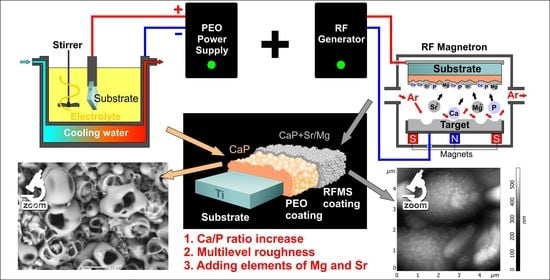
Porous CaP Coatings Formed by Combination of Plasma Electrolytic Oxidation and RF-Magnetron Sputtering
Abstract
1. Introduction
2. Materials and Methods
2.1. Coatings Formation
2.2. Coating Thickness
2.3. Scanning Electron Microscopy (SEM)
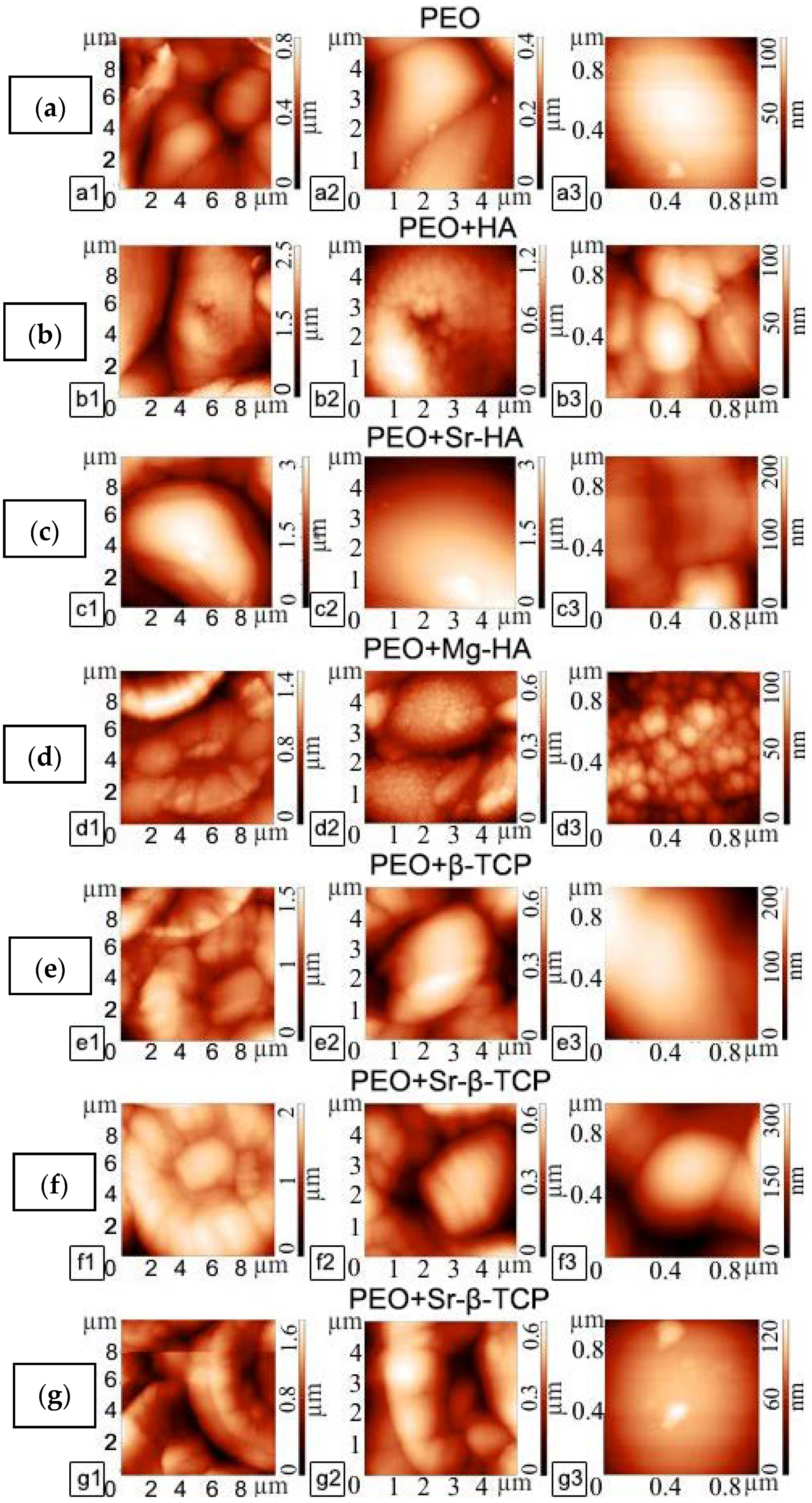
2.4. Atomic Force Microscopy (AFM)
2.5. X-ray Photoelectron Spectroscopy (XPS)
2.6. X-ray Diffraction (XRD) Analysis
2.7. Nanoindentation
3. Results
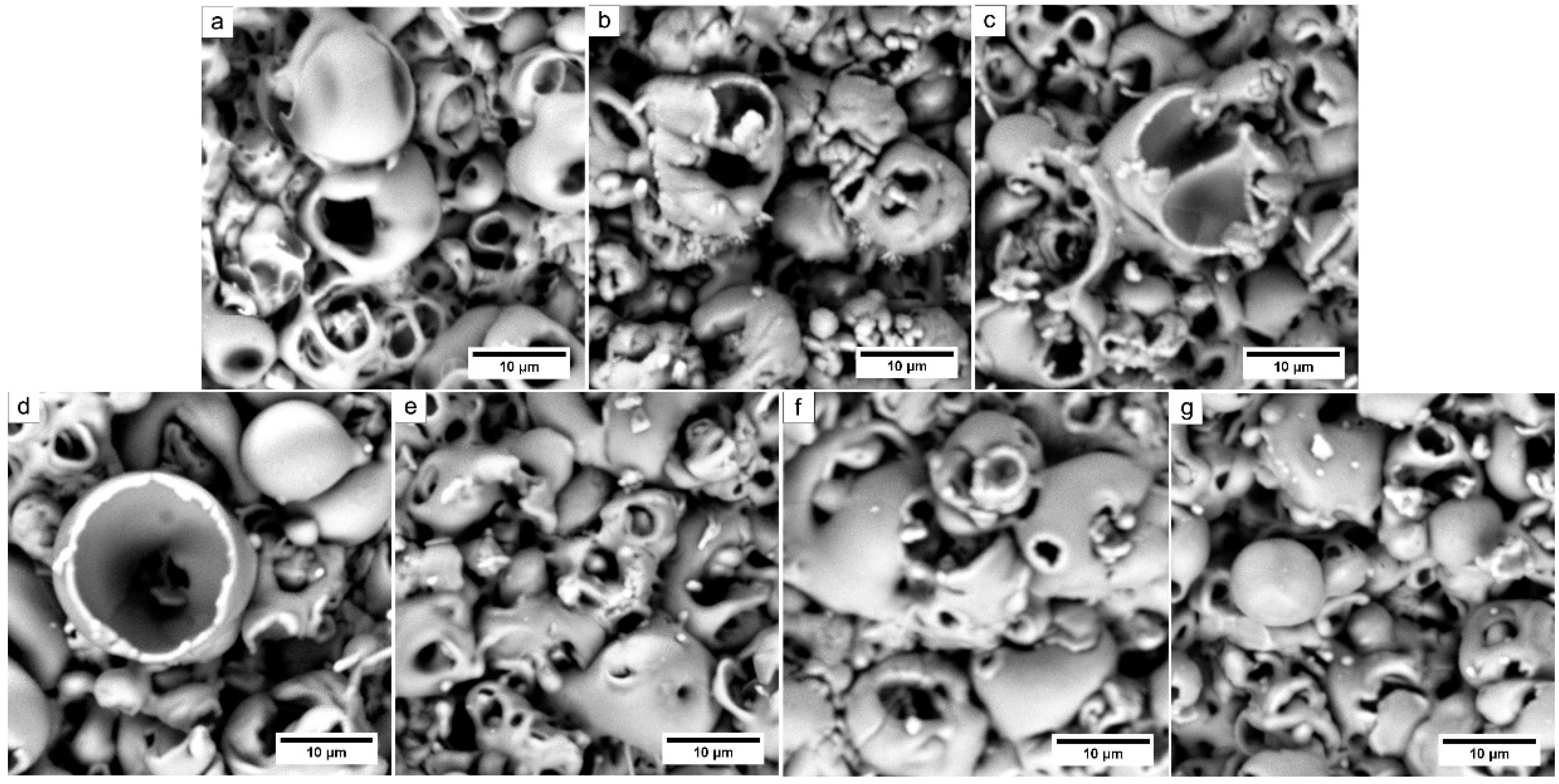
3.1. Composite Coating Morphology
3.2. Coating Phase Composition
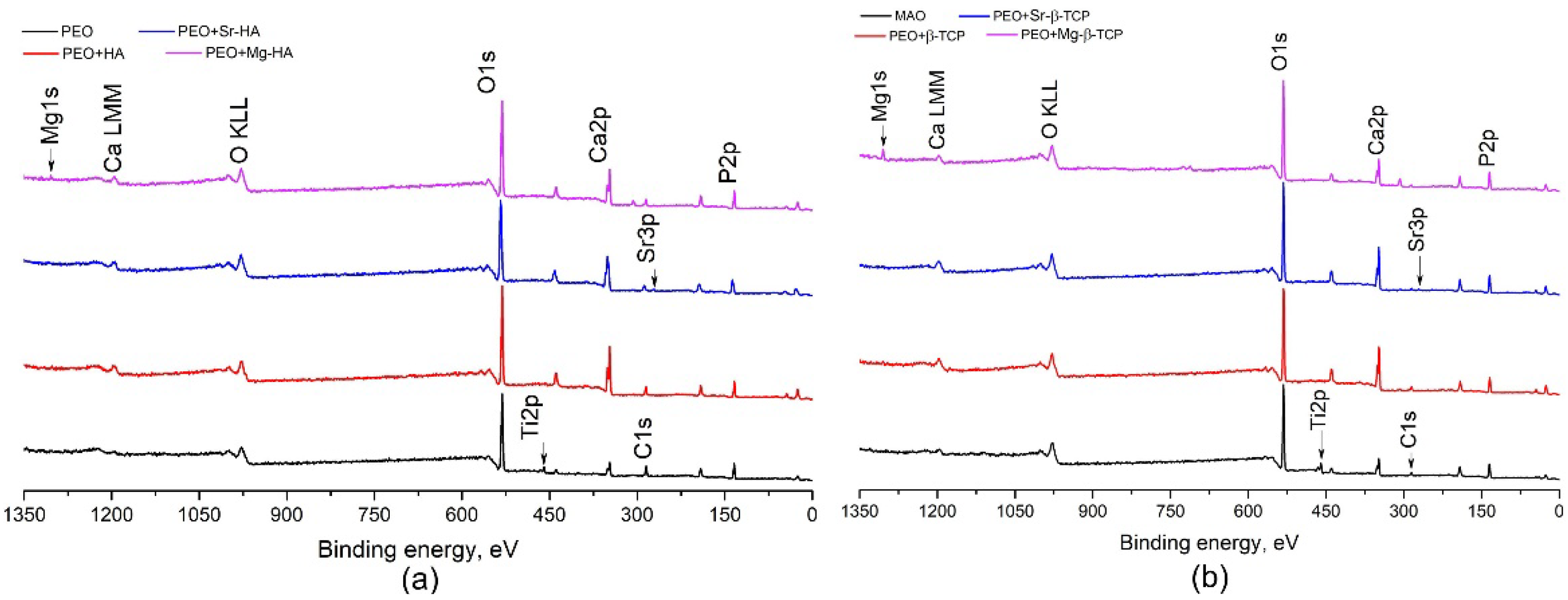
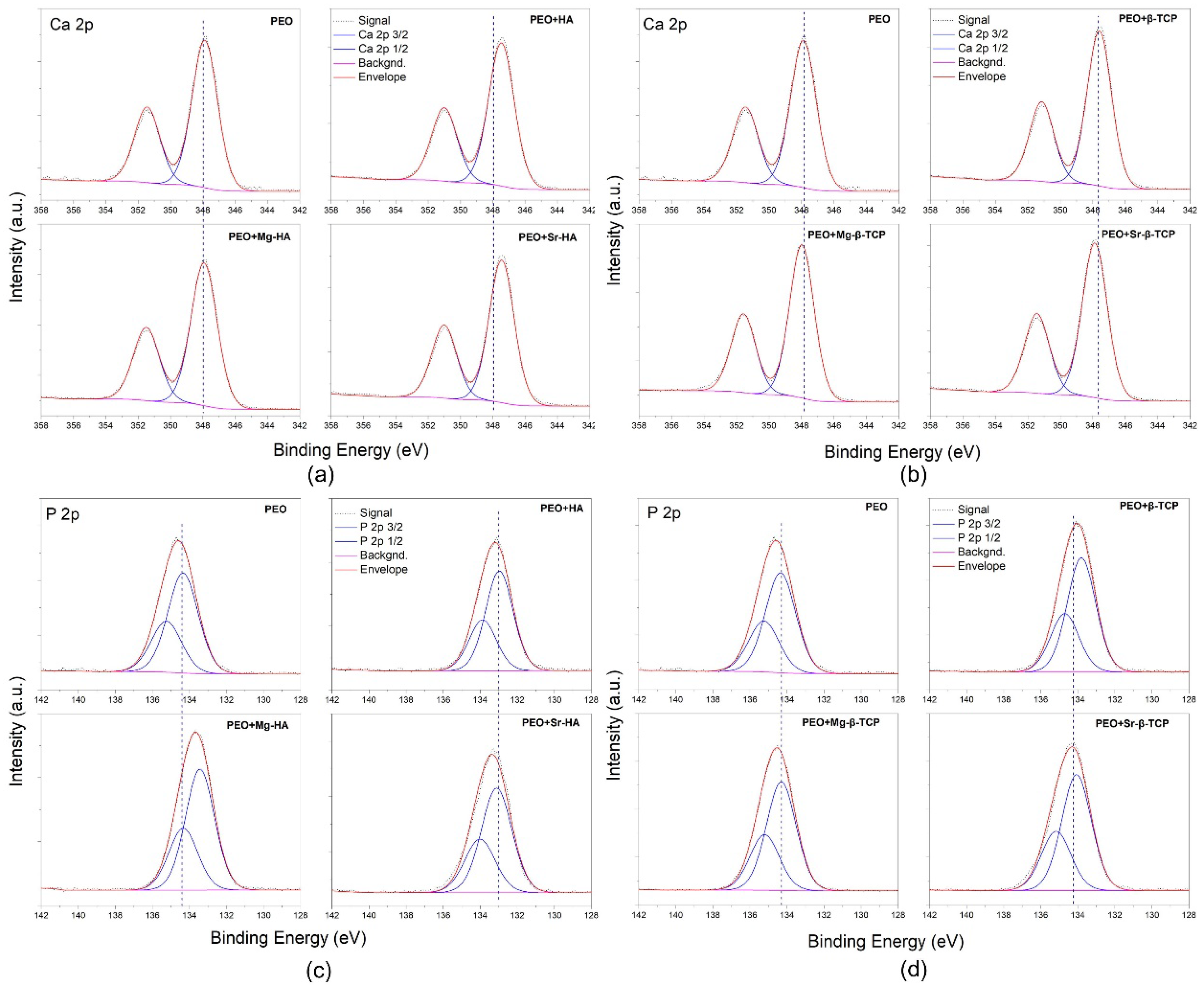
3.3. Coating Chemical Composition
- PEO and PEO + Mg-β-TCP coatings show the highest P2p binding energy;
- PEO + Mg-β-TCP and PEO+Mg-HA coatings show the highest Ca2p binding energy;
- Binding energy increases by the following progression: PEO + Mg-β-TCP < PEO + Sr-β-TCP < PEO + β-TCP and PEO + Mg-HA < PEO + Sr-HA < PEO + HA.
3.4. Young’s Modulus
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, F.; Song, Y.; Wang, F.; Shimizu, T.; Igarashi, K.; Zhao, L. Formation characterization of hydroxyapatite on titanium by microarc oxidation and hydrothermal treatment. J. Biosci. Bioeng. 2005, 100, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Parfenov, E.; Parfenova, L.; Mukaeva, V.; Farrakhov, R.; Stotskiy, A.; Raab, A.; Danilko, K.; Rameshbabu, N.; Valiev, R. Biofunctionalization of PEO coatings on titanium implants with inorganic and organic substances. Surf. Coat. Technol. 2020, 404, 126486. [Google Scholar] [CrossRef]
- Yang, W.; Wang, P.; Guo, Y.; Jiang, B.; Yang, F.; Li, J. Microstructure and corrosion resistance of modified AZ31 magnesium alloy using microarc oxidation combined with electrophoresis process. J. Wuhan Univ. Technol. Sci. Ed. 2013, 28, 612–616. [Google Scholar] [CrossRef]
- Rehman, Z.U.; Choi, D.; Koo, B.H. A three layer corrosion protection system for Mg–9Al–Zn alloy offered by complex fluoride ions and precipitates-based electrolytic oxidation. Surf. Coat. Technol. 2020, 393, 125804. [Google Scholar] [CrossRef]
- Ur Rehman, Z.; Heun Koo, B.; Choi, D. Influence of complex SiF62− Ions on the PEO coatings formed on Mg–Al6–Zn1 alloy for enhanced wear and corrosion protection. Coatings 2020, 10, 94. [Google Scholar] [CrossRef]
- Ur Rehman, Z.; Choi, D. Investigation of ZrO2 nanoparticles concentration and processing time effect on the localized PEO coatings formed on AZ91 alloy. J. Magnes. Alloy. 2019, 7, 555–565. [Google Scholar] [CrossRef]
- Gu, Y.; Xiong, W.; Ning, C.; Zhang, J. Residual stresses in microarc oxidation ceramic coatings on biocompatible AZ31 magnesium alloys. J. Mater. Eng. Perform. 2011. [Google Scholar] [CrossRef]
- Akin, F.A.; Zreiqat, H.; Jordan, S.; Wijesundara, M.B.J.; Hanley, L. Preparation and analysis of macroporous TiO2 films on Ti surfaces for bone–tissue implants. J. Biomed. Mater. Res. Part A 2001, 57, 588–596. [Google Scholar] [CrossRef]
- Yavari, S.A.; Necula, B.S.; Fratila-Apachitei, L.E.; Duszczyk, J.; Apachitei, I. Biofunctional surfaces by plasma electrolytic oxidation on titanium biomedical alloys. Surf. Eng. 2016, 32, 411–417. [Google Scholar] [CrossRef]
- Krząkała, A.; Kazek-Kęsik, A.; Simka, W. Application of plasma electrolytic oxidation to bioactive surface formation on titanium and its alloys. RSC Adv. 2013, 3, 19725. [Google Scholar] [CrossRef]
- Barati Darband, G.; Aliofkhazraei, M.; Hamghalam, P.; Valizade, N. Plasma electrolytic oxidation of magnesium and its alloys: Mechanism, properties and applications. J. Magnes. Alloy. 2017, 5, 74–132. [Google Scholar] [CrossRef]
- Jiang, B.L.; Wang, Y.M. Plasma electrolytic oxidation treatment of aluminium and titanium alloys. In Surface Engineering of Light Alloys; Elsevier: Amsterdam, The Netherlands, 2010; pp. 110–154. [Google Scholar]
- Depla, D.; Mahieu, S.; Greene, J.E. Sputter Deposition Processes. In B Handbook of Deposition Technologies for Films and Coatings; Elsevier: Amsterdam, The Netherlands, 2010; pp. 253–296. [Google Scholar]
- Juhasz, J.A.; Best, S.M. Surface modification of biomaterials by calcium phosphate deposition. In Surface Modification of Biomaterials; Elsevier: Amsterdam, The Netherlands, 2011; pp. 143–169. [Google Scholar]
- Park, S.-Y.; Choe, H.-C. Mn-coatings on the micro-pore formed Ti–29Nb–xHf alloys by RF-magnetron sputtering for dental applications. Appl. Surf. Sci. 2018, 432, 278–284. [Google Scholar] [CrossRef]
- Hwang, I.-J.; Choe, H.-C. Surface morphology and cell behavior of Zn-coated Ti–6Al–4V alloy by RF-sputtering after PEO-treatment. Surf. Coat. Technol. 2019, 361, 386–395. [Google Scholar] [CrossRef]
- Lu, J.; Yu, H.; Chen, C. Biological properties of calcium phosphate biomaterials for bone repair: A review. RSC Adv. 2018, 8, 2015–2033. [Google Scholar] [CrossRef]
- Urquia Edreira, E.R.; Wolke, J.G.C.; Aldosari, A.A.; Al-Johany, S.S.; Anil, S.; Jansen, J.A.; van den Beucken, J.J.J.P. Effects of calcium phosphate composition in sputter coatings on in vitro and in vivo performance. J. Biomed. Mater. Res. Part A 2015, 103, 300–310. [Google Scholar] [CrossRef]
- Eliaz, N.; Metoki, N. Calcium phosphate bioceramics: A review of their history, structure, properties, coating technologies and biomedical applications. Materials 2017, 10, 334. [Google Scholar] [CrossRef]
- Zeng, J.; Guo, J.; Sun, Z.; Deng, F.; Ning, C.; Xie, Y. Osteoblastic and anti-osteoclastic activities of strontium-substituted silicocarnotite ceramics: In vitro and in vivo studies. Bioact. Mater. 2020, 5, 435–446. [Google Scholar] [CrossRef]
- BS EN ISO 14577 International Organization for Standardization. Metallic Materials: Instrumented Indentation Test for Hardness and Materials Parameters. Verification and Calibration of Testing Machines; ISO: Geneva, Switzerland, 2002. [Google Scholar]
- Kozelskaya, A.I.; Kulkova, S.E.; Fedotkin, A.Y.; Bolbasov, E.N.; Zhukov, Y.M.; Stipniece, L.; Bakulin, A.V.; Useinov, A.S.; Shesterikov, E.V.; Locs, J.; et al. Radio frequency magnetron sputtering of Sr- and Mg-substituted β-tricalcium phosphate: Analysis of the physicochemical properties and deposition rate of coatings. Appl. Surf. Sci. 2020, 509, 144763. [Google Scholar] [CrossRef]
- Callen, B.W.; Lowenberg, B.F.; Lugowski, S.; Sodhi, R.N.S.; Davies, J.E. Nitric acid passivation of Ti6A14V reduces thickness of surface oxide layer and increases trace element release. J. Biomed. Mater. Res. 1995, 29, 279–290. [Google Scholar] [CrossRef]
- França, R.; Samani, T.D.; Bayade, G.; Yahia, L.; Sacher, E. Nanoscale surface characterization of biphasic calcium phosphate, with comparisons to calcium hydroxyapatite and β-tricalcium phosphate bioceramics. J. Colloid Interface Sci. 2014, 420, 182–188. [Google Scholar] [CrossRef]
- Lu, H.B.; Campbell, C.T.; Graham, D.J.; Ratner, B.D. Surface characterization of hydroxyapatite and related calcium phosphates by XPS and TOF-SIMS. Anal. Chem. 2000, 72, 2886–2894. [Google Scholar] [CrossRef]
- Wu, D.; Isaksson, P.; Ferguson, S.J.; Persson, C. Young’s modulus of trabecular bone at the tissue level: A review. Acta Biomater. 2018, 78, 1–12. [Google Scholar] [CrossRef]

| Group | PEO Subcoating Thickness, μm | RFMS Upper Coating Thickness, μm | Total Composite Coating Thickness, μm |
|---|---|---|---|
| PEO | 35 ± 1.7 | – | 35.0 ± 1.7 |
| PEO + HA | 36 ± 1.0 | 2.4 ± 0.1 | 38.4 ± 1.1 * |
| PEO + Sr-HA | 36 ± 1.0 | 1.3 ± 0.1 † | 37.3 ± 1.1 |
| PEO + Mg-HA | 34 ± 0.5 | 1.1 ± 0.1 † | 35.1 ± 0.6 |
| PEO + β-TCP | 35 ± 1.5 | 0.7 ± 0.1 | 35.7 ± 1.6 |
| PEO + Sr-β-TCP | 36 ± 1.5 | 1.0 ± 0.1 † | 37.0 ± 1.6 |
| PEO + Mg-β-TCP | 35 ± 1.5 | 0.6 ± 0.1 | 35.6 ± 1.6 |
| Sample | The Content of Elements in the Coating (at.%) | |||||||
|---|---|---|---|---|---|---|---|---|
| O | Ca | P | Ti | C | Sr | Mg | Ca/P | |
| PEO | 61.5 ± 0.6 | 6.0 ± 0.3 | 17.6 ± 0.2 | 3.7 ± 0.3 | 11.4 ± 1.4 | – | – | 0.34 ± 0.02 |
| PEO + HA | 54.9 ± 0.8 * | 17.9 ± 0.6 * | 14.7 ± 0.5 * | – | 12.5 ± 0.5 | – | – | 1.22 ± 0.06 * |
| PEO + Sr-HA | 57.7 ± 1.8 * | 17.1 ± 0.5 * | 17.0 ± 0.5 | – | 7.7 ± 2.8 | 0.5 ± 0.04 * | – | 1.01 ± 0.04 * |
| PEO + Mg-HA | 60.0 ± 2.0 | 13.4 ± 0.4 * | 17.4 ± 0.6 | – | 7.9 ± 2.9 | – | 1.3 ± 0.3 * | 0.77 ± 0.04 * |
| PEO + β-TCP | 59.4 ± 1.0 *† | 15.1 ± 0.1 *† | 16.2 ± 0.4 *† | – | 9.3 ± 1.4 † | – | – | 0.93 ± 0.02 *† |
| PEO + Sr-β-TCP | 58.3 ± 2.5 | 13.2 ± 0.8 *† | 19.0 ± 1.3 *† | – | 9.0 ± 4.7 | 0.5 ± 0.2 * | – | 0.70 ± 0.06 *† |
| PEO + Mg-β-TCP | 62.8 ± 0.5 *† | 8.5 ± 0.1 *† | 18.3 ± 0.3 * | – | 7.4 ± 1.2 * | – | 2.9 ± 0.4 *† | 0.46 ± 0.01 *† |
| Coating | Ca 2p3/2 (eV) | P 2p3/2 (eV) |
|---|---|---|
| PEO | 347.88 | 134.31 |
| PEO + HA | 347.46 | 133.00 |
| PEO + Sr-HA | 347.44 | 133.10 |
| PEO + Mg-HA | 347.92 | 133.43 |
| PEO + β-TCP | 347.59 | 133.80 |
| PEO + Sr-β-TCP | 347.87 | 134.07 |
| PEO + Mg-β-TCP | 347.97 | 134.31 |
| Coating | EIT, GPa |
|---|---|
| PEO | 15.4 ± 3.8 |
| PEO + HA | 21.3 ± 3.7 |
| PEO + Sr-HA | 36.6 ± 9.5 *’† |
| PEO + Mg-HA | 29.8 ± 6.8 * |
| PEO + β-TCP | 25.6 ± 5.8 * |
| PEO + Sr-β-TCP | 19.5 ± 4.1 |
| PEO + Mg-β-TCP | 17.4 ± 3.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozelskaya, A.; Dubinenko, G.; Vorobyev, A.; Fedotkin, A.; Korotchenko, N.; Gigilev, A.; Shesterikov, E.; Zhukov, Y.; Tverdokhlebov, S. Porous CaP Coatings Formed by Combination of Plasma Electrolytic Oxidation and RF-Magnetron Sputtering. Coatings 2020, 10, 1113. https://doi.org/10.3390/coatings10111113
Kozelskaya A, Dubinenko G, Vorobyev A, Fedotkin A, Korotchenko N, Gigilev A, Shesterikov E, Zhukov Y, Tverdokhlebov S. Porous CaP Coatings Formed by Combination of Plasma Electrolytic Oxidation and RF-Magnetron Sputtering. Coatings. 2020; 10(11):1113. https://doi.org/10.3390/coatings10111113
Chicago/Turabian StyleKozelskaya, Anna, Gleb Dubinenko, Alexandr Vorobyev, Alexander Fedotkin, Natalia Korotchenko, Alexander Gigilev, Evgeniy Shesterikov, Yuriy Zhukov, and Sergei Tverdokhlebov. 2020. "Porous CaP Coatings Formed by Combination of Plasma Electrolytic Oxidation and RF-Magnetron Sputtering" Coatings 10, no. 11: 1113. https://doi.org/10.3390/coatings10111113
APA StyleKozelskaya, A., Dubinenko, G., Vorobyev, A., Fedotkin, A., Korotchenko, N., Gigilev, A., Shesterikov, E., Zhukov, Y., & Tverdokhlebov, S. (2020). Porous CaP Coatings Formed by Combination of Plasma Electrolytic Oxidation and RF-Magnetron Sputtering. Coatings, 10(11), 1113. https://doi.org/10.3390/coatings10111113