Structural and Bioactivity Characterization of Filipin Derivatives from Engineered Streptomyces filipinensis Strains Reveals Clues for Reduced Haemolytic Action
Abstract
1. Introduction
2. Results
2.1. Structural Characterization of Filipin III Precursors
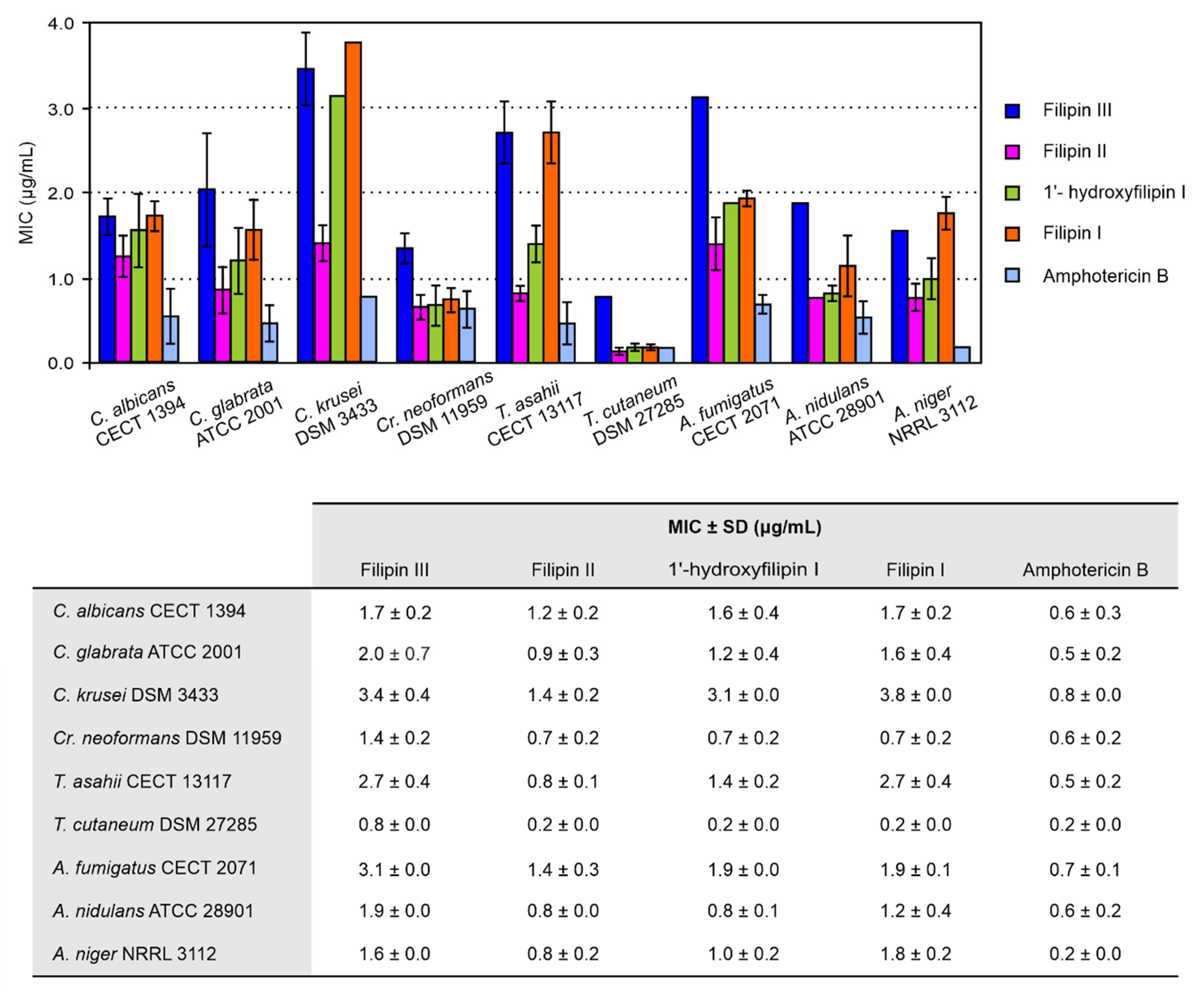
2.2. Antifungal Activity
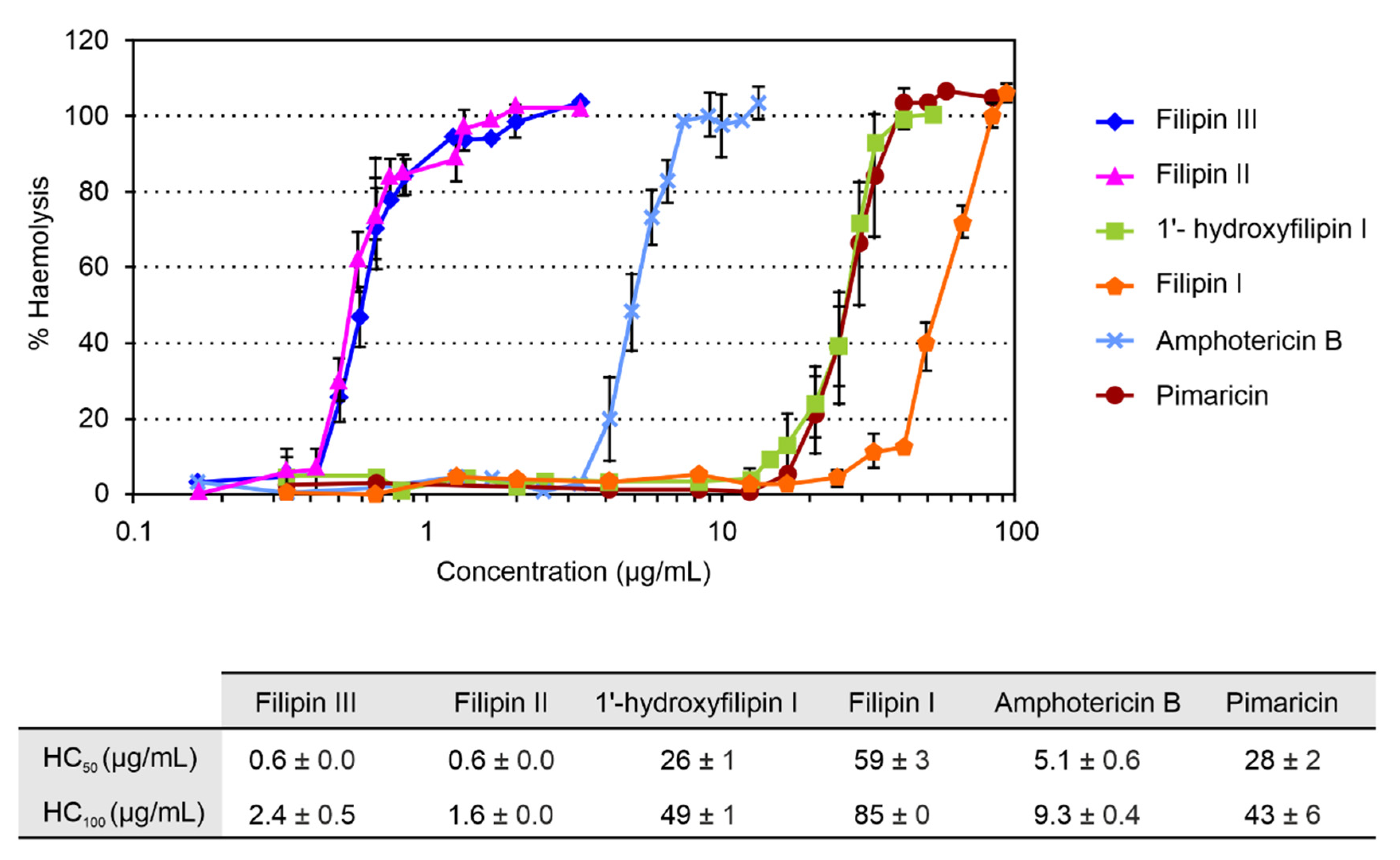
2.3. Haemolytic Activity
3. Discussion
4. Materials and Methods
4.1. Microbial Strains and Cultivation
4.2. Filipin Derivative Purification
4.3. Structural Elucidation of Compounds
4.4. MIC and Minimal Fungicide Concentration (MFC) Determination
4.5. Haemolytic Activity Assay
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bongomin, F.; Gago, S.; Oladele, R.O.; Denning, D.W. Global and multi-national prevalence of fungal diseases-estimate precision. J. Fungi. 2017, 3, 57. [Google Scholar] [CrossRef] [PubMed]
- Howard, K.C.; Dennis, E.K.; Watt, D.S.; Garneau-Tsodikova, S. A comprehensive overview of the medicinal chemistry of antifungal drugs: Perspectives and promise. Chem. Soc. Rev. 2020, 49, 2426–2480. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.D.; Denning, D.W.; Levitz, S.M. Tackling human fungal infections. Science 2012, 336, 647. [Google Scholar] [CrossRef]
- Di Mambro, T.; Guerriero, I.; Aurisicchio, L.; Magnani, M.; Marra, E. The Yin and Yang of current antifungal therapeutic strategies: How can we harness our natural defenses? Front. Pharmacol. 2019, 10, 80. [Google Scholar] [CrossRef] [PubMed]
- Wiederhold, N.P. Antifungal resistance: Current trends and future strategies to combat. Infect. Drug Resist. 2017, 10, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Yapar, N. Epidemiology and risk factors for invasive candidiasis. Ther. Clin. Risk Manag. 2014, 10, 95–105. [Google Scholar] [CrossRef]
- Dabas, Y.; Bakhshi, S.; Xess, I. Fatal cases of bloodstream infection by Fusarium solani and review of published literature. Mycopathologia 2016, 181, 291–296. [Google Scholar] [CrossRef]
- Almeida Júnior, J.N.; Hennequin, C. Invasive Trichosporon infection: A systematic review on a re-emerging fungal pathogen. Front. Microbiol. 2016, 7, 1629. [Google Scholar] [CrossRef]
- Gimpl, G.; Gehrig-Burger, K. Probes for studying cholesterol binding and cell biology. Steroids 2011, 76, 216–231. [Google Scholar] [CrossRef]
- Vanier, M.T.; Latour, P. Laboratory diagnosis of Niemann-Pick disease type C: The filipin staining test. Methods Cell Biol. 2015, 126, 357–375. [Google Scholar]
- te Welscher, Y.M.; ten Napel, H.H.; Balagué, M.M.; Souza, C.M.; Riezman, H.; de Kruijff, B.; Breukink, E. Natamycin blocks fungal growth by binding specifically to ergosterol without permeabilizing the membrane. J Biol. Chem. 2008, 283, 6393–6401. [Google Scholar] [CrossRef]
- Payero, T.D.; Vicente, C.M.; Rumbero, A.; Barreales, E.G.; Santos-Aberturas, J.; de Pedro, A.; Aparicio, J.F. Functional analysis of filipin tailoring genes from Streptomyces filipinensis reveals alternative routes in filipin III biosynthesis and yields bioactive derivatives. Microb. Cell Fact. 2015, 14, 114. [Google Scholar] [CrossRef]
- Balakrishnan, A.R.; Easwaran, K.R. Conformation of polyene antibiotic, filipin III: CD and 1H NMR studies. J. Biomol. Struct. Dyn. 1993, 11, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Rychnovsky, S.D.; Richardson, T.I. Relative and Absolute configuration of filipin III. Angew. Chem. Int. Ed. Engl. 1995, 34, 1227–1230. [Google Scholar] [CrossRef]
- Richardson, T.I.; Rychnovsky, S.D. Filipin III: Configuration assignment and confirmation by synthetic correlation. J. Org. Chem. 1996, 61, 4219–4231. [Google Scholar] [CrossRef]
- Richardson, T.I.; Rychnovsky, S.D. Total synthesis of filipin III. J. Am. Chem. Soc. 1997, 119, 12360–12361. [Google Scholar] [CrossRef]
- Rochet, P.; Lancelin, J.-M. Revised 1H and 13C NMR assignments of the polyene antibiotic filipin III. Magn. Reson. Chem. 1997, 35, 538–542. [Google Scholar] [CrossRef]
- Volpon, L.; Lancelin, J.-M. Solution NMR structures of the polyene macrolide antibiotic filipin III. FEBS Lett. 2000, 478, 137–140. [Google Scholar] [CrossRef]
- Aparicio, J.F.; Barreales, E.G.; Payero, T.D.; Vicente, C.M.; de Pedro, A.; Santos-Aberturas, J. Biotechnological production and application of the antibiotic pimaricin: Biosynthesis and its regulation. Appl. Microbiol. Biotechnol. 2016, 100, 61–78. [Google Scholar] [CrossRef] [PubMed]
- Poša, M.; Kuhajda, K. Hydrophobicity and haemolytic potential of oxo derivatives of cholic, deoxycholic and chenodeoxycholic acids. Steroids 2010, 75, 424–431. [Google Scholar] [CrossRef]
- Wilcock, B.C.; Endo, M.M.; Uno, B.E.; Burke, M.D. C2′-OH of amphotericin B plays an important role in binding the primary sterol of human cells but not yeast cells. J. Am. Chem. Soc. 2013, 135, 8488–8491. [Google Scholar] [CrossRef]
- Brautaset, T.; Sletta, H.; Degnes, K.F.; Sekurova, O.N.; Bakke, I.; Volokhan, O.; Andreassen, T.; Ellingsen, T.E.; Zotchev, S.B. New nystatin-related antifungal polyene macrolides with altered polyol region generated via biosynthetic engineering of Streptomyces noursei. Appl. Environ. Microbiol. 2011, 77, 6636–6643. [Google Scholar] [CrossRef] [PubMed]
- Maskarinec, S.A.; Johnson, M.D.; Perfect, J.R. Genetic susceptibility to fungal infections: What is in the genes? Curr. Clin. Microbiol. Rep. 2016, 3, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Pianalto, K.M.; Alspaugh, J.A. New horizons in antifungal therapy. J. Fungic. 2016, 2, 26. [Google Scholar] [CrossRef] [PubMed]
- Gray, K.C.; Palacios, D.S.; Dailey, I.; Endo, M.M.; Uno, B.E.; Wilcock, B.C.; Burke, M.D. Amphotericin primarily kills yeast by simply binding ergosterol. Proc. Natl. Acad. Sci. USA 2012, 109, 2234–2239. [Google Scholar] [CrossRef] [PubMed]
- E Klepser, M. The value of amphotericin B in the treatment of invasive fungal infections. J. Crit. Care 2011, 26, 225.e1–225.e10. [Google Scholar] [CrossRef]
- Ostrosky-Zeichner, L.; Casadevall, A.; Galgiani, J.N.; Odds, F.C.; Rex, J.H. An insight into the antifungal pipeline: Selected new molecules and beyond. Nat. Rev. Drug Discov. 2010, 9, 719–727. [Google Scholar] [CrossRef]
- Baginski, M.; Czub, J. Amphotericin B and its new derivatives-mode of action. Curr. Drug Metab. 2009, 10, 459–469. [Google Scholar] [CrossRef]
- Park, B.J.; Wannemuehler, K.A.; Marston, B.J.; Govender, N.; Pappas, P.G.; Chiller, T.M. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS 2009, 23, 525–530. [Google Scholar] [CrossRef]
- Mendes, M.V.; Recio, E.; Fouces, R.; Luiten, R.; Martin, J.F.; Aparicio, J.F. Engineered biosynthesis of novel polyenes: A pimaricin derivative produced by targeted gene disruption in Streptomyces natalensis. Chem. Biol. 2001, 8, 635–644. [Google Scholar] [CrossRef]
- Santos-Aberturas, J.; Engel, J.; Dickerhoff, J.; Dörr, M.; Rudroff, F.; Weisz, K.; Bornscheuer, U.T. Exploration of the substrate promiscuity of biosynthetic tailoring enzymes as a new source of structural diversity for polyene macrolide antifungals. ChemCatChem 2015, 7, 490–500. [Google Scholar] [CrossRef]
- Kieser, T.; Bibb, M.J.; Buttner, M.J.; Chater, K.F.; Hopwood, D.A. Practical Streptomyces Genetics; John Innes Foundation: Norwich, UK, 2000. [Google Scholar]
- Recio, E.; Aparicio, J.F.; Rumbero, A.; Martín, J.F. Glycerol, ethylene glycol and propanediol elicit pimaricin biosynthesis in the PI-factor-defective strain Streptomyces natalensis npi287 and increase polyene production in several wild-type actinomycetes. Microbiology 2006, 152, 3147–3156. [Google Scholar] [CrossRef] [PubMed]
- Rex, J.H.; Alexander, B.D.; Andes, D.; Arthington-Skaggs, B.; Brown, S.D.; Chaturvedi, V.; Ghannoum, M.A.; Espinel-Ingroff, A.; Knapp, C.C.; Ostrosky-Zeichner, L.; et al. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts, Approved Standard, 3rd ed.; M27-A3; Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA, 2008.
- John, H.R. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi, Approved Standard, 2nd ed.; M38-A2; Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA, 2008.
- Meletiadis, J.; Antachopoulos, C.; Stergiopoulou, T.; Pournaras, S.; Roilides, E.; Walsh, T.J. Differential fungicidal activities of amphotericin B and voriconazole against Aspergillus species determined by microbroth methodology. Antimicrob. Agents Chemother. 2007, 51, 3329–3337. [Google Scholar] [CrossRef] [PubMed]
- Seco, E.M.; Cuesta, T.; Fotso, S.; Laatsch, H.; Malpartida, F. Two polyene amides produced by genetically modified Streptomyces diastaticus var. 108. Chem. Biol. 2005, 12, 535–543. [Google Scholar] [CrossRef]
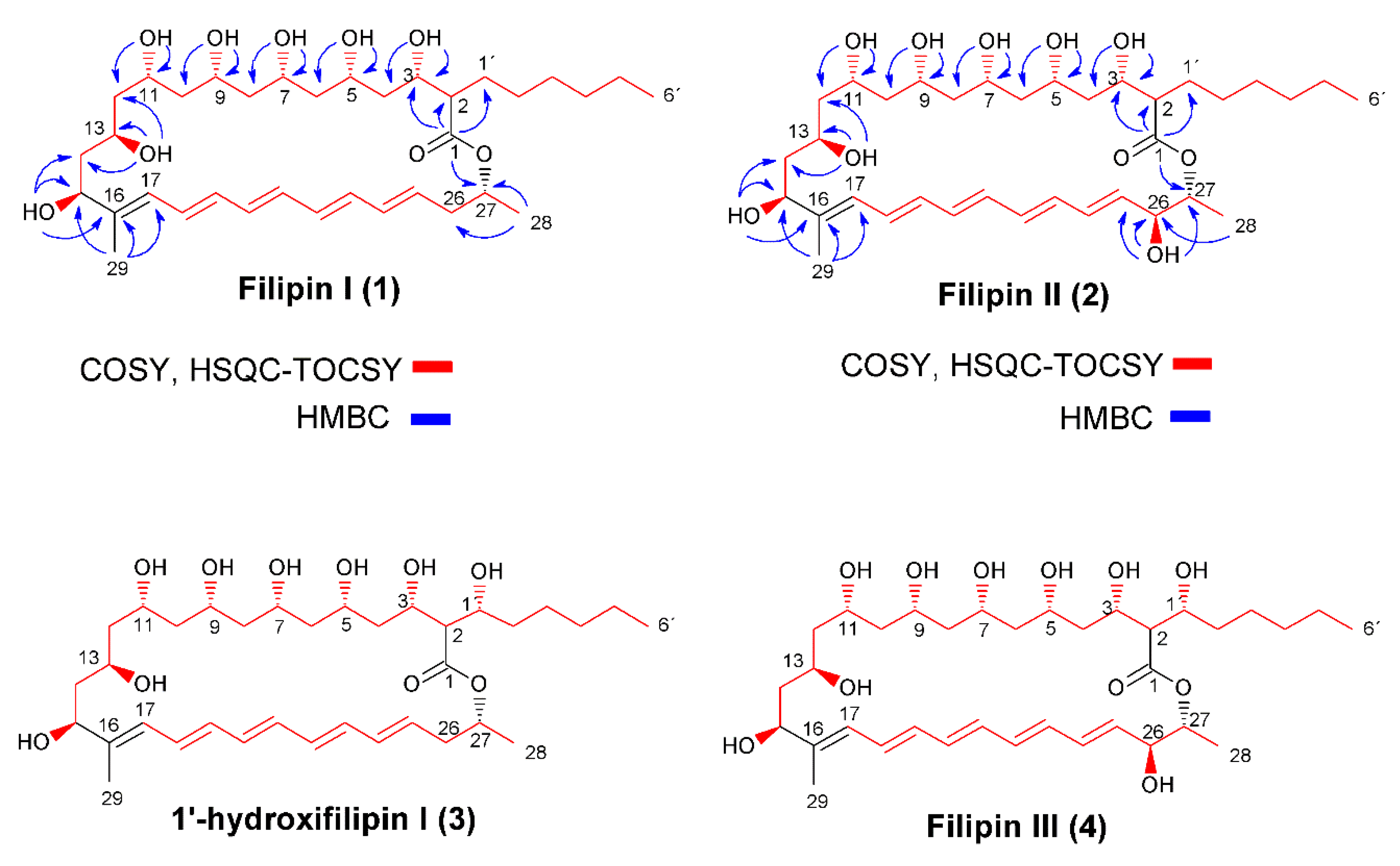
| C | Filipin I | Filipin II | ||
|---|---|---|---|---|
| δC (DEPT) | δH | δC (DEPT) | δH | |
| 1 | 172.80 (C) | 173.70 (C) | ||
| 2 | 53.21 (CH) | 2.21 (m) | 53.00 (CH) | 2.25 (m) |
| 3 | 71.26 (CH) | 3.61 (m); 5.05 (d, J = 4.8 Hz, OH) | 71.32 (CH) | 3.63 (m); 5.02 (d, J = 4.4 Hz, OH) |
| 4 | 42.47 (CH2) | 1.30 (m) | 42.17 (CH2) | 1.32 (m) |
| 5 | 69.98 (CH) | 3.83 (m); 4.97 (d, J = 2.2 Hz OH) | 70.01 (CH) | 3.88 (m); 5.00 (d, J = 2.0 Hz, OH) |
| 6 | 44.08 (CH2) | 1.29 (m) | 44.08 (CH2) | 1.30 (m) |
| 7 | 70.23 (CH) | 3.85 (m); 4.89 (d, J = 1.8 Hz, OH) | 70.33 (CH) | 3.88 (m); 4.95 (d, J = 1.8 Hz, OH) |
| 8 | 44.32 (CH2) | A: 1.38 (m) B: 1.28 (m) | 44.32 (CH2) | A: 1.40 (m) B: 1.30 (m) |
| 9 | 71.53 (CH) | 3.82 (m); 5.13 (s br, OH) | 71.49 (CH) | 3.85 (m); 5.15 (d, J = 1.4 Hz, OH) |
| 10 | 42.94 (CH2) | A: 1.35 (m) B: 1.22 (m) | 43.05 (CH2) | A: 1.36 (m) B: 1.28 (m) |
| 11 | 69.44 (CH) | 3.79 (m); 4.84 (d, J = 2.2 Hz, OH) | 69.45 (CH) | 3.83 (m); 4.83 (d, J = 2.5 Hz, OH) |
| 12 | 44.94 (CH2) | A: 1.59 (m) B: 1.28 (m) | 44.86 (CH2) | A: 1.61 (m) B: 1.28 (m) |
| 13 | 65.67 (CH) | 3.18 (m); 4.46 (d, J = 4.8 Hz, OH) | 65.76 (CH) | 3.09 (m); 4.43 (d, J = 4.7 Hz, OH) |
| 14 | 42.99 (CH2) | A: 1.71 (m) B: 1.52 (m) | 43.19 (CH2) | A: 1.71 (m) B: 1.53 (m) |
| 15 | 73.74 (CH) | 3.98 (m); 4.82 (d, J = 3.7 Hz, OH) | 73.77 (CH) | 3.98 (m); 4.79 (d, J = 3.7 Hz, OH) |
| 16 | 140.96 (C) | 141.09 (C) | ||
| 17 | 126.28 (CH) | 5.93 (d, J = 11.2 Hz) | 126.10 (CH) | 5.93 (d, J = 11.2 Hz) |
| 18 | 128.60 (CH) | 6.46 (dd, J = 14.5, 11.2 Hz) | 128.71 (CH) | 6.48 (dd, J = 14.5, 11.2 Hz) |
| 19 | 133.22 (CH) | 6.23 (m) | 133.18 (CH) | 6.22 (m) |
| 20 | 133.01 (CH) | 6.35 (m) | 133.08 (CH) | 6.33–6.36 (m) |
| 21 | 133.27 (CH) | 6.31 (m) | 132.26 (CH) | 6.27 (m) |
| 22 | 131.70 (CH) | 6.18 (m) | 133.15 (CH) | 6.33–6.36 (m) |
| 23 | 133.39 (CH) | 6.28 (m) | 133.37 (CH) | 6.33–6.36 (m) |
| 24 | 132.74 (CH) | 6.13 (dd, J = 14.5, 11.2 Hz) | 129.86 (CH) | 6.31 (m) |
| 25 | 130.90 (CH) | 5.75 (dt, J = 14.5, 6.9 Hz) | 135.62 (CH) | 5.92 (d, J = 14.5 Hz) |
| 26 | 37.98 (CH2) | A: 2.35 (m) B: 2.25 (m) | 72.11 (CH) | 3.93 (m); 5.26 (d, J = 5.6 Hz, OH) |
| 27 | 70.17 (CH) | 5.02 (m) | 73.40 (CH) | 4.63 (m) |
| 28 | 20.29 (CH3) | 1.21 (d, J = 6.30 Hz) | 18.54 (CH3) | 1.22 (d, J = 6.20 Hz) |
| 29 | 11.33 (CH3) | 1.68 (s br) | 11.46 (CH3) | 1.69 (s br) |
| 1′ | 29.47 (CH2) | A: 1.68 (m) B: 1.44 (m) | 29.60 (CH2) | A: 1.68 (m) B: 1.43 (m) |
| 2′ | 28.98 (CH2) | 1.24 (m) | 29.04 (CH2) | 1.24 (m) |
| 3′ | 27.13 (CH2) | 1.19 (m) | 27.05 (CH2) | 1.19 (m) |
| 4′ | 31.55 (CH2) | 1.22 (m) | 31.59 (CH2) | 1.22 (m) |
| 5′ | 22.41 (CH2) | 1.24 (m) | 22.45 (CH2) | 1.25 (m) |
| 6′ | 14.38 (CH3) | 0.85 (t, J = 6.8 Hz) | 14.40 (CH3) | 0.86 (t, J = 6.8 Hz) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barreales, E.G.; Rumbero, Á.; Payero, T.D.; de Pedro, A.; Jambrina, E.; Aparicio, J.F. Structural and Bioactivity Characterization of Filipin Derivatives from Engineered Streptomyces filipinensis Strains Reveals Clues for Reduced Haemolytic Action. Antibiotics 2020, 9, 413. https://doi.org/10.3390/antibiotics9070413
Barreales EG, Rumbero Á, Payero TD, de Pedro A, Jambrina E, Aparicio JF. Structural and Bioactivity Characterization of Filipin Derivatives from Engineered Streptomyces filipinensis Strains Reveals Clues for Reduced Haemolytic Action. Antibiotics. 2020; 9(7):413. https://doi.org/10.3390/antibiotics9070413
Chicago/Turabian StyleBarreales, Eva G., Ángel Rumbero, Tamara D. Payero, Antonio de Pedro, Ester Jambrina, and Jesús F. Aparicio. 2020. "Structural and Bioactivity Characterization of Filipin Derivatives from Engineered Streptomyces filipinensis Strains Reveals Clues for Reduced Haemolytic Action" Antibiotics 9, no. 7: 413. https://doi.org/10.3390/antibiotics9070413
APA StyleBarreales, E. G., Rumbero, Á., Payero, T. D., de Pedro, A., Jambrina, E., & Aparicio, J. F. (2020). Structural and Bioactivity Characterization of Filipin Derivatives from Engineered Streptomyces filipinensis Strains Reveals Clues for Reduced Haemolytic Action. Antibiotics, 9(7), 413. https://doi.org/10.3390/antibiotics9070413







