Impact of Case Definitions on Efficacy Estimation in Clinical Trials—A Proof-of-Principle Based on Historical Examples
Abstract
1. Introduction
2. Materials and Methods
2.1. Summaries of Historical Studies Used to Exemplify the Approach
2.1.1. The CAPRISA 004 Trial
2.1.2. The Study by van Nood and Colleagues
- An initial vancomycin regimen of 500 mg orally four times per day for 4 days, followed by bowel lavage and subsequent infusion of a solution of donor feces through a nasoduodenal tube;
- A standard vancomycin regimen of 500 mg orally four times per day for 14 days; or
- A standard vancomycin regimen with bowel lavage.
3. Results and Discussion
3.1. Effectiveness and Safety of Tenofovir Gel, an Antiretroviral Microbicide, for the Prevention of HIV Infection in Women
3.2. Duodenal Infusion of Donor Feces for Recurrent Clostridium difficile
Author Contributions
Funding
Conflicts of Interest
References
- Hahn, A.; Meyer, C.G.; Frickmann, H. Impact of diagnostic methods on efficacy estimation—A proof-of-principle based on historical examples. Trop. Med. Int. Health 2020, 25, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Abdool Karim, Q.; Abdool Karim, S.S.; Frohlich, J.A.; Grobler, A.C.; Baxter, C.; Mansoor, L.E.; Kharsany, A.B.; Sibeko, S.; Mlisana, K.P.; Omar, Z.; et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science 2010, 329, 1168–1174. [Google Scholar] [CrossRef]
- van Nood, E.; Vrieze, A.; Nieuwdorp, M.; Fuentes, S.; Zoetendal, E.G.; de Vos, W.M.; Visser, C.E.; Kuijper, E.J.; Bartelsman, J.F.; Tijssen, J.G.; et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N. Engl. J. Med. 2013, 368, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Zautner, A.E.; Groß, U.; Emele, M.F.; Hagen, R.M.; Frickmann, H. More pathogenicity or just more pathogens?—On the interpretation problem of multiple pathogen detections with diagnostic multiplex assays. Front. Microbiol. 2017, 8, 1210. [Google Scholar] [CrossRef] [PubMed]
- Gart, J.J.; Buck, A.A. Comparison of a screening test and a reference test in epidemiologic studies. II. A probabilistic model for the comparison of diagnostic tests. Am. J. Epidemiol. 1966, 83, 593–602. [Google Scholar] [CrossRef]
- Buck, A.A.; Gart, J.J. Comparison of a screening test and a reference test in epidemiologic studies. I. Indices of agreement and their relation to prevalence. Am. J. Epidemiol. 1966, 83, 586–592. [Google Scholar] [CrossRef]
- Rogan, W.J.; Gladen, B. Estimating prevalence from the results of a screening test. Am. J. Epidemiol. 1978, 107, 71–76. [Google Scholar] [CrossRef]
- Lachenbruch, P.A. Sensitivity, specificity, and vaccine efficacy. Control Clin. Trials. 1998, 19, 569–574. [Google Scholar] [CrossRef]
- Gart, J.J. The estimation problem in a probabilistic model for the comparison of diagnostic tests (Abstract). Biometrics 1965, SJ, 764. [Google Scholar]
- Neyman, J. Outline of statistical treatment of the problem of diagnosis. Pub. Health Rep. 1947, 63, 1449–1456. [Google Scholar] [CrossRef]
- Hui, S.L.; Walter, S.D. Estimating the error rates of diagnostic tests. Biometrics 1980, 36, 167–171. [Google Scholar] [CrossRef]
- Hui, S.L.; Zhou, X.H. Evaluation of diagnostic tests without gold standards. Statist. Meth. Med. Res. 1998, 7, 354–370. [Google Scholar] [CrossRef] [PubMed]
- Hadgu, A.; Qu, Y. A biomedical application of latent class models with random effects. Appl. Statist. 1998, 47, 603–616. [Google Scholar] [CrossRef]
- Lu, D.; Zhou, C.; Tang, L.; Tan, M.; Yuan, A.; Chan, L. Evaluating accuracy of diagnostic tests without conditional independence assumption. Stat. Med. 2018, 37, 2809–2821. [Google Scholar] [CrossRef] [PubMed]
- Torrance-Rynard, V.L.; Walter, S.D. Effects of dependent errors in the assessment of diagnostic test performance. Statist. Med. 1997, 16, 2157–2175. [Google Scholar] [CrossRef]
- Weng, T.S. Evaluation of a new diagnostic test against a reference test less than perfect in accuracy. Commun. Statist. Simul. Comput. 1996, 35, 533–555. [Google Scholar] [CrossRef]
- Vacek, P.M. The effect of conditional dependence on the evaluation of diagnostic tests. Biometrics 1985, 41, 959–968. [Google Scholar] [CrossRef]
- Valenstein, P.N. Evaluating diagnostic tests with imperfect standards. Am. J. Clin. Pathol. 1990, 93, 252–258. [Google Scholar] [CrossRef]
- Enøe, C.; Andersen, S.; Thomsen, L.K.; Mousing, J.; Leontides, L.; Sùrensen, V.; Willeberg, P. Estimation of the sensitivity and the specificity of two diagnostic tests for the detection of antibodies against Actinobacillus pleuropneumoniae serotype 2 in pigs by maximum-likelihood-estimation and Gibbs sampling. Epidémiologie St. Anim. 1997, 31/32, 12. [Google Scholar]
- Enøe, C.; Georgiadis, M.P.; Johnson, W.O. Estimation of sensitivity and specificity of diagnostic tests and disease prevalence when the true disease state is unknown. Prev. Vet. Med. 2000, 45, 61–81. [Google Scholar] [CrossRef]
- Piwowar-Manning, E.M.; Tustin, N.B.; Sikateyo, P.; Kamwendo, D.; Chipungu, C.; Maharaj, R.; Mushanyu, J.; Richardson, B.A.; Hillier, S.; Brooks Jackson, J. Validation of rapid HIV antibody tests in 5 African countries. J. Int. Assoc. Physicians AIDS Care 2010, 9, 170–172. [Google Scholar] [CrossRef] [PubMed]
- Karim, S.S.; Karim, Q.A. Antiretroviral prophylaxis: A defining moment in HIV control. Lancet 2011, 378, e23–e25. [Google Scholar] [CrossRef][Green Version]
- Valley-Omar, Z.; Sibeko, S.; Anderson, J.; Goodier, S.; Werner, L.; Arney, L.; Naranbhai, V.; Treurnicht, F.; Abrahams, M.R.; Bandawe, G.; et al. CAPRISA 004 tenofovir microbicide trial: No impact of tenofovir gel on the HIV transmission bottleneck. J. Infect. Dis. 2012, 206, 35–40. [Google Scholar] [CrossRef][Green Version]
- MacQueen, K.M.; Weaver, M.A.; van Loggerenberg, F.; Succop, S.; Majola, N.; Taylor, D.; Karim, Q.A.; Karim, S.A. Assessing adherence in the CAPRISA 004 tenofovir gel HIV prevention trial: Results of a nested case-control study. AIDS Behav. 2014, 18, 826–832. [Google Scholar] [CrossRef] [PubMed]
- Mansoor, L.E.; Abdool Karim, Q.; Yende-Zuma, N.; MacQueen, K.M.; Baxter, C.; Madlala, B.T.; Grobler, A.; Abdool Karim, S.S. Adherence in the CAPRISA 004 tenofovir gel microbicide trial. AIDS Behav. 2014, 18, 811–819. [Google Scholar] [CrossRef] [PubMed]
- McCormack, S.; Dunn, D.T.; Desai, M.; Dolling, D.I.; Gafos, M.; Gilson, R.; Sullivan, A.K.; Clarke, A.; Reeves, I.; Schembri, G.; et al. Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): Effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet 2016, 387, 53–60. [Google Scholar] [CrossRef]
- Molina, J.M.; Capitant, C.; Spire, B.; Pialoux, G.; Cotte, L.; Charreau, I.; Tremblay, C.; Le Gall, J.M.; Cua, E.; Pasquet, A.; et al. On-demand preexposure prophylaxis in men at high risk for HIV-1 infection. N. Engl. J. Med. 2015, 373, 2237–2246. [Google Scholar] [CrossRef]
- Novak-Weekley, S.M.; Hollingsworth, M.H. Comparison of the premier toxin A and B assay and the TOX A/B II assay for diagnosis of Clostridium difficile infection. Clin. Vaccine Immunol. 2008, 15, 575–578. [Google Scholar] [CrossRef][Green Version]
- van Nood, E.; Speelman, P.; Nieuwdorp, M.; Keller, J. Fecal microbiota transplantation: Facts and controversies. Curr. Opin. Gastroenterol. 2014, 30, 34–39. [Google Scholar] [CrossRef]
- Lindsay, B.; Ochieng, J.B.; Ikumapayi, U.N.; Toure, A.; Ahmed, D.; Li, S.; Panchalingam, S.; Levine, M.M.; Kotloff, K.; Rasko, D.A.; et al. Quantitative PCR for detection of Shigella improves ascertainment of Shigella burden in children with moderate-to-severe diarrhea in low-income countries. J. Clin. Microbiol. 2013, 51, 1740–1746. [Google Scholar] [CrossRef]
- Liu, J.; Kabir, F.; Manneh, J.; Lertsethtakarn, P.; Begum, S.; Gratz, J.; Becker, S.M.; Operario, D.J.; Taniuchi, M.; Janaki, L.; et al. Development and assessment of molecular diagnostic tests for 15 enteropathogens causing childhood diarrhoea: A multicentre study. Lancet Infect. Dis. 2014, 14, 716–724. [Google Scholar] [CrossRef]
- Platts-Mills, J.A.; Gratz, J.; Mduma, E.; Svensen, E.; Amour, C.; Liu, J.; Maro, A.; Saidi, Q.; Swai, N.; Kumburu, H.; et al. Association between stool enteropathogen quantity and disease in Tanzanian children using TaqMan array cards: A nested case—control study. Am. J. Trop. Med. Hyg. 2014, 90, 133–138. [Google Scholar] [CrossRef] [PubMed]

| Specificity Assumption | Efficacy Estimation | 0.95 Confidence Interval |
|---|---|---|
| 1 | 0.385 | 0.090, 0.584 |
| 0.995 | 0.408 | 0.094, 0.613 |
| 0.985 | 0.461 | 0.101, 0.677 |
| 0.975 | 0.532 | 0.102, 0.756 |
| 0.965 | 0.627 | 0.067, 0.851 |
| 0.955 | 0.764 | −0.246, 0.955 |
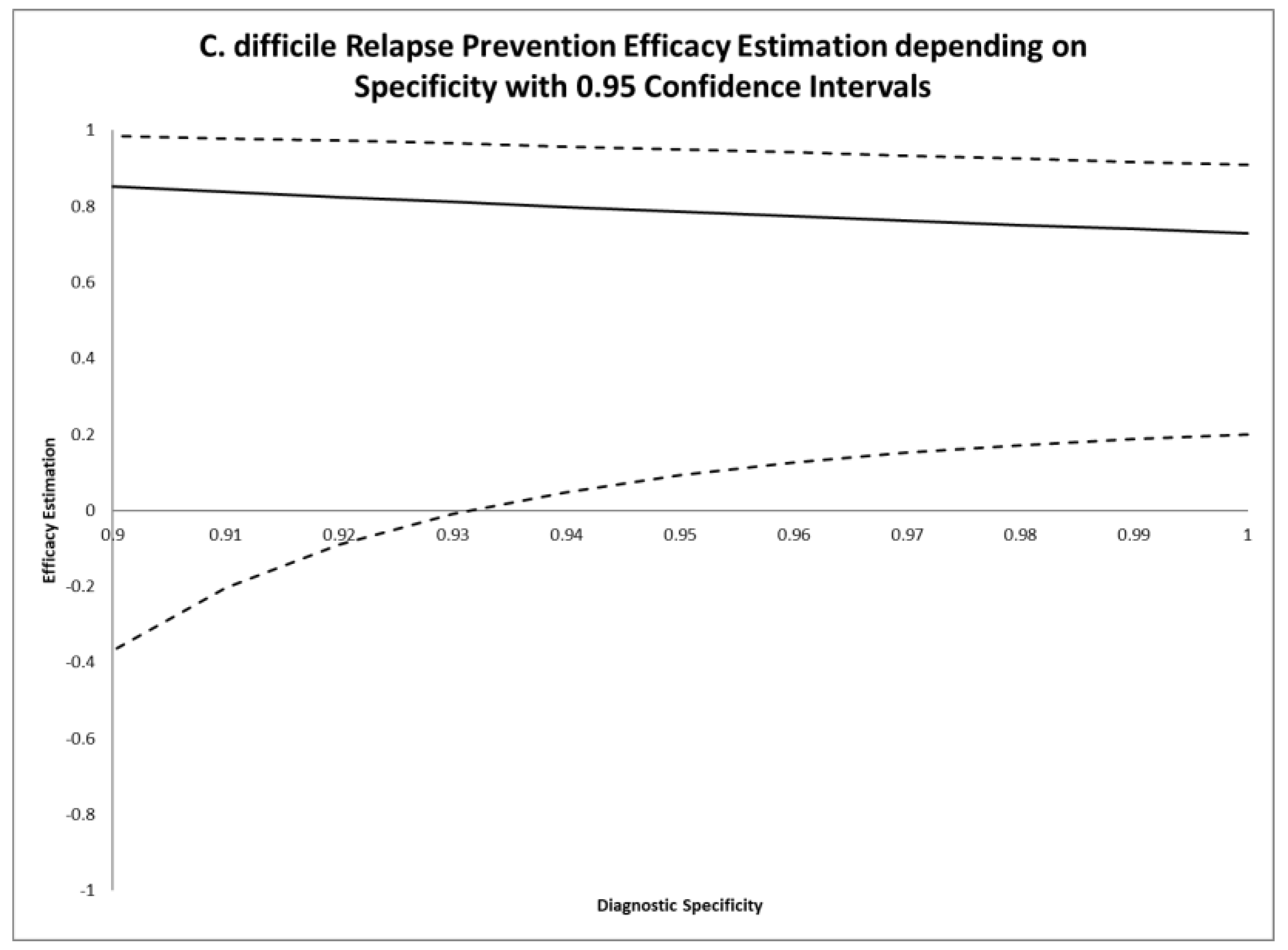
| Specificity Assumption | Efficacy Estimation | 0.95 Confidence Interval |
|---|---|---|
| 1 | 0.729 | 0.201, 0.908 |
| 0.98 | 0.751 | 0.172, 0.925 |
| 0.96 | 0.774 | 0.126, 0.942 |
| 0.94 | 0.798 | 0.048, 0.957 |
| 0.927 | 0.815 | −0.031, 0.967 |
| 0.92 | 0.824 | −0.090, 0.972 |
| 0.90 | 0.852 | −0.370, 0.984 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hahn, A.; Frickmann, H.; Zautner, A.E. Impact of Case Definitions on Efficacy Estimation in Clinical Trials—A Proof-of-Principle Based on Historical Examples. Antibiotics 2020, 9, 379. https://doi.org/10.3390/antibiotics9070379
Hahn A, Frickmann H, Zautner AE. Impact of Case Definitions on Efficacy Estimation in Clinical Trials—A Proof-of-Principle Based on Historical Examples. Antibiotics. 2020; 9(7):379. https://doi.org/10.3390/antibiotics9070379
Chicago/Turabian StyleHahn, Andreas, Hagen Frickmann, and Andreas E. Zautner. 2020. "Impact of Case Definitions on Efficacy Estimation in Clinical Trials—A Proof-of-Principle Based on Historical Examples" Antibiotics 9, no. 7: 379. https://doi.org/10.3390/antibiotics9070379
APA StyleHahn, A., Frickmann, H., & Zautner, A. E. (2020). Impact of Case Definitions on Efficacy Estimation in Clinical Trials—A Proof-of-Principle Based on Historical Examples. Antibiotics, 9(7), 379. https://doi.org/10.3390/antibiotics9070379





