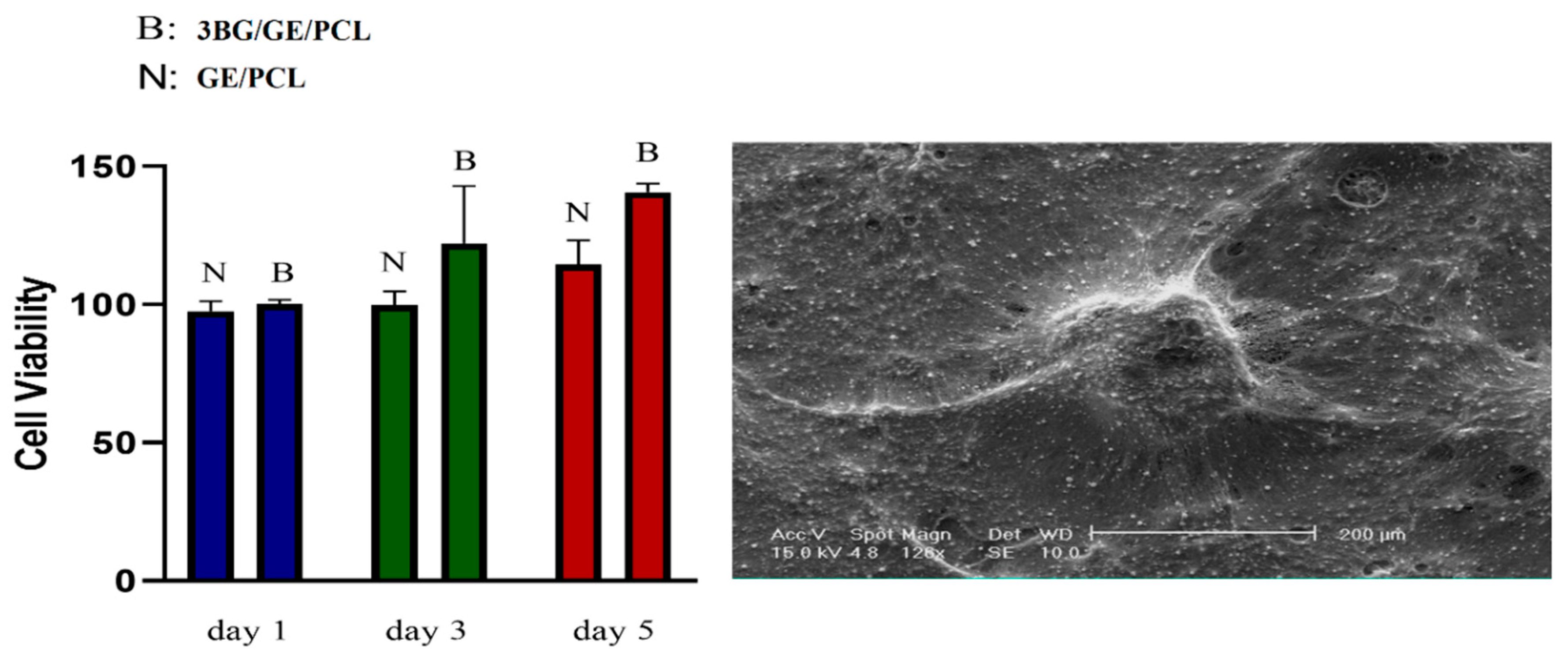
3.6. MTT Analysis
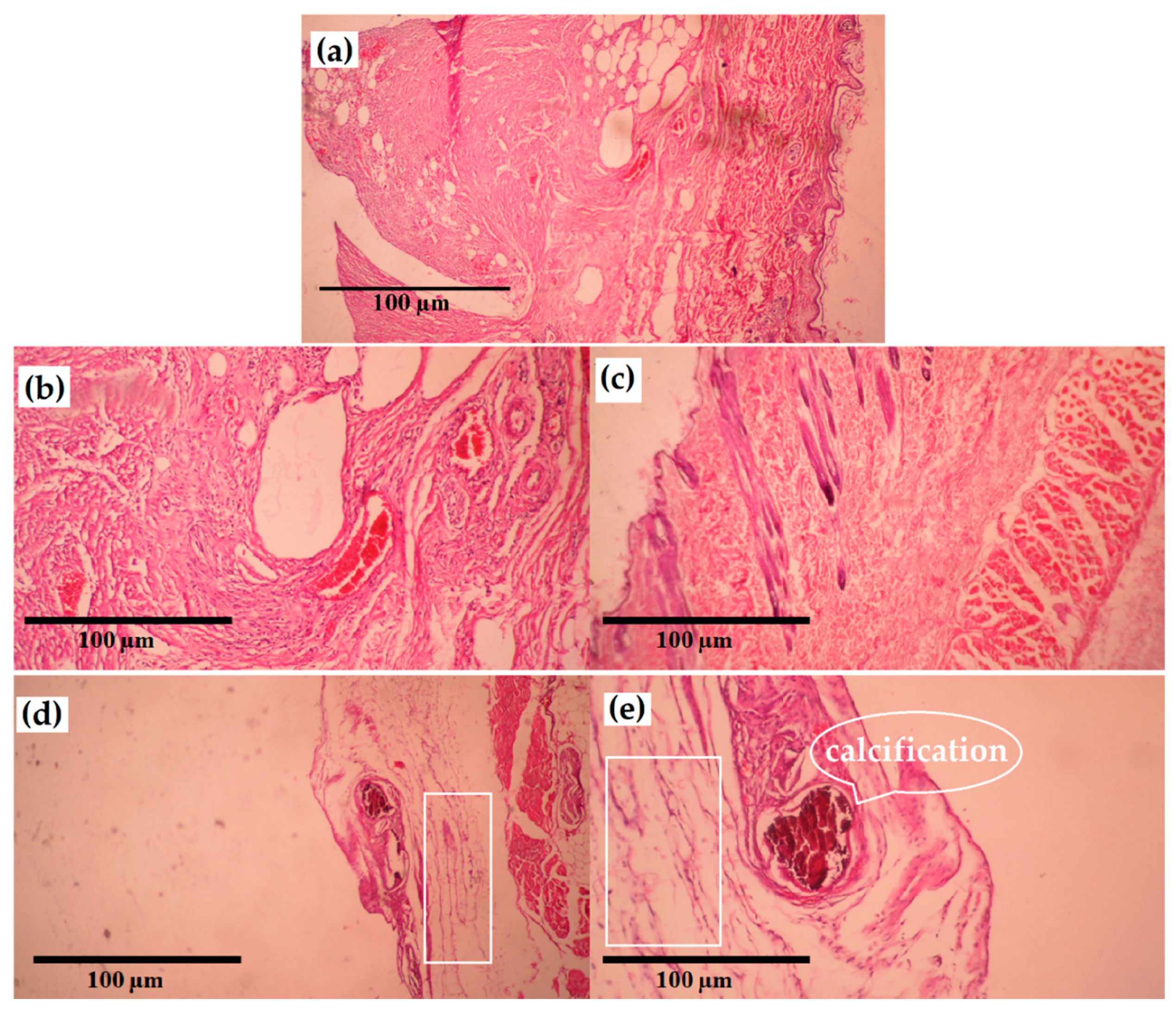
3.7. In Vivo Study
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bordjih, K.; Jouzeau, J.-Y.; Mainard, D.; Payan, E.; Delagoutte, J.-P.; Netter, P. Evaluation of the effect of three surface treatments on the biocompatibility of 316L stainless steel using human differentiated cells. Biomaterials 1996, 17, 491–500. [Google Scholar] [CrossRef]
- Talha, M.; Behera, C.; Sinha, O. Effect of nitrogen and cold working on structural and mechanical behavior of Ni-free nitrogen containing austenitic stainless steels for biomedical applications. Mater. Sci. Eng. C 2015, 47, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Gadkari, S.; Gu, S.; Sadhukhan, J. Two-dimensional mathematical model of an air-cathode microbial fuel cell with graphite fiber brush anode. J. Power Sources 2019, 441, 227145. [Google Scholar] [CrossRef]
- Kocijan, A.; Conradi, M.; Hočevar, M. The influence of surface wettability and topography on the bioactivity of TiO2/epoxy coatings on AISI 316L stainless steel. Materials 2019, 12, 1877. [Google Scholar] [CrossRef]
- Vickers, N.J. Animal communication: When i’m calling you, will you answer too? Curr. Biol. 2017, 27, R713–R715. [Google Scholar] [CrossRef]
- Bekmurzayeva, A.; Duncanson, W.J.; Azevedo, H.S.; Kanayeva, D. Surface modification of stainless steel for biomedical applications: Revisiting a century-old material. Mater. Sci. Eng. C 2018, 93, 1073–1089. [Google Scholar] [CrossRef]
- Avcu, E.; Baştan, F.E.; Abdullah, H.Z.; Rehman, M.A.U.; Avcu, Y.Y.; Boccaccini, A.R. Electrophoretic deposition of chitosan-based composite coatings for biomedical applications: A review. Prog. Mater. Sci. 2019, 103, 69–108. [Google Scholar] [CrossRef]
- Kubisztal, J.; Kubisztal, M.; Stach, S.; Haneczok, G. Corrosion resistance of anodic coatings studied by scanning microscopy and electrochemical methods. Surf. Coat. Technol. 2018, 350, 419–427. [Google Scholar] [CrossRef]
- Alizadeh-Osgouei, M.; Li, Y.; Wen, C. A comprehensive review of biodegradable synthetic polymer-ceramic composites and their manufacture for biomedical applications. Bioact. Mater. 2019, 4, 22–36. [Google Scholar] [CrossRef]
- Zahran, M.; Marei, A.H. Innovative natural polymer metal nanocomposites and their antimicrobial activity. Int. J. Biol. Macromol. 2019, 136, 586–596. [Google Scholar] [CrossRef]
- Abrisham, M.; Noroozi, M.; Panahi-Sarmad, M.; Arjmand, M.; Goodarzi, V.; Shakeri, Y.; Golbaten-Mofrad, H.; Dehghan, P.; Sahzabi, A.S.; Sadri, M.; et al. The Role of Polycaprolactone-Triol (PCL-T) in Biomedical Applications: A state-of-the-art review. Eur. Polym. J. 2020, 131, 109701. [Google Scholar] [CrossRef]
- Jia, L.; Han, F.; Wang, H.; Zhu, C.; Guo, Q.; Li, J.; Zhao, Z.; Zhang, Q.; Zhu, X.; Li, B. Polydopamine-assisted surface modification for orthopaedic implants. J. Orthop. Transl. 2019, 17, 82–95. [Google Scholar] [CrossRef] [PubMed]
- Catauro, M.; Bollino, F.; Giovanardi, R.; Veronesi, P. Modification of Ti6Al4V implant surfaces by biocompatible TiO2/PCL hybrid layers prepared via sol-gel dip coating: Structural characterization, mechanical and corrosion behavior. Mater. Sci. Eng. C 2017, 74, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Catauro, M.; Bollino, F.; Papale, F.; Marciano, S.; Pacifico, S. TiO2/PCL hybrid materials synthesized via sol-gel technique for biomedical applications. Mater. Sci. Eng. C 2015, 47, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Grossen, P.; Witzigmann, D.; Sieber, S.; Huwyler, J. PEG-PCL-based nanomedicines: A biodegradable drug delivery system and its application. J. Control. Release 2017, 260, 46–60. [Google Scholar] [CrossRef] [PubMed]
- Pokorski, J.K.; Hore, M.J. Structural characterization of protein–polymer conjugates for biomedical applications with small-angle scattering. Curr. Opin. Colloid Interface Sci. 2019, 42, 157–168. [Google Scholar] [CrossRef]
- Lv, L.-C.; Huang, Q.-Y.; Ding, W.; Xiao, X.-H.; Zhang, H.-Y.; Xiong, L.-X. Fish gelatin: The novel potential applications. J. Funct. Foods 2019, 63, 103581. [Google Scholar] [CrossRef]
- Govindan, R.; Gu, F.; Karthi, S.; Girija, E. Effect of phosphate glass reinforcement on the mechanical and biological properties of freeze-dried gelatin composite scaffolds for bone tissue engineering applications. Mater. Today Commun. 2020, 22, 100765. [Google Scholar] [CrossRef]
- Divya, M.; Vaseeharan, B.; Abinaya, M.; Vijayakumar, S.; Govindarajan, M.; Alharbi, N.S.; Kadaikunnan, S.; Khaled, J.M.; Benelli, G. Biopolymer gelatin-coated zinc oxide nanoparticles showed high antibacterial, antibiofilm and anti-angiogenic activity. J. Photochem. Photobiol. B Biol. 2018, 178, 211–218. [Google Scholar] [CrossRef]
- Sun, Z.; Qin, R.; Li, D.; Ji, K.; Wang, T.; Cui, Z.; Huang, Y. A novel bacterial type II l-asparaginase and evaluation of its enzymatic acrylamide reduction in French fries. Int. J. Biol. Macromol. 2016, 92, 232–239. [Google Scholar] [CrossRef]
- Torkaman, R.; Darvishi, S.; Jokar, M.; Kharaziha, M.; Karbasi, M. Electrochemical and in vitro bioactivity of nanocomposite gelatin-forsterite coatings on AISI 316L stainless steel. Prog. Org. Coat. 2017, 103, 40–47. [Google Scholar] [CrossRef]
- Cañaveral, S.; Morales, D.; Vargas, A.F. Synthesis and characterization of a 58S bioglass modified with manganese by a sol-gel route. Mater. Lett. 2019, 255, 126575. [Google Scholar] [CrossRef]
- Khosravi, F.; Khorasani, S.N.; Khalili, S.; Neisiany, R.E.; Ghomi, E.R.; Ejeian, F.; Das, O.; Nasr-Esfahani, M.H. Development of a Highly Proliferated Bilayer Coating on 316L Stainless Steel Implants. Polymers 2020, 12, 1022. [Google Scholar] [CrossRef] [PubMed]
- Macuvele, D.L.P.; Nones, J.; Matsinhe, J.V.; Lima, M.M.; Soares, C.; Fiori, M.A.; Riella, H.G. Advances in ultra high molecular weight polyethylene/hydroxyapatite composites for biomedical applications: A brief review. Mater. Sci. Eng. C 2017, 76, 1248–1262. [Google Scholar] [CrossRef] [PubMed]
- Mesquita-Guimarães, J.; Ramos, L.; Detsch, R.; Henriques, B.; Fredel, M.; Silva, F.; Boccaccini, A.R. Evaluation of in vitro properties of 3D micro-macro porous zirconia scaffolds coated with 58S bioactive glass using MG-63 osteoblast-like cells. J. Eur. Ceram. Soc. 2019, 39, 2545–2558. [Google Scholar] [CrossRef]
- Boodagh, P.; Johnson, R.; Maly, C.; Ding, Y.; Tan, W. Soft-sheath, stiff-core microfiber hydrogel for coating vascular implants. Colloids Surf. B Biointerfaces 2019, 183, 110395. [Google Scholar] [CrossRef]
- Hanas, T.; Kumar, T.S.; Perumal, G.; Doble, M.; Ramakrishna, S. Electrospun PCL/HA coated friction stir processed AZ31/HA composites for degradable implant applications. J. Mater. Process. Technol. 2018, 252, 398–406. [Google Scholar]
- Yang, G.; Chen, H.; Qin, H.; Feng, Y. Amination of activated carbon for enhancing phenol adsorption: Effect of nitrogen-containing functional groups. Appl. Surf. Sci. 2014, 293, 299–305. [Google Scholar] [CrossRef]
- Ananth, K.P.; Nathanael, A.J.; Jose, S.P.; Oh, T.H.; Mangalaraj, D. A novel silica nanotube reinforced ionic incorporated hydroxyapatite composite coating on polypyrrole coated 316L SS for implant application. Mater. Sci. Eng. C 2016, 59, 1110–1124. [Google Scholar] [CrossRef]
- de Souza, E.A.; Giz, M.J.; Camara, G.A.; Antolini, E.; Passos, R.R. Ethanol electro-oxidation on partially alloyed Pt-Sn-Rh/C catalysts. Electrochim. Acta 2014, 147, 483–489. [Google Scholar] [CrossRef]
- Kumar, A.M.; Nagarajan, S.; Ramakrishna, S.; Sudhagar, P.; Kang, Y.S.; Kim, H.; Gasem, Z.M.; Rajendran, N. Electrochemical and in vitro bioactivity of polypyrrole/ceramic nanocomposite coatings on 316L SS bio-implants. Mater. Sci. Eng. C 2014, 43, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.M.; Sudhagar, P.; Fujishima, A.; Gasem, Z.M. Hierarchical polymer nanocomposite coating material for 316L SS implants: Surface and electrochemical aspects of PPy/f-CNTs coatings. Polymer 2014, 55, 5417–5424. [Google Scholar] [CrossRef]
- Kichi, M.K.; Torkaman, R.; Mohammadi, H.; Toutounchi, A.; Kharaziha, M.; Alihosseini, F. Electrochemical and in vitro bioactivity behavior of Poly (ε-caprolactone)(PCL)-Gelatin-Forsterite Nano Coating on Titanium for Biomedical Application. Mater. Today Commun. 2020, 24, 101326. [Google Scholar] [CrossRef]
- Matsuo, T. Trehalose protects corneal epithelial cells from death by drying. Br. J. Ophthalmol. 2001, 85, 610–612. [Google Scholar] [CrossRef] [PubMed]
- Won, Y.-W.; Patel, A.N.; Bull, D.A. Cell surface engineering to enhance mesenchymal stem cell migration toward an SDF-1 gradient. Biomaterials 2014, 35, 5627–5635. [Google Scholar] [CrossRef] [PubMed]
- Iso, B.; Standard, B. Biological evaluation of medical devices. Part 2009, 1, 10993. [Google Scholar]
- Yang, Y.; Zheng, K.; Liang, R.; Mainka, A.; Taccardi, N.; Roether, J.A.; Detsch, R.; Goldmann, W.H.; Virtanen, S.; Boccaccini, A.R. Cu-releasing bioactive glass/polycaprolactone coating on Mg with antibacterial and anticorrosive properties for bone tissue engineering. Biomed. Mater. 2017, 13, 015001. [Google Scholar] [CrossRef]
- Gorustovich, A.A.; Roether, J.A.; Boccaccini, A.R. Effect of bioactive glasses on angiogenesis: A review of in vitro and in vivo evidences. Tissue Eng. Part B Rev. 2010, 16, 199–207. [Google Scholar] [CrossRef]
- Rahaman, M.N.; Day, D.E.; Bal, B.S.; Fu, Q.; Jung, S.B.; Bonewald, L.F.; Tomsia, A.P. Bioactive glass in tissue engineering. Acta Biomater. 2011, 7, 2355–2373. [Google Scholar] [CrossRef]
- Sterling, D.L.; Thornton, J.D.; Swafford, A.; Gottlieb, S.; Bishop, S.; Stanley, A.; Downey, J.M. Hyperbaric oxygen limits infarct size in ischemic rabbit myocardium in vivo. Circulation 1993, 88, 1931–1936. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Raeissi, K.; Karimzadeh, F.; Golozar, M. A study on corrosion behavior of graphene oxide coating produced on stainless steel by electrophoretic deposition. Surf. Coat. Technol. 2019, 372, 327–342. [Google Scholar] [CrossRef]
- Deligianni, D.D.; Katsala, N.D.; Koutsoukos, P.G.; Missirlis, Y.F. Effect of surface roughness of hydroxyapatite on human bone marrow cell adhesion, proliferation, differentiation and detachment strength. Biomaterials 2000, 22, 87–96. [Google Scholar] [CrossRef]
- Lu, A.; Gao, Y.; Jin, T.; Luo, X.; Zeng, Q.; Shang, Z. Effects of surface roughness and texture on the bacterial adhesion on the bearing surface of bio-ceramic joint implants: An in vitro study. Ceram. Int. 2000, 46, 6550–6559. [Google Scholar] [CrossRef]
- Coan, T.; Barroso, G.; Machado, R.; de Souza, F.; Spinelli, A.; Motz, G. A novel organic-inorganic PMMA/polysilazane hybrid polymer for corrosion protection. Prog. Org. Coat. 2015, 89, 220–230. [Google Scholar] [CrossRef]
- Tan, O.; Cimen, O.; Yolcu, P.; ÇİÇEK, B. Development and characterisation of solvent-borne thermally cured cross-linked TiO2 reinforced Polyceramic coatings for long service-life on industrial metal substrates. La Metall. Ital. 2020, 6, 6–20. [Google Scholar]
- Tamjid, E.; Bagheri, R.; Vossoughi, M.; Simchi, A. Effect of particle size on the in vitro bioactivity, hydrophilicity and mechanical properties of bioactive glass-reinforced polycaprolactone composites. Mater. Sci. Eng. C 2011, 31, 1526–1533. [Google Scholar] [CrossRef]
- Tabesh, E.; Kharaziha, M.; Mahmoudi, M.; Shahnam, E.; Rozbahani, M. Biological and corrosion evaluation of Laponite®: Poly (caprolactone) nanocomposite coating for biomedical applications. Colloids Surf. A Physicochem. Eng. Asp. 2019, 583, 123945. [Google Scholar] [CrossRef]
- Tabesh, E.; Salimijazi, H.; Kharaziha, M.; Mahmoudi, M.; Hejazi, M. Development of an in-situ chitosan-copper nanoparticle coating by electrophoretic deposition. Surf. Coat. Technol. 2019, 364, 239–247. [Google Scholar] [CrossRef]
- Ataei, S.; Khorasani, S.N.; Torkaman, R.; Neisiany, R.E.; Koochaki, M.S. Self-healing performance of an epoxy coating containing microencapsulated alkyd resin based on coconut oil. Prog. Org. Coat. 2018, 120, 160–166. [Google Scholar] [CrossRef]
- Li, S.; Ren, G.; Hoque, M.N.F.; Dong, Z.; Warzywoda, J.; Fan, Z. Carbonized cellulose paper as an effective interlayer in lithium-sulfur batteries. Appl. Surf. Sci. 2017, 396, 637–643. [Google Scholar] [CrossRef]
- Wang, L.; Wei, Z.-L.; Chen, Z.-Z.; Liu, C.; Dong, W.-K.; Ding, Y.-J. A chemical probe capable for fluorescent and colorimetric detection to Cu2+ and CN− based on coordination and nucleophilic addition mechanism. Microchem. J. 2020, 155, 104801. [Google Scholar] [CrossRef]
- Barrère, F.; van Blitterswijk, C.A.; de Groot, K. Bone regeneration: Molecular and cellular interactions with calcium phosphate ceramics. Int. J. Nanomed. 2006, 1, 317. [Google Scholar]
- Dziadek, M.; Zagrajczuk, B.; Menaszek, E.; Cholewa-Kowalska, K. A new insight into in vitro behaviour of poly (ε-caprolactone)/bioactive glass composites in biologically related fluids. J. Mater. Sci. 2018, 53, 3939–3958. [Google Scholar] [CrossRef]
- Islam, M.T.; Felfel, R.M.; Neel, E.A.A.; Grant, D.M.; Ahmed, I.; Hossain, K.M.Z. Bioactive calcium phosphate–based glasses and ceramics and their biomedical applications: A review. J. Tissue Eng. 2017, 8, 2041731417719170. [Google Scholar] [CrossRef] [PubMed]
- Coletti, C.; Jaroszeski, M.J.; Hoff, A.; Saddow, S.E. SiC In Vitro Biocompatibility: Epidermal and Connective Tissue Cells. In Silicon Carbide Biotechnology: A Biocompatible Semiconductor for Advanced Biomedical Devices and Applications; Elsevier: Amsterdam, The Netherlands, 2011; pp. 119–151. [Google Scholar]
- Makino, H.; Emi, H.; Yamaguchi, A.; Iritani, E.; Namiki, N.; Myojo, T.; Yamamoto, K. Environmental and safety issues with nanoparticles. In Nanoparticle Technology Handbook; Elsevier: Amsterdam, The Netherlands, 2008; pp. 385–417. [Google Scholar]
- Mishra, D.; Iyyanki, T.; Hubenak, J.; Zhang, Q.; Mathur, A.B. Silk fibroin nanoparticles and cancer therapy. In Nanotechnology in Cancer; Elsevier: Amsterdam, The Netherlands, 2017; pp. 19–44. [Google Scholar]
- Cadena-Herrera, D.; Lara, J.E.E.-D.; Ramírez-Ibañez, N.D.; López-Morales, C.A.; Pérez, N.O.; Flores-Ortiz, L.F.; Medina-Rivero, E. Validation of three viable-cell counting methods: Manual, semi-automated, and automated. Biotechnol. Rep. 2015, 7, 9–16. [Google Scholar] [CrossRef]
- Baserga, C.; Massarelli, O.; Bolzoni, A.R.; Rossi, D.S.; Beltramini, G.A.; Baj, A.; Giannì, A.B. Fibula free flap pedicle ossification: Experience of two centres and a review of the literature. J. Cranio Maxillofac. Surg. 2018, 46, 1674–1678. [Google Scholar] [CrossRef]
- Bemenderfer, T.B.; Davis, W.H.; Anderson, R.B.; Wing, K.; Escudero, M.I.; Waly, F.; Penner, M. Heterotopic ossification in total ankle arthroplasty: Case series and systematic review. J. Foot Ankle Surg. 2020, 59, 716–721. [Google Scholar] [CrossRef]
| Designation | Polycaprolactone (wt.%) | Gelatin (wt.%) | Bioglass (wt.%) |
|---|---|---|---|
| GE/PCL | 90 | 10 | - |
| 1BG/GE/PCL | 89.1 | 9.9 | 1 |
| 3BG/GE/PCL | 87.3 | 9.7 | 3 |
| 5BG/GE/PCL | 85.5 | 9.5 | 5 |
| Samples | Surface Roughness (µm) | ||
|---|---|---|---|
| Ra (the Average Roughness) | Rq | Rz | |
| 5BG/GE/PCL | 5.08 | 6.62 | 40.37 |
| 3BG/GE/PCL | 2.57 | 3.16 | 17.26 |
| 1BG/GE/PCL | 1.47 | 1.78 | 8.65 |
| GE/PCL | 0.71 | 0.97 | 8.09 |
| Samples | Classification | % of Area Removed |
|---|---|---|
| 5BG/GE/PCL | 6B | Less than 5% |
| 3BG/GE/PCL | 6B | Less than 5% |
| 1BG/GE/PCL | 3B | 5–15% |
| GE/PCL | 3B | 5–15% |
| Samples | Rs (Ω cm2) | CPEcoat (µF·cm−2) | n | Rcoat (Ω·cm2) | CPEdl (µF·cm−2) | n | Rct (Ω·cm2) | χ−2 (Chi-Squared) |
|---|---|---|---|---|---|---|---|---|
| GE/PCL | 41.56 | 9.37 × 10−5 | 0.72 | 2141 | 1.21 × 10−4 | 0.89 | 11,543 | 0.00028 |
| 3BG/GE/PCL | 45.4 | 6.52 × 10−5 | 0.43 | 10,790 | 1.21 × 10−7 | 0.86 | 49,634 | 0.00027 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mojarad Shafiee, B.; Torkaman, R.; Mahmoudi, M.; Emadi, R.; Derakhshan, M.; Karamian, E.; Tavangarian, F. Surface Modification of 316L SS Implants by Applying Bioglass/Gelatin/Polycaprolactone Composite Coatings for Biomedical Applications. Coatings 2020, 10, 1220. https://doi.org/10.3390/coatings10121220
Mojarad Shafiee B, Torkaman R, Mahmoudi M, Emadi R, Derakhshan M, Karamian E, Tavangarian F. Surface Modification of 316L SS Implants by Applying Bioglass/Gelatin/Polycaprolactone Composite Coatings for Biomedical Applications. Coatings. 2020; 10(12):1220. https://doi.org/10.3390/coatings10121220
Chicago/Turabian StyleMojarad Shafiee, Behzad, Reza Torkaman, Mohammad Mahmoudi, Rahmatollah Emadi, Maryam Derakhshan, Ebrahim Karamian, and Fariborz Tavangarian. 2020. "Surface Modification of 316L SS Implants by Applying Bioglass/Gelatin/Polycaprolactone Composite Coatings for Biomedical Applications" Coatings 10, no. 12: 1220. https://doi.org/10.3390/coatings10121220
APA StyleMojarad Shafiee, B., Torkaman, R., Mahmoudi, M., Emadi, R., Derakhshan, M., Karamian, E., & Tavangarian, F. (2020). Surface Modification of 316L SS Implants by Applying Bioglass/Gelatin/Polycaprolactone Composite Coatings for Biomedical Applications. Coatings, 10(12), 1220. https://doi.org/10.3390/coatings10121220






