Review on Current Status of Echinocandins Use
Abstract
1. Introduction
2. Echinocandins
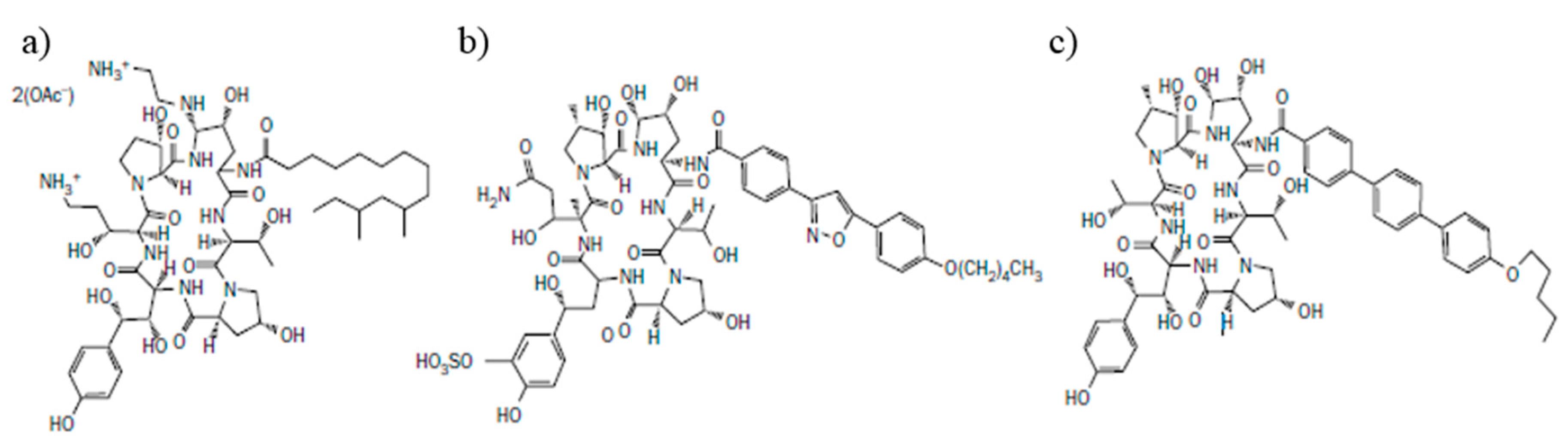
2.1. Semi-Synthetic Echinocandin Derivatives
2.1.1. Caspofungin
2.1.2. Micafungin
2.1.3. Anidulafungin
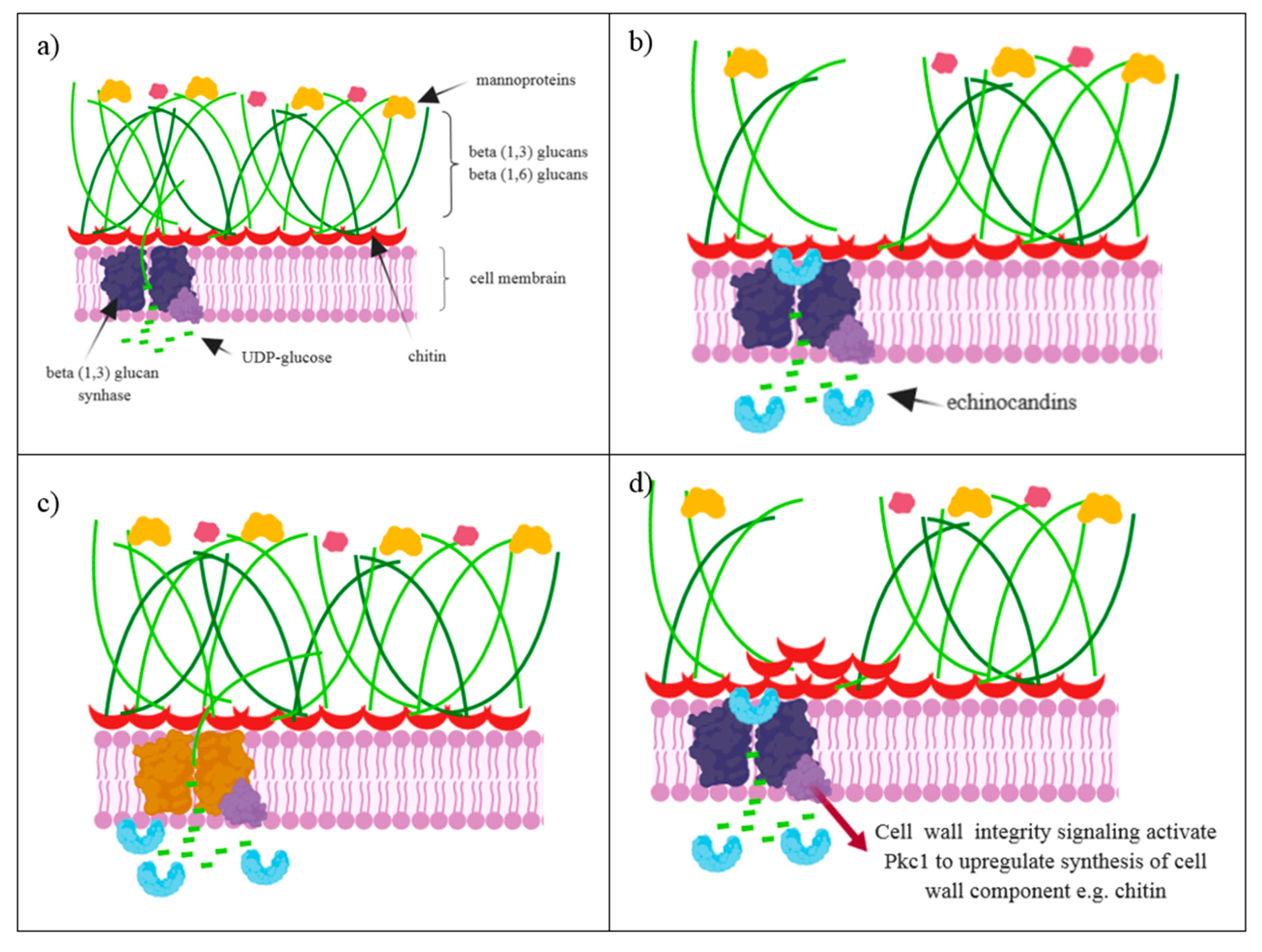
3. The Mechanism of Action
3.1. Antifungal Activity of Echinocandins
3.2. Pharmacokinetics and Pharmacodynamics
3.3. Side Effects of Echinocandins
4. Resistance to Echinocandins
Reasons for the Occurrence of Echinocandin Resistance
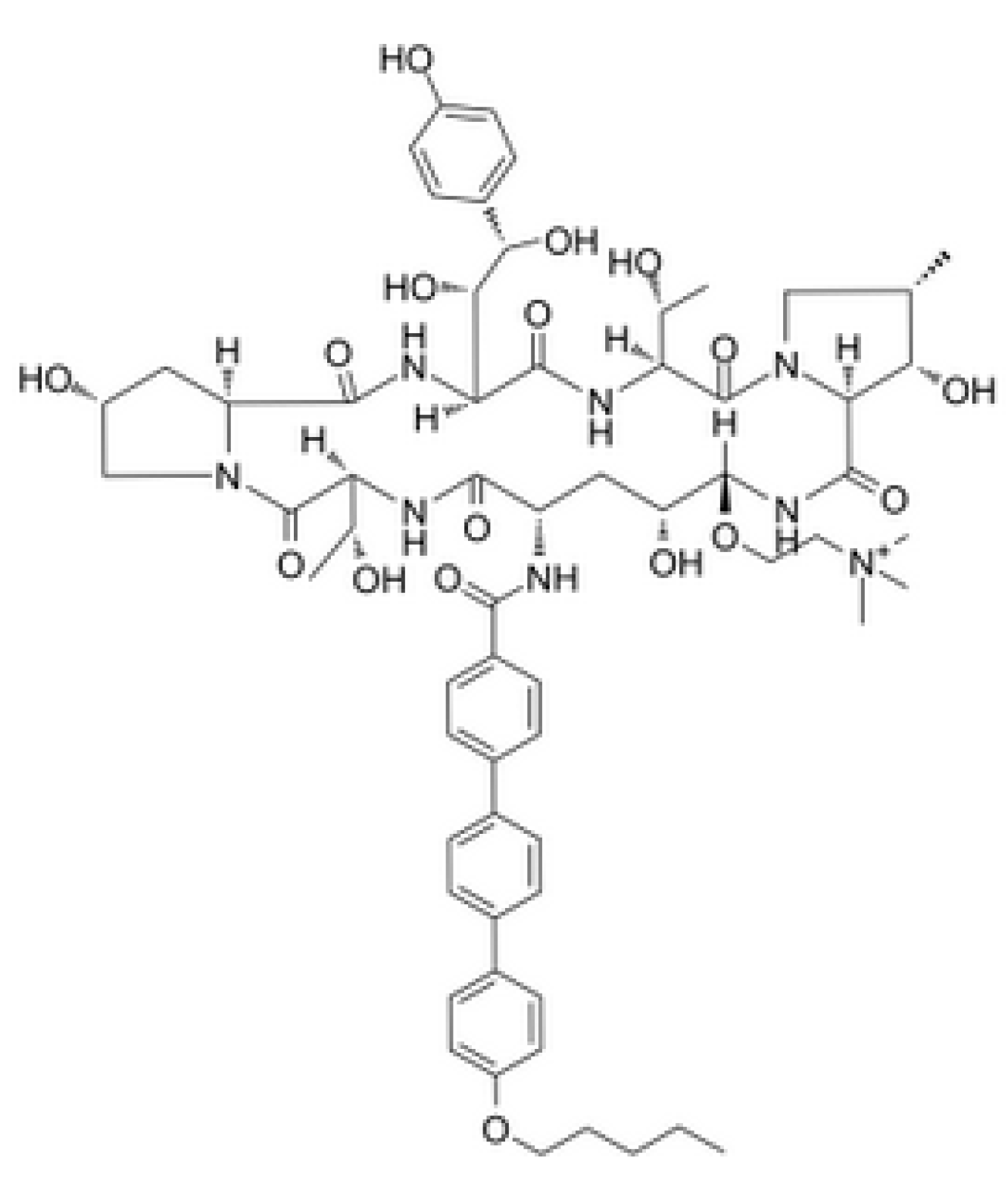
5. Next-Generation Echinocandins
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Brown, G.D.; Denning, D.W.; Gow, N.A.R.; Levitz, S.M.; Netea, M.G.; White, T.C. Hidden killers: Human fungal infections. Sci. Transl. Med. 2012, 4, 165rv13. [Google Scholar] [CrossRef]
- Sanguinetti, M.; Posteraro, B.; Lass-Flörl, C. Antifungal drug resistance among Candida species: Mechanisms and clinical impact. Mycoses 2015, 58, 2–13. [Google Scholar] [CrossRef]
- Perlin, D.S. Mechanisms of echinocandin antifungal drug resistance. Ann. N. Y. Acad. Sci. 2015, 1354, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Odds, F.C.; Brown, A.J.P.; Gow, N.A.R. Antifungal agents: Mechanisms of action. Trends Microbiol. 2003, 11, 272–279. [Google Scholar] [CrossRef]
- Pegu, R.; Borah, R.; Pratihar, S. Synthetic Compounds for Antifungal Chemotherapy. In Recent Trends in Antifungal Agents and Antifungal Therapy; Basak, A., Chakraborty, R., Mandal, S.M., Eds.; Springer: New Delhi, India, 2016; pp. 191–215. ISBN 978-81-322-2782-3. [Google Scholar]
- Denning, D.W. Echinocandin antifungal drugs. Lancet 2003, 362, 1142–1151. [Google Scholar] [CrossRef]
- Aguilar-Zapata, D.; Petraitiene, R.; Petraitis, V. Echinocandins: The Expanding Antifungal Armamentarium. Clin. Infect. Dis. 2015, 61, S604–S611. [Google Scholar] [CrossRef] [PubMed]
- Perlin, D.S. Resistance to echinocandin-class antifungal drugs. Drug Resist. Updat. 2007, 10, 121–130. [Google Scholar] [CrossRef]
- Cornely, O.A.; Bassetti, M.; Calandra, T.; Garbino, J.; Kullberg, B.J.; Lortholary, O.; Meersseman, W.; Akova, M.; Arendrup, M.C.; Arikan-Akdagli, S.; et al. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: Non-neutropenic adult patients. Clin. Microbiol. Infect. 2012, 18, 19–37. [Google Scholar] [CrossRef]
- Balkovec, J.M.; Hughes, D.L.; Masurekar, P.S.; Sable, C.A.; Schwartz, R.E.; Singh, S.B. Discovery and development of first in class antifungal caspofungin (CANCIDAS®)—A case study. Nat. Prod. Rep. 2014, 31, 15–34. [Google Scholar] [CrossRef]
- Joseph, J.M.; Jain, R.; Danziger, L.H. Micafungin: A New Echinocandin Antifungal. Pharmacotherapy 2007, 27, 53–67. [Google Scholar] [CrossRef]
- U.S. FDA Approves Supplemental New Drug Application (sNDA) for Expanded Indication of MYCAMINE® (micafungin for injection) for the Treatment of Invasive Candidiasis in Pediatric Patients Less Than 4 Months of Age; Astellas Pharma US, Inc.: Tokyo, Japan. Available online: https://www.astellas.com/us/news/4761 (accessed on 14 April 2020).
- Roilides, E.; Carlesse, F.; Tawadrous, M.; Leister-Tebbe, H.; Conte, U.; Raber, S.; Swanson, R.; Yan, J.L.; Aram, J.A.; Queiroz-Telles, F.; et al. Safety, Efficacy and Pharmacokinetics of Anidulafungin in Patients 1 Month to <2 Years of Age With Invasive Candidiasis, Including Candidemia. Pediatr. Infect. Dis. J. 2020, 39, 305–309. [Google Scholar] [CrossRef]
- Roilides, E.; Carlesse, F.; Leister-Tebbe, H.; Conte, U.; Yan, J.L.; Liu, P.; Tawadrous, M.; Aram, J.A.; Queiroz-Telles, F. A Prospective, Open-label Study to Assess the Safety, Tolerability and Efficacy of Anidulafungin in the Treatment of Invasive Candidiasis in Children 2 to <18 Years of Age. Pediatr. Infect. Dis. J. 2019, 38, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, J.A. Anidulafungin: A new echinocandin with a novel profile. Clin. Ther. 2005, 27, 657–673. [Google Scholar] [CrossRef] [PubMed]
- Sanguinetti, M.; Posteraro, P.; Posteraro, B. Echinocandin Antifungal Drug Resistance in Candida Species: A Cause for Concern? Curr. Infect. Dis. Rep. 2010, 12, 437–443. [Google Scholar] [CrossRef]
- Cappelletty, D.; Eiselstein-McKitrick, K. The Echinocandins. Pharmacotherapy 2007, 27, 369–388. [Google Scholar] [CrossRef]
- Sucher, A.J.; Chahine, E.B.; Balcer, H.E. Echinocandins: The Newest Class of Antifungals. Ann. Pharmacother. 2009, 43, 1647–1657. [Google Scholar] [CrossRef] [PubMed]
- Douglas, C.M. Fungal b (1,3)-D-glucan synthesis. Med. Mycol. 2001, 39, 55–66. [Google Scholar] [CrossRef]
- Qadota, H.; Python, C.P.; Inoue, S.B.; Arisawa, M.; Anraku, Y.; Zheng, Y.; Watanabe, T.; Levin, D.E.; Ohya, Y. Identification of yeast Rho1p GTPase as a regulatory subunit of 1,3-beta-glucan synthase. Science 1996, 272, 279–281. [Google Scholar] [CrossRef] [PubMed]
- Suwunnakorn, S.; Wakabayashi, H.; Kordalewska, M.; Perlin, D.S.; Rustchenko, E. FKS2 and FKS3 Genes of Opportunistic Human Pathogen Candida albicans Influence Echinocandin Susceptibility. Antimicrob. Agents Chemother. 2018, 62, e02299-17. [Google Scholar] [CrossRef]
- Arendrup, M.C.; Cuenca-Estrella, M.; Lass-Flörl, C.; Hope, W. EUCAST technical note on the EUCAST definitive document EDef 7.2: Method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for yeasts EDef 7.2 (EUCAST-AFST)*. Clin. Microbiol. Infect. 2012, 18, E246–E247. [Google Scholar] [CrossRef]
- European Committee on Antimicrobial Susceptibility Testing Clinical Breakpoints for Antifungals. Available online: http://www.eucast.org/astoffungi/clinicalbreakpointsforantifungals/ (accessed on 13 February 2020).
- Arendrup, M.C.; Jørgensen, K.M.; Hare, R.K.; Cuenca-Estrella, M.; Zaragoza, O. EUCAST Reference Testing of Rezafungin Susceptibility and Impact of Choice of Plastic Plates. Antimicrob. Agents Chemother. 2019, 63, e00659-19. [Google Scholar] [CrossRef] [PubMed]
- Cleveland, A.A.; Farley, M.M.; Harrison, L.H.; Stein, B.; Hollick, R.; Lockhart, S.R.; Magill, S.S.; Derado, G.; Park, B.J.; Chiller, T.M. Changes in Incidence and Antifungal Drug Resistance in Candidemia: Results From Population-Based Laboratory Surveillance in Atlanta and Baltimore, 2008–2011. Clin. Infect. Dis. 2012, 55, 1352–1361. [Google Scholar] [CrossRef] [PubMed]
- Chowdhary, A.; Sharma, C.; Meis, J.F. Candida auris: A rapidly emerging cause of hospital-acquired multidrug-resistant fungal infections globally. PLoS Pathog. 2017, 13, e1006290. [Google Scholar] [CrossRef] [PubMed]
- Chowdhary, A.; Voss, A.; Meis, J.F. Multidrug-resistant Candida auris: ‘new kid on the block’ in hospital-associated infections? J. Hosp. Infect. 2016, 94, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Walker, L.A.; Gow, N.A.R.; Munro, C.A. Fungal echinocandin resistance. Fungal Genet. Biol. 2010, 47, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Staab, J.F.; Kahn, J.N.; Marr, K.A. Differential Aspergillus lentulus Echinocandin Susceptibilities Are Fksp Independent. Antimicrob. Agents Chemother. 2010, 54, 4992–4998. [Google Scholar] [CrossRef] [PubMed]
- Perlin, D.S. Current perspectives on echinocandin class drugs. Future Microbiol. 2011, 6, 441–457. [Google Scholar] [CrossRef] [PubMed]
- Walker, L.A.; Lee, K.K.; Munro, C.A.; Gow, N.A.R. Caspofungin Treatment of Aspergillus fumigatus Results in ChsG-Dependent Upregulation of Chitin Synthesis and the Formation of Chitin-Rich Microcolonies. Antimicrob. Agents Chemother. 2015, 59, 5932–5941. [Google Scholar] [CrossRef]
- Ikeda, R.; Sugita, T.; Jacobson, E.S.; Shinoda, T. Effects of Melanin upon Susceptibility of Cryptococcus to Antifungals. Microbiol. Immunol. 2003, 47, 271–277. [Google Scholar] [CrossRef]
- Maligie, M.A.; Selitrennikoff, C.P. Cryptococcus neoformans Resistance to Echinocandins: (1,3)β-Glucan Synthase Activity Is Sensitive to Echinocandins. Antimicrob. Agents Chemother. 2005, 49, 2851–2856. [Google Scholar] [CrossRef]
- McBride, J.A.; Gauthier, G.M.; Klein, B.S. Clinical manifestations and treatment of blastomycosis. Clin. Chest Med. 2017, 38, 435–449. [Google Scholar] [CrossRef]
- Anderson, T. Echinocandin Antifungals. In Drug Dosing in Obesity: Volume I: Antimicrobials; Zaidi, S.T.R., Roberts, J.A., Eds.; Springer: Cham, Switzerland, 2016; pp. 97–107. ISBN 978-3-319-44034-7. [Google Scholar]
- Iii, G.R.T.; Barker, B.M.; Wiederhold, N.P. Large-Scale Evaluation of In Vitro Amphotericin B, Triazole, and Echinocandin Activity against Coccidioides Species from U.S. Institutions. Antimicrob. Agents Chemother. 2017, 61, e02634-16. [Google Scholar] [CrossRef]
- González, G.M.; Tijerina, R.; Najvar, L.K.; Bocanegra, R.; Luther, M.; Rinaldi, M.G.; Graybill, J.R. Correlation between Antifungal Susceptibilities of Coccidioides immitis In Vitro and Antifungal Treatment with Caspofungin in a Mouse Model. Antimicrob. Agents Chemother. 2001, 45, 1854–1859. [Google Scholar] [CrossRef]
- Castanheira, M.; Messer, S.A.; Jones, R.N.; Farrell, D.J.; Pfaller, M.A. Activity of echinocandins and triazoles against a contemporary (2012) worldwide collection of yeast and moulds collected from invasive infections. Int. J. Antimicrob. Agents 2014, 44, 320–326. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Carvalhaes, C.; Messer, S.A.; Rhomberg, P.R.; Castanheira, M. Activity of a Long-Acting Echinocandin, Rezafungin, and Comparator Antifungal Agents Tested against Contemporary Invasive Fungal Isolates (SENTRY Program, 2016 to 2018). Antimicrob. Agents Chemother. 2020, 64, e00099-20. [Google Scholar] [CrossRef]
- Martos, A.I.; Romero, A.; González, M.T.; González, A.; Serrano, C.; Castro, C.; Pemán, J.; Cantón, E.; Martín-Mazuelos, E. Evaluation of the Etest method for susceptibility testing of Aspergillus spp. and Fusarium spp. to three echinocandins. Med. Mycol. 2010, 48, 858–861. [Google Scholar] [CrossRef]
- Perdomo, H.; Sutton, D.A.; García, D.; Fothergill, A.W.; Cano, J.; Gené, J.; Summerbell, R.C.; Rinaldi, M.G.; Guarro, J. Spectrum of Clinically Relevant Acremonium Species in the United States. J. Clin. Microbiol. 2011, 49, 243–256. [Google Scholar] [CrossRef]
- Da Cunha, K.C.; Sutton, D.A.; Fothergill, A.W.; Gené, J.; Cano, J.; Madrid, H.; de Hoog, S.; Crous, P.W.; Guarro, J. In vitro antifungal susceptibility and molecular identity of 99 clinical isolates of the opportunistic fungal genus Curvularia. Diagn. Microbiol. Infect. Dis. 2013, 76, 168–174. [Google Scholar] [CrossRef]
- Singh, J.; Rimek, D.; Kappe, R. In vitro susceptibility of 15 strains of zygomycetes to nine antifungal agents as determined by the NCCLS M38-A microdilution method. Mycoses 2005, 48, 246–250. [Google Scholar] [CrossRef]
- Sandoval-Denis, M.; Sutton, D.A.; Cano-Lira, J.F.; Gene, J.; Fothergill, A.W.; Wiederhold, N.P.; Guarro, J. Phylogeny of the Clinically Relevant Species of the Emerging Fungus Trichoderma and Their Antifungal Susceptibilities. J. Clin. Microbiol. 2014, 52, 2112–2125. [Google Scholar] [CrossRef]
- Song, J.C.; Stevens, D.A. Caspofungin: Pharmacodynamics, pharmacokinetics, clinical uses and treatment outcomes. Crit. Rev. Microbiol. 2016, 42, 813–846. [Google Scholar] [CrossRef]
- Stevens, D.A.; Espiritu, M.; Parmar, R. Paradoxical Effect of Caspofungin: Reduced Activity against Candida albicans at High Drug Concentrations. Antimicrob. Agents Chemother. 2004, 48, 3407–3411. [Google Scholar] [CrossRef]
- Chamilos, G.; Lewis, R.E.; Albert, N.; Kontoyiannis, D.P. Paradoxical Effect of Echinocandins across Candida Species In Vitro: Evidence for Echinocandin-Specific and Candida Species-Related Differences. Antimicrob. Agents Chemother. 2007, 51, 2257–2259. [Google Scholar] [CrossRef]
- Stevens, D.A.; White, T.C.; Perlin, D.S.; Selitrennikoff, C.P. Studies of the paradoxical effect of caspofungin at high drug concentrations. Diagn. Microbiol. Infect. Dis. 2005, 51, 173–178. [Google Scholar] [CrossRef]
- Stevens, D.A.; Ichinomiya, M.; Koshi, Y.; Horiuchi, H. Escape of Candida from Caspofungin Inhibition at Concentrations above the MIC (Paradoxical Effect) Accomplished by Increased Cell Wall Chitin; Evidence for β-1,6-Glucan Synthesis Inhibition by Caspofungin. Antimicrob. Agents Chemother. 2006, 50, 3160–3161. [Google Scholar] [CrossRef]
- Wagener, J.; Loiko, V. Recent Insights into the Paradoxical Effect of Echinocandins. J. Fungi (Basel) 2018, 4, 5. [Google Scholar] [CrossRef]
- Juvvadi, P.R.; Muñoz, A.; Lamoth, F.; Soderblom, E.J.; Moseley, M.A.; Read, N.D.; Steinbach, W.J. Calcium-Mediated Induction of Paradoxical Growth following Caspofungin Treatment Is Associated with Calcineurin Activation and Phosphorylation in Aspergillus fumigatus. Antimicrob. Agents Chemother. 2015, 59, 4946–4955. [Google Scholar] [CrossRef]
- Klont, R.R.; Mennink-Kersten, M.A.S.H.; Ruegebrink, D.; Rijs, A.J.M.M.; Blijlevens, N.M.A.; Donnelly, J.P.; Verweij, P.E. Paradoxical Increase in Circulating Aspergillus Antigen during Treatment with Caspofungin in a Patient with Pulmonary Aspergillosis. Clin. Infect. Dis. 2006, 43, e23–e25. [Google Scholar] [CrossRef]
- Fortún, J.; Martín-Dávila, P.; Montejo, M.; Muñoz, P.; Cisneros, J.; Ramos, A.; Aragón, C.; Blanes, M.; Juan, R.S.; Gavaldá, J.; et al. Prophylaxis With Caspofungin for Invasive Fungal Infections in High-Risk Liver Transplant Recipients. Transplantation 2009, 87, 424–435. [Google Scholar] [CrossRef]
- Hoffman, J.A.; Walsh, T.J. Echinocandins in Children. Pediatr. Infect. Dis. J. 2011, 30, 508–509. [Google Scholar] [CrossRef]
- Perea, S.; Gonzalez, G.; Fothergill, A.W.; Kirkpatrick, W.R.; Rinaldi, M.G.; Patterson, T.F. In Vitro Interaction of Caspofungin Acetate with Voriconazole against Clinical Isolates of Aspergillus spp. Antimicrob. Agents Chemother. 2002, 46, 3039–3041. [Google Scholar] [CrossRef]
- Petraitis, V.; Petraitiene, R.; Sarafandi, A.A.; Kelaher, A.M.; Lyman, C.A.; Casler, H.E.; Sein, T.; Groll, A.H.; Bacher, J.; Avila, N.A.; et al. Combination Therapy in Treatment of Experimental Pulmonary Aspergillosis: Synergistic Interaction between an Antifungal Triazole and an Echinocandin. J. Infect. Dis. 2003, 187, 1834–1843. [Google Scholar] [CrossRef]
- Calvo, E.; Pastor, F.J.; Salas, V.; Mayayo, E.; Guarro, J. Combined Therapy of Voriconazole and Anidulafungin in Murine Infections by Aspergillus flavus. Mycopathologia 2012, 173, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Marr, K.A.; Schlamm, H.T.; Herbrecht, R.; Rottinghaus, S.T.; Bow, E.J.; Cornely, O.A.; Heinz, W.J.; Jagannatha, S.; Koh, L.P.; Kontoyiannis, D.P.; et al. Combination Antifungal Therapy for Invasive Aspergillosis: A Randomized Trial. Ann. Intern. Med. 2015, 162, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Marr, K.A.; Boeckh, M.; Carter, R.A.; Kim, H.W.; Corey, L. Combination antifungal therapy for invasive aspergillosis. Clin. Infect. Dis. 2004, 39, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, W.R.; Perea, S.; Coco, B.J.; Patterson, T.F. Efficacy of Caspofungin Alone and in Combination with Voriconazole in a Guinea Pig Model of Invasive Aspergillosis. Antimicrob. Agents Chemother. 2002, 46, 2564–2568. [Google Scholar] [CrossRef]
- Raad, I.I.; Zakhem, A.E.; Helou, G.E.; Jiang, Y.; Kontoyiannis, D.P.; Hachem, R. Clinical experience of the use of voriconazole, caspofungin or the combination in primary and salvage therapy of invasive aspergillosis in haematological malignancies. Int. J. Antimicrob. Agents 2015, 45, 283–288. [Google Scholar] [CrossRef]
- Perlin, D.S.; Hope, W.W. Echinocandins. In Aspergillosis: From Diagnosis to Prevention; Comarú Pasqualotto, A., Ed.; Springer: Dordrecht, The Netherlands, 2010; pp. 263–279. ISBN 978-90-481-2408-4. [Google Scholar]
- Kartsonis, N.A.; Nielsen, J.; Douglas, C.M. Caspofungin: The first in a new class of antifungal agents. Drug Resist. Updat. 2003, 6, 197–218. [Google Scholar] [CrossRef]
- Grover, N.D. Echinocandins: A ray of hope in antifungal drug therapy. Indian J. Pharmacol. 2010, 42, 9–11. [Google Scholar] [CrossRef]
- Wiederhold, N.P.; Lewis, J.S. The echinocandin micafungin: A review of the pharmacology, spectrum of activity, clinical efficacy and safety. Expert Opin. Pharmacother. 2007, 8, 1155–1166. [Google Scholar] [CrossRef]
- Park, S.; Kelly, R.; Kahn, J.N.; Robles, J.; Hsu, M.-J.; Register, E.; Li, W.; Vyas, V.; Fan, H.; Abruzzo, G.; et al. Specific Substitutions in the Echinocandin Target Fks1p Account for Reduced Susceptibility of Rare Laboratory and Clinical Candida sp. Isolates. Antimicrob. Agents Chemother. 2005, 49, 3264–3273. [Google Scholar] [CrossRef] [PubMed]
- Grossman, N.T.; Chiller, T.M.; Lockhart, S.R. Epidemiology of echinocandin resistance in Candida. Curr. Fungal Infect. Rep. 2014, 8, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Castanheira, M.; Woosley, L.N.; Diekema, D.J.; Messer, S.A.; Jones, R.N.; Pfaller, M.A. Low Prevalence of fks1 Hot Spot 1 Mutations in a Worldwide Collection of Candida Strains. Antimicrob. Agents Chemother. 2010, 54, 2655–2659. [Google Scholar] [CrossRef] [PubMed]
- Alexander, B.D.; Johnson, M.D.; Pfeiffer, C.D.; Jiménez-Ortigosa, C.; Catania, J.; Booker, R.; Castanheira, M.; Messer, S.A.; Perlin, D.S.; Pfaller, M.A. Increasing Echinocandin Resistance in Candida glabrata: Clinical Failure Correlates With Presence of FKS Mutations and Elevated Minimum Inhibitory Concentrations. Clin. Infect. Dis. 2013, 56, 1724–1732. [Google Scholar] [CrossRef]
- Hakki, M.; Staab, J.F.; Marr, K.A. Emergence of a Candida krusei Isolate with Reduced Susceptibility to Caspofungin during Therapy. Antimicrob. Agents Chemother. 2006, 50, 2522–2524. [Google Scholar] [CrossRef]
- Kahn, J.N.; Garcia-Effron, G.; Hsu, M.-J.; Park, S.; Marr, K.A.; Perlin, D.S. Acquired Echinocandin Resistance in a Candida krusei Isolate Due to Modification of Glucan Synthase. Antimicrob. Agents Chemother. 2007, 51, 1876–1878. [Google Scholar] [CrossRef]
- Forastiero, A.; Garcia-Gil, V.; Rivero-Menendez, O.; Garcia-Rubio, R.; Monteiro, M.C.; Alastruey-Izquierdo, A.; Jordan, R.; Agorio, I.; Mellado, E. Rapid Development of Candida krusei Echinocandin Resistance during Caspofungin Therapy. Antimicrob. Agents Chemother. 2015, 59, 6975–6982. [Google Scholar] [CrossRef]
- Spellberg, B.J.; Filler, S.G.; Edwards, J.E. Current Treatment Strategies for Disseminated Candidiasis. Clin. Infect. Dis. 2006, 42, 244–251. [Google Scholar] [CrossRef]
- Infection Prevention and Control for Candida auris|Candida auris|Fungal Diseases|CDC. Available online: https://www.cdc.gov/fungal/candida-auris/c-auris-infection-control.html (accessed on 8 March 2020).
- Satoh, K.; Makimura, K.; Hasumi, Y.; Nishiyama, Y.; Uchida, K.; Yamaguchi, H. Candida auris sp. nov., a novel ascomycetous yeast isolated from the external ear canal of an inpatient in a Japanese hospital. Microbiol. Immunol. 2009, 53, 41–44. [Google Scholar] [CrossRef]
- Lee, W.G.; Shin, J.H.; Uh, Y.; Kang, M.G.; Kim, S.H.; Park, K.H.; Jang, H.-C. First three reported cases of nosocomial fungemia caused by Candida auris. J. Clin. Microbiol. 2011, 49, 3139–3142. [Google Scholar] [CrossRef]
- Chowdhary, A.; Sharma, C.; Duggal, S.; Agarwal, K.; Prakash, A.; Singh, P.K.; Jain, S.; Kathuria, S.; Randhawa, H.S.; Hagen, F.; et al. New Clonal Strain of Candida auris, Delhi, India. Emerg. Infect. Dis. 2013, 19, 1670–1673. [Google Scholar] [CrossRef] [PubMed]
- Schelenz, S.; Hagen, F.; Rhodes, J.L.; Abdolrasouli, A.; Chowdhary, A.; Hall, A.; Ryan, L.; Shackleton, J.; Trimlett, R.; Meis, J.F.; et al. First hospital outbreak of the globally emerging Candida auris in a European hospital. Antimicrob. Resist. Infect. Control. 2016, 5, 35. [Google Scholar] [CrossRef] [PubMed]
- Calvo, B.; Melo, A.S.A.; Perozo-Mena, A.; Hernandez, M.; Francisco, E.C.; Hagen, F.; Meis, J.F.; Colombo, A.L. First report of Candida auris in America: Clinical and microbiological aspects of 18 episodes of candidemia. J. Infect. 2016, 73, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Sears, D.; Schwartz, B.S. Candida auris: An emerging multidrug-resistant pathogen. Int. J. Infect. Dis. 2017, 63, 95–98. [Google Scholar] [CrossRef]
- Al Maani, A.; Paul, H.; Al-Rashdi, A.; Al Wahaibi, A.; Al-Jardani, A.; Al Abri, A.M.A.; AlBalushi, M.A.H.; Al Abri, S.; Al Reesi, M.; Al Maqbali, A.; et al. Ongoing Challenges with Healthcare-Associated Candida auris Outbreaks in Oman. J. Fungi (Basel) 2019, 5, 101. [Google Scholar] [CrossRef]
- Chowdhary, A.; Prakash, A.; Sharma, C.; Kordalewska, M.; Kumar, A.; Sarma, S.; Tarai, B.; Singh, A.; Upadhyaya, G.; Upadhyay, S.; et al. A multicentre study of antifungal susceptibility patterns among 350 Candida auris isolates (2009–17) in India: Role of the ERG11 and FKS1 genes in azole and echinocandin resistance. J. Antimicrob. Chemother. 2018, 73, 891–899. [Google Scholar] [CrossRef]
- Identification of Candida auris|Candida auris|Fungal Diseases|CDC. Available online: https://www.cdc.gov/fungal/candida-auris/recommendations.html (accessed on 8 March 2020).
- Beyda, N.D.; John, J.; Kilic, A.; Alam, M.J.; Lasco, T.M.; Garey, K.W. FKS Mutant Candida glabrata: Risk Factors and Outcomes in Patients With Candidemia. Clin. Infect. Dis. 2014, 59, 819–825. [Google Scholar] [CrossRef]
- Fekkar, A.; Dannaoui, E.; Meyer, I.; Imbert, S.; Brossas, J.Y.; Uzunov, M.; Mellon, G.; Nguyen, S.; Guiller, E.; Caumes, E.; et al. Emergence of echinocandin-resistant Candida spp. in a hospital setting: A consequence of 10 years of increasing use of antifungal therapy? Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 1489–1496. [Google Scholar] [CrossRef]
- Shields, R.K.; Nguyen, M.H.; Clancy, C.J. Clinical perspectives on echinocandin resistance among Candida species. Curr. Opin. Infect. Dis. 2015, 28, 514–522. [Google Scholar] [CrossRef]
- Ruggero, M.A.; Topal, J.E. Development of echinocandin-resistant Candida albicans candidemia following brief prophylactic exposure to micafungin therapy. Transpl. Infect. Dis. 2014, 16, 469–472. [Google Scholar] [CrossRef]
- Bizerra, F.C.; Jimenez-Ortigosa, C.; Souza, A.C.R.; Breda, G.L.; Queiroz-Telles, F.; Perlin, D.S.; Colombo, A.L. Breakthrough Candidemia Due to Multidrug-Resistant Candida glabrata during Prophylaxis with a Low Dose of Micafungin. Antimicrob. Agents Chemother. 2014, 58, 2438–2440. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, K.F.; Taff, H.T.; Cuevas, M.A.; Reinicke, E.L.; Sanchez, H.; Andes, D.R. Role of Matrix β-1,3 Glucan in Antifungal Resistance of Non-albicans Candida Biofilms. Antimicrob. Agents Chemother. 2013, 57, 1918–1920. [Google Scholar] [CrossRef] [PubMed]
- Perlin, D.S. Echinocandin Resistance in Candida. Clin. Infect. Dis. 2015, 61, S612–S617. [Google Scholar] [CrossRef] [PubMed]
- Cowen, L.E.; Steinbach, W.J. Stress, Drugs, and Evolution: The Role of Cellular Signaling in Fungal Drug Resistance. Eukaryot. Cell 2008, 7, 747–764. [Google Scholar] [CrossRef]
- Perlin, D.S. Echinocandin Resistance, Susceptibility Testing and Prophylaxis: Implications for Patient Management. Drugs 2014, 74, 1573–1585. [Google Scholar] [CrossRef]
- Shields, R.K.; Nguyen, M.H.; Press, E.G.; Cumbie, R.; Driscoll, E.; Pasculle, A.W.; Clancy, C.J. Rate of FKS Mutations among Consecutive Candida Isolates Causing Bloodstream Infection. Antimicrob. Agents Chemother. 2015, 59, 7465–7470. [Google Scholar] [CrossRef]
- Ben-Ami, R.; Garcia-Effron, G.; Lewis, R.E.; Gamarra, S.; Leventakos, K.; Perlin, D.S.; Kontoyiannis, D.P. Fitness and Virulence Costs of Candida albicans FKS1 Hot Spot Mutations Associated With Echinocandin Resistance. J. Infect. Dis. 2011, 204, 626–635. [Google Scholar] [CrossRef]
- Gardiner, R.E.; Souteropoulos, P.; Park, S.; Perlin, D.S. Characterization of Aspergillus fumigatus mutants with reduced susceptibility to caspofungin. Med. Mycol. 2005, 43, S299–S305. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Diekema, D.J.; Messer, S.A.; Hollis, R.J.; Jones, R.N. In Vitro Activities of Caspofungin Compared with Those of Fluconazole and Itraconazole against 3,959 Clinical Isolates of Candida spp., Including 157 Fluconazole-Resistant Isolates. Antimicrob. Agents Chemother. 2003, 47, 1068–1071. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Messer, S.A.; Boyken, L.; Rice, C.; Tendolkar, S.; Hollis, R.J.; Diekema, D.J. Caspofungin Activity against Clinical Isolates of Fluconazole-Resistant Candida. J. Clin. Microbiol. 2003, 41, 5729–5731. [Google Scholar] [CrossRef]
- Niimi, K.; Maki, K.; Ikeda, F.; Holmes, A.R.; Lamping, E.; Niimi, M.; Monk, B.C.; Cannon, R.D. Overexpression of Candida albicans CDR1, CDR2, or MDR1 Does Not Produce Significant Changes in Echinocandin Susceptibility. Antimicrob. Agents Chemother. 2006, 50, 1148–1155. [Google Scholar] [CrossRef] [PubMed]
- Lesage, G.; Bussey, H. Cell Wall Assembly in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 2006, 70, 317–343. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Alampalli, S.V.; Nageshan, R.K.; Chettiar, S.T.; Joshi, S.; Tatu, U.S. Draft genome of a commonly misdiagnosed multidrug resistant pathogen Candida auris. BMC Genomics 2015, 16, 686. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, B.R.; James, K.D.; Polowy, K.; Bryant, B.J.; Vaidya, A.; Smith, S.; Laudeman, C.P. CD101, a novel echinocandin with exceptional stability properties and enhanced aqueous solubility. J. Antibiot. 2017, 70, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Messer, S.A.; Rhomberg, P.R.; Jones, R.N.; Castanheira, M. Activity of a long-acting echinocandin, CD101, determined using CLSI and EUCAST reference methods, against Candida and Aspergillus spp., including echinocandin- and azole-resistant isolates. J. Antimicrob. Chemother. 2016, 71, 2868–2873. [Google Scholar] [CrossRef] [PubMed]
- Ong, V.; Hough, G.; Schlosser, M.; Bartizal, K.; Balkovec, J.M.; James, K.D.; Krishnan, B.R. Preclinical Evaluation of the Stability, Safety, and Efficacy of CD101, a Novel Echinocandin. Antimicrob. Agents Chemother. 2016, 60, 6872–6879. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Messer, S.A.; Rhomberg, P.R.; Castanheira, M. CD101, a long-acting echinocandin, and comparator antifungal agents tested against a global collection of invasive fungal isolates in the SENTRY 2015 Antifungal Surveillance Program. Int. J. Antimicrob. Agents 2017, 50, 352–358. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Messer, S.A.; Rhomberg, P.R.; Castanheira, M. Activity of a Long-Acting Echinocandin (CD101) and Seven Comparator Antifungal Agents Tested against a Global Collection of Contemporary Invasive Fungal Isolates in the SENTRY 2014 Antifungal Surveillance Program. Antimicrob. Agents Chemother. 2017, 61, e02045-16. [Google Scholar] [CrossRef]
- Boikov, D.A.; Locke, J.B.; James, K.D.; Bartizal, K.; Sobel, J.D. In vitro activity of the novel echinocandin CD101 at pH 7 and 4 against Candida spp. isolates from patients with vulvovaginal candidiasis. J. Antimicrob. Chemother. 2017, 72, 1355–1358. [Google Scholar] [CrossRef]
- Arendrup, M.C.; Meletiadis, J.; Zaragoza, O.; Jørgensen, K.M.; Marcos-Zambrano, L.J.; Kanioura, L.; Cuenca-Estrella, M.; Mouton, J.W.; Guinea, J. Multicentre determination of rezafungin (CD101) susceptibility of Candida species by the EUCAST method. Clin. Microbiol. Infect. 2018, 24, 1200–1204. [Google Scholar] [CrossRef]
- Chandra, J.; Ghannoum, M.A. CD101, a Novel Echinocandin, Possesses Potent Antibiofilm Activity against Early and Mature Candida albicans Biofilms. Antimicrob. Agents Chemother. 2017, 62, e01750-17. [Google Scholar] [CrossRef] [PubMed]
- Locke, J.B.; Almaguer, A.L.; Zuill, D.E.; Bartizal, K. Characterization of in vitro Resistance Development to the Novel Echinocandin CD101 in Candida Species. Antimicrob. Agents Chemother. 2016, 60, 6100–6107. [Google Scholar] [CrossRef] [PubMed]
- Berkow, E.L.; Lockhart, S.R. Activity of CD101, a long-acting echinocandin, against clinical isolates of Candida auris. Diagn. Microbiol. Infect. Dis. 2018, 90, 196–197. [Google Scholar] [CrossRef] [PubMed]
- Hager, C.L.; Larkin, E.L.; Long, L.A.; Ghannoum, M.A. Evaluation of the efficacy of rezafungin, a novel echinocandin, in the treatment of disseminated Candida auris infection using an immunocompromised mouse model. J. Antimicrob. Chemother. 2018, 73, 2085–2088. [Google Scholar] [CrossRef]
- Wring, S.A.; Randolph, R.; Park, S.; Abruzzo, G.; Chen, Q.; Flattery, A.; Garrett, G.; Peel, M.; Outcalt, R.; Powell, K.; et al. Preclinical Pharmacokinetics and Pharmacodynamic Target of SCY-078, a First-in-Class Orally Active Antifungal Glucan Synthesis Inhibitor, in Murine Models of Disseminated Candidiasis. Antimicrob. Agents Chemother. 2017, 61, e02068-16. [Google Scholar] [CrossRef]
- Ghannoum, M.; Long, L.; Larkin, E.L.; Isham, N.; Sherif, R.; Borroto-Esoda, K.; Barat, S.; Angulo, D. Evaluation of the Antifungal Activity of the Novel Oral Glucan Synthase Inhibitor SCY-078, Singly and in Combination, for the Treatment of Invasive Aspergillosis. Antimicrob. Agents Chemother. 2018, 62, e00244-18. [Google Scholar] [CrossRef]
- Scorneaux, B.; Angulo, D.; Borroto-Esoda, K.; Ghannoum, M.; Peel, M.; Wring, S. SCY-078 Is Fungicidal against Candida Species in Time-Kill Studies. Antimicrob. Agents Chemother. 2017, 61, e01961-16. [Google Scholar] [CrossRef]
- Nunnally, N.S.; Etienne, K.A.; Angulo, D.; Lockhart, S.R.; Berkow, E.L. In Vitro Activity of Ibrexafungerp, a Novel Glucan Synthase Inhibitor against Candida glabrata Isolates with FKS Mutations. Antimicrob. Agents Chemother. 2019, 63, e01692-19. [Google Scholar] [CrossRef]
- Jiménez-Ortigosa, C.; Paderu, P.; Motyl, M.R.; Perlin, D.S. Enfumafungin derivative MK-3118 shows increased in vitro potency against clinical echinocandin-resistant Candida Species and Aspergillus species isolates. Antimicrob. Agents Chemother. 2014, 58, 1248–1251. [Google Scholar] [CrossRef]
- Berkow, E.L.; Angulo, D.; Lockhart, S.R. In Vitro Activity of a Novel Glucan Synthase Inhibitor, SCY-078, against Clinical Isolates of Candida auris. Antimicrob. Agents Chemother. 2017, 61, e00435-17. [Google Scholar] [CrossRef]
- Cornely, O.A.; Sidhu, M.; Odeyemi, I.; van Engen, A.K.; van der Waal, J.M.; Schoeman, O. Economic analysis of micafungin versus liposomal amphotericin B for treatment of candidaemia and invasive candidiasis in Germany. Curr. Med. Res. Opin. 2008, 24, 1743–1753. [Google Scholar] [CrossRef] [PubMed]
- Bruynesteyn, K.; Gant, V.; McKenzie, C.; Pagliuca, T.; Poynton, C.; Kumar, R.N.; Jansen, J.P. A cost-effectiveness analysis of caspofungin vs. liposomal amphotericin B for treatment of suspected fungal infections in the UK. Eur. J. Haematol. 2007, 78, 532–539. [Google Scholar] [CrossRef] [PubMed]
- Neoh, C.F.; Liew, D.; Slavin, M.; Marriott, D.; Chen, S.C.-A.; Morrissey, O.; Stewart, K.; Kong, D.C.M. Cost-effectiveness analysis of anidulafungin versus fluconazole for the treatment of invasive candidiasis. J. Antimicrob. Chemother. 2011, 66, 1906–1915. [Google Scholar] [CrossRef] [PubMed]
| Antifungal Agent | Standard | C. albicans | C. glabrata | C. krusei | C. parapsilosis | C. tropicalis | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| S≤ | R> | S≤ | R> | S≤ | R> | S≤ | R> | S≤ | R> | ||
| AND | EUCAST | 0.03 | 0.03 | 0.06 | 0.06 | 0.06 | 0.06 | 4 | 4 | 0.06 | 0.06 |
| CLSI | 0.25 | 0.5 | 0.12 | 0.25 | 0.25 | 0.5 | 2 | 4 | 0.25 | 0.5 | |
| CAS | EUCAST | N | N | N | N | N | N | N | N | N | N |
| CLSI | 0.25 | 0.5 | 0.125 | 0.25 | 0.25 | 0.5 | 2 | 4 | 0.25 | 0.5 | |
| MCF | EUCAST | 0.016 | 0.016 | 0.03 | 0.03 | IE | IE | 2 | 2 | IE | IE |
| CLSI | 0.25 | 0.5 | 0.06 | 0.12 | 0.25 | 0.5 | 2 | 4 | 0.25 | 0.5 | |
| Antifungal Agent | ||||
|---|---|---|---|---|
| Organism | AND | CSP | MCF | Reference |
| Candida albicans | + | + | + | [38,39] |
| Candida glabrata | + | + | + | [39] |
| Candida parapsilosis | + | + | + | [39] |
| Candida tropicalis | + | + | + | [39] |
| Candida krusei | + | + | + | [39] |
| Candida lusitaniae | + | + | + | [38] |
| Aspergillus fumigatus | + | + | + | [40] |
| Aspergillus flavus | + | + | + | [40] |
| Aspergillus niger | + | + | + | [40] |
| Aspergillus terreus | + | + | + | [40] |
| Acremonium | - | - | - | [35,41] |
| Alternaria | - | - | - | [35] |
| Blastomyces spp. | +/− | +/− | +/− | [34] |
| Coccidioides spp. | +/− | +/− | +/− | [36] |
| Cryptococcus neoformans | - | - | - | [32,33] |
| Curvularia | + | + | + | [42] |
| Fusarium spp. | - | - | - | [40] |
| Histoplasma spp. | +/− | +/− | +/− | [17] |
| Mucorales | - | - | - | [43] |
| Rizpous | - | - | - | [43] |
| Scedosporium spp. | - | - | - | [17] |
| Trichoderma | + | + | + | [44] |
| Trichosporon | - | - | - | [17,45] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mroczyńska, M.; Brillowska-Dąbrowska, A. Review on Current Status of Echinocandins Use. Antibiotics 2020, 9, 227. https://doi.org/10.3390/antibiotics9050227
Mroczyńska M, Brillowska-Dąbrowska A. Review on Current Status of Echinocandins Use. Antibiotics. 2020; 9(5):227. https://doi.org/10.3390/antibiotics9050227
Chicago/Turabian StyleMroczyńska, Martyna, and Anna Brillowska-Dąbrowska. 2020. "Review on Current Status of Echinocandins Use" Antibiotics 9, no. 5: 227. https://doi.org/10.3390/antibiotics9050227
APA StyleMroczyńska, M., & Brillowska-Dąbrowska, A. (2020). Review on Current Status of Echinocandins Use. Antibiotics, 9(5), 227. https://doi.org/10.3390/antibiotics9050227





