Screening and Molecular Docking of Novel Benzothiazole Derivatives as Potential Antimicrobial Agents
Abstract
1. Introduction
2. Results
2.1. Antimicrobial Activity
2.2. Effect of Benzothiazole Compounds on the Activity of E. coli Dihydroorotase
2.3. Effect of Benzothiazole Compounds on Dimorphic Transition of C. Albicans
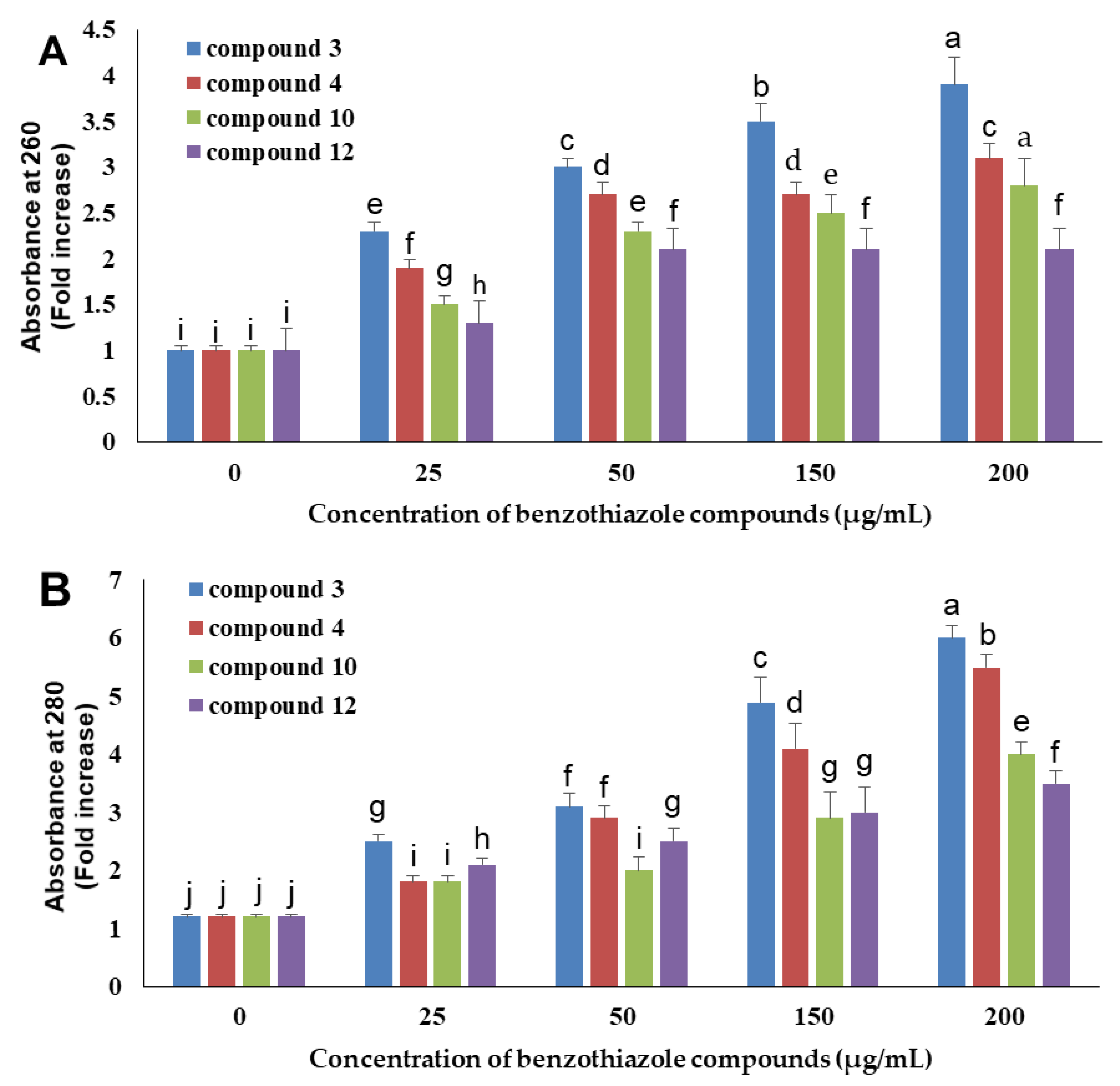
2.4. Determination of DNA and Protein Leakage
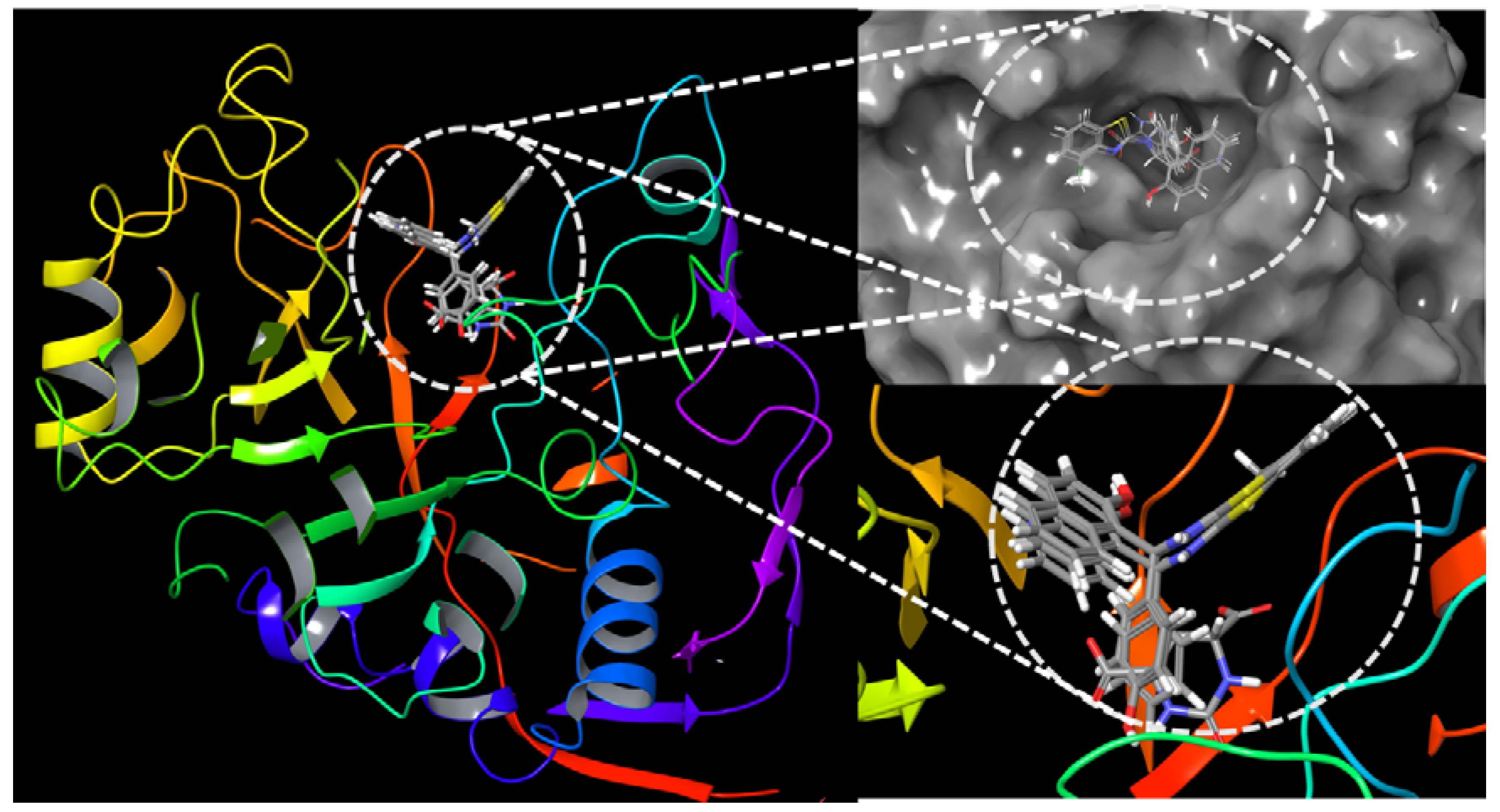
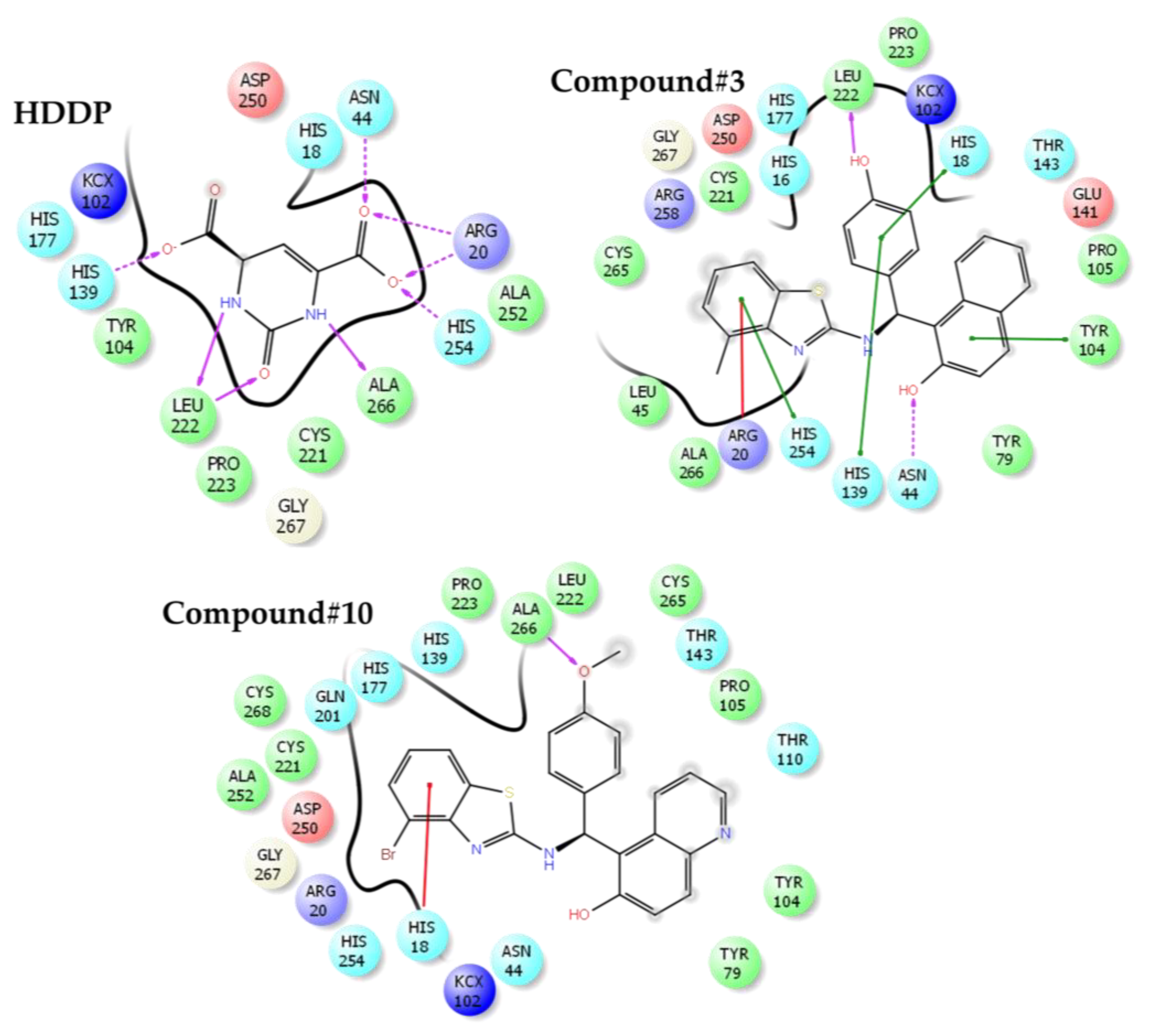
2.5. Interactions of Benzothiazole Compounds with E. coli Dihydroorotase
3. Discussion
4. Materials and Methods
4.1. Biological Activity
4.1.1. Antibacterial Activity of Benzothiazole Compounds
4.1.2. Antifungal Activity of Benzothiazole Compounds
4.1.3. Effect of Benzothiazole Compounds on Dihydroorotase Activity
4.1.4. Effect of Benzothiazole Compounds on C. albicans Hyphal Development in Liquid Media
4.1.5. Effect of Benzothiazole Compounds on the Leakage of DNA and Proteins
4.2. Computational Studies
4.3. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Cantas, L.; Shah, S.Q.; Cavaco, L.M.; Manaia, C.M.; Walsh, F.; Popowska, M.; Garelick, H.; Burgmann, H.; Sorum, H. A brief multi-disciplinary review on antimicrobial resistance in medicine and its linkage to the global environmental microbiota. Front. Microbiol. 2013, 4, 96. [Google Scholar] [CrossRef] [PubMed]
- Blair, J.M.; Webber, M.A.; Baylay, A.J.; Ogbolu, D.O.; Piddock, L.J. Molecular mechanisms of antibiotic resistance. Nat. Rev. Microbiol. 2015, 13, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Fauci, A.S.; Morens, D.M. The Perpetual Challenge of Infectious Diseases. New Engl. J. Med. 2012, 366, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Morens, D.M.; Folkers, G.K.; Fauci, A.S. The challenge of emerging and re-emerging infectious diseases. Nature 2004, 430, 242–249. [Google Scholar] [CrossRef]
- Singh, M.; Gangwar, M.; Nath, G.; Singh, S.K. Synthesis, DNA cleavage and antimicrobial activity of 4-thiazolidinones-benzothiazole conjugates. Indian J. Exp. Biol 2014, 52, 1062–1070. [Google Scholar]
- Shaikh, F.M.; Patel, N.B.; Sanna, G.; Busonera, B.; Colla, P.; Rajani, D.P. Synthesis of some new 2-amino-6-thiocyanato benzothiazole derivatives bearing 2,4-thiazolidinediones and screening of their in vitro antimicrobial, antitubercular and antiviral activities. Med. Chem. Res. 2015, 24, 3129–3142. [Google Scholar] [CrossRef]
- Abuzar, S.; Sharma, S. Synthesis of 6-N-Aryl and heteroaryl benzthiazoles as potential anthelmintics. Z. Fuer Nat. 1981, 36, 108–111. [Google Scholar] [CrossRef]
- Puranik, N.V.; Puntambekar, H.M.; Srivastava, P. Antidiabetic potential and enzyme kinetics of benzothiazole derivatives and their non-bonded interactions with α-glucosidase and α-amylase. Med. Chem. Res. 2016, 25, 805–816. [Google Scholar] [CrossRef]
- Nagoshi, N.; Nakashima, H.; Fehlings, M.G. Riluzole as a neuroprotective drug for spinal cord injury: From bench to bedside. Molecules 2015, 20, 7775–7789. [Google Scholar] [CrossRef]
- Venugopala, K.N.; Krishnappa, M.; Nayak, S.K.; Subrahmanya, B.K.; Vaderapura, J.P.; Chalannavar, R.K.; Gleiser, R.M.; Odhav, B. Synthesis and antimosquito properties of 2,6-substituted benzo[d]thiazole and 2,4-substituted benzo[d]thiazole analogues against Anopheles arabiensis. Eur. J. Med. Chem. 2013, 65, 295–303. [Google Scholar] [CrossRef]
- Shah, F.; Wu, Y.; Gut, J.; Pedduri, Y.; Legac, J.; Rosenthal, P.J.; Avery, M.A. Design, synthesis and biological evaluation of novel benzothiazole and triazole analogs as falcipain inhibitors. MedChemComm 2011, 2, 1201–1207. [Google Scholar] [CrossRef]
- Rida, S.M.; Youssef, A.M.; Badr, M.H.; Malki, A.; Sherif, Z.A.; Sultan, A.S. Design, synthesis and evaluation of novel benzimidazoles, benzothiazoles and benzofurans incorporating pyrazole moiety as antiangiogenic agents. Arzneimittelforschung 2012, 62, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Lad, N.P.; Manohar, Y.; Mascarenhas, M.; Pandit, Y.B.; Kulkarni, M.R.; Sharma, R.; Salkar, K.; Suthar, A.; Pandit, S.S. Methylsulfonyl benzothiazoles (MSBT) derivatives: Search for new potential antimicrobial and anticancer agents. Bioorganic Med. Chem. Lett. 2017, 27, 1319–1324. [Google Scholar] [CrossRef] [PubMed]
- Padalkar, V.S.; Borse, B.N.; Gupta, V.D.; Phatangare, K.R.; Patil, V.S.; Umape, P.G.; Sekar, N. Synthesis and antimicrobial activity of novel 2-substituted benzimidazole, benzoxazole and benzothiazole derivatives. Arab. J. Chem. 2016, 9, S1125–S1130. [Google Scholar] [CrossRef]
- Trapani, A.; Catalano, A.; Carocci, A.; Carrieri, A.; Mercurio, A.; Rosato, A.; Mandracchia, D.; Tripodo, G.; Schiavone, B.I.P.; Franchini, C.; et al. Effect of Methyl-beta-Cyclodextrin on the antimicrobial activity of a new series of poorly water-soluble benzothiazoles. Carbohydr. Polym. 2019, 207, 720–728. [Google Scholar] [CrossRef] [PubMed]
- Al-Talib, M.; Al-Soud, Y.A.; Abussaud, M.; Khshashneh, S. Synthesis and biological evaluation of new benzothiazoles as antimicrobial agents. Arab. J. Chem. 2016, 9, S926–S930. [Google Scholar] [CrossRef]
- Yurttaş, L.; Özkay, Y.; Duran, M.; Turan-Zitouni, G.; Özdemir, A.; Cantürk, Z.; Küçükoğlu, K.; Kaplancıklı, Z.A. Synthesis and antimicrobial activity evaluation of new dithiocarbamate derivatives bearing thiazole/benzothiazole rings. Phosphorus Sulfur Silicon Relat. Elem. 2016, 191, 1166–1173. [Google Scholar] [CrossRef]
- Maddili, S.K.; Li, Z.Z.; Kannekanti, V.K.; Bheemanaboina, R.R.Y.; Tuniki, B.; Tangadanchu, V.K.R.; Zhou, C.H. Azoalkyl ether imidazo[2,1-b]benzothiazoles as potentially antimicrobial agents with novel structural skeleton. Bioorganic Med. Chem. Lett. 2018, 28, 2426–2431. [Google Scholar] [CrossRef]
- Kumari, B.; Chauhan, K.; Trivedi, J.; Jaiswal, V.; Kanwar, S.S.; Pokharel, Y.R. Benzothiazole-Based-Bioconjugates with Improved Antimicrobial, Anticancer and Antioxidant Potential. ChemistrySelect 2018, 3, 11326–11332. [Google Scholar] [CrossRef]
- Mishra, V.R.; Ghanavatkar, C.W.; Mali, S.N.; Qureshi, S.I.; Chaudhari, H.K.; Sekar, N. Design, synthesis, antimicrobial activity and computational studies of novel azo linked substituted benzimidazole, benzoxazole and benzothiazole derivatives. Comput. Biol. Chem. 2019, 78, 330–337. [Google Scholar] [CrossRef]
- Naaz, F.; Srivastava, R.; Singh, A.; Singh, N.; Verma, R.; Singh, V.K.; Singh, R.K. Molecular modeling, synthesis, antibacterial and cytotoxicity evaluation of sulfonamide derivatives of benzimidazole, indazole, benzothiazole and thiazole. Bioorganic Med. Chem. 2018, 26, 3414–3428. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, S.S.; Thakor, P.; Ray, A.; Doshi, H.; Thakkar, V.R. Benzothiazole analogues: Synthesis, characterization, MO calculations with PM6 and DFT, in silico studies and in vitro antimalarial as DHFR inhibitors and antimicrobial activities. Bioorganic Med. Chem. 2017, 25, 5396–5406. [Google Scholar] [CrossRef]
- Er, M.; Özer, A.; Direkel, Ş.; Karakurt, T.; Tahtaci, H. Novel substituted benzothiazole and Imidazo [2, 1-b][1, 3, 4] Thiadiazole derivatives: Synthesis, characterization, molecular docking study, and investigation of their in vitro antileishmanial and antibacterial activities. J. Mol. Struct. 2019, 1194, 284–296. [Google Scholar] [CrossRef]
- Venugopala, K.N.; Chandrashekharappa, S.; Pillay, M.; Bhandary, S.; Kandeel, M.; Mahomoodally, F.M.; Morsy, M.A.; Chopra, D.; Aldhubiab, B.E.; Attimarad, M.; et al. Synthesis and Structural Elucidation of Novel Benzothiazole Derivatives as Anti-tubercular Agents: In-silico Screening for Possible Target Identification. Med. Chem. 2019, 15, 311–326. [Google Scholar] [CrossRef] [PubMed]
- Telvekar, V.N.; Bairwa, V.K.; Satardekar, K.; Bellubi, A. Novel 2-(2-(4-aryloxybenzylidene) hydrazinyl) benzothiazole derivatives as anti-tubercular agents. Bioorganic Med. Chem. Lett. 2012, 22, 649–652. [Google Scholar] [CrossRef]
- Rouf, A.; Tanyeli, C. Bioactive thiazole and benzothiazole derivatives. Eur. J. Med. Chem. 2015, 97, 911–927. [Google Scholar] [CrossRef]
- Gjorgjieva, M.; Tomasic, T.; Kikelj, D.; Masic, L.P. Benzothiazole-based Compounds in Antibacterial Drug Discovery. Curr. Med. Chem. 2018, 25, 5218–5236. [Google Scholar] [CrossRef]
- Bradbury, B.J.; Pucci, M.J. Recent advances in bacterial topoisomerase inhibitors. Curr. Opin. Pharmacol. 2008, 8, 574–581. [Google Scholar] [CrossRef]
- Luo, B.; Li, D.; Zhang, A.L.; Gao, J.M. Synthesis, Antifungal Activities and Molecular Docking Studies of Benzoxazole and Benzothiazole Derivatives. Molecules 2018, 23, 2457. [Google Scholar] [CrossRef]
- Maddila, S.; Gorle, S.; Seshadri, N.; Lavanya, P.; Jonnalagadda, S.B. Synthesis, antibacterial and antifungal activity of novel benzothiazole pyrimidine derivatives. Arab. J. Chem. 2016, 9, 681–687. [Google Scholar] [CrossRef]
- Singh, M.K.; Tilak, R.; Nath, G.; Awasthi, S.K.; Agarwal, A. Design, synthesis and antimicrobial activity of novel benzothiazole analogs. Eur. J. Med. Chem. 2013, 63, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Saeed, S.; Rashid, N.; Jones, P.G.; Ali, M.; Hussain, R. Synthesis, characterization and biological evaluation of some thiourea derivatives bearing benzothiazole moiety as potential antimicrobial and anticancer agents. Eur. J. Med. Chem. 2010, 45, 1323–1331. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Sellamuthu, S.; Singh, S.K.; Gangwar, M.; Nath, G.; Singh, S.K. Antimicrobial Potency and Molecular Mechanism of Benzothiazole Schiff Base Hybrids. Saudi J. Med. Pharm. Sci. 2017, 3, 1360–1369. [Google Scholar] [CrossRef]
- Singh, M.; Singh, S.K.; Gangwar, M.; Nath, G.; Singh, S.K. Design, synthesis and mode of action of some benzothiazole derivatives bearing an amide moiety as antibacterial agents. RSC Adv. 2014, 4, 19013–19023. [Google Scholar] [CrossRef]
- Carradori, S.; Chimenti, P.; Fazzari, M.; Granese, A.; Angiolella, L. Antimicrobial activity, synergism and inhibition of germ tube formation by Crocus sativus-derived compounds against Candida spp. J. Enzym. Inhib Med. Chem. 2016, 31, 189–193. [Google Scholar] [CrossRef]
- Modrzewska, B.; Kurnatowski, P. Adherence of Candida sp. to host tissues and cells as one of its pathogenicity features. Ann. Parasitol. 2015, 61, 3–9. [Google Scholar] [PubMed]
- Bujdáková, H.; Múcková, M. Antifungal activity of a new benzothiazole derivative against Candida in vitro and in vivo. Int. J. Antimicrob. Agents 1994, 4, 303–308. [Google Scholar] [CrossRef]
- Fábry, S.; Gáborová, S.; Bujdáková, H.; Klobusický, M.; Volleková, A.; Kuchta, T. Inhibition of germ tube formation, filamentation and ergosterol biosynthesis in Candida albicans treated with 6-amino-2-n-pentylthiobenzothiazole. Folia Microbiol. 1999, 44, 523–526. [Google Scholar] [CrossRef]
- Lee, M.; Chan, C.W.; Graham, S.C.; Christopherson, R.I.; Guss, J.M.; Maher, M.J. Structures of Ligand-free and Inhibitor Complexes of Dihydroorotase from Escherichia coli: Implications for Loop Movement in Inhibitor Design. J. Mol. Biol. 2007, 370, 812–825. [Google Scholar] [CrossRef]
- Vitali, J.; Singh, A.K.; Colaneri, M.J. Characterization of the Dihydroorotase from Methanococcus jannaschii. Protein J. 2017, 36, 361–373. [Google Scholar] [CrossRef]
- Rice, A.J.; Lei, H.; Santarsiero, B.D.; Lee, H.; Johnson, M.E. Ca-asp bound X-ray structure and inhibition of Bacillus anthracis dihydroorotase (DHOase). Bioorganic Med. Chem. 2016, 24, 4536–4543. [Google Scholar] [CrossRef] [PubMed]
- Truong, L.; Hevener, K.E.; Rice, A.J.; Patel, K.; Johnson, M.E.; Lee, H. High-level expression, purification, and characterization of Staphylococcus aureus dihydroorotase (PyrC) as a cleavable His-SUMO fusion. Protein Expr. Purif. 2013, 88, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Aleksenko, A.; Liu, W.; Gojkovic, Z.; Nielsen, J.; Piskur, J. Structural and transcriptional analysis of the pyrABCN, pyrD and pyrF genes in Aspergillus nidulans and the evolutionary origin of fungal dihydroorotases. Mol. Microbiol. 1999, 33, 599–611. [Google Scholar] [CrossRef]
- Khalil, N.M.; Shalaby, E.A.; Ali, D.M.; Ali, E.M.; Aboul-Enein, A.M. Biological activities of secondary metabolites from Emericella nidulans EGCU 312. Afr. J. Microbiol. Res. 2014, 8, 2011–2021. [Google Scholar]
- Hossain, M.A.; Shah, M.D.; Sang, S.V.; Sakari, M. Chemical composition and antibacterial properties of the essential oils and crude extracts of Merremia borneensis. J. King Saud Univ. Sci. 2012, 24, 243–249. [Google Scholar] [CrossRef]
- Patel, J.B.; Tenover, F.C.; Turnidge, J.D.; Jorgensen, J.H. Susceptibility test methods: Dilution and disk diffusion methods. In Manual of Clinical Microbiology, 10th ed.; Versalovic, J., Carroll, K., Funke, G., Jorgensen, J., Landry, M., Warnock, D., Eds.; ASM Press: Washington, DC, USA, 2011; pp. 1122–1143. [Google Scholar] [CrossRef]
- Johnson, E.M.; Cavling-Arendrup, M. Susceptibility test methods: Yeasts and filamentous fungi. In Manual of Clinical Microbiology, 11th ed.; Jorgensen, J., Pfaller, M., Carroll, K., Funke, G., Landry, M., Richter, S., Warnock, D., Eds.; ASM Press: Washington, DC, USA, 2015; pp. 2255–2281. [Google Scholar] [CrossRef]
- Ali, E.M. Phytochemical composition, antifungal, antiaflatoxigenic, antioxidant, and anticancer activities of Glycyrrhiza glabra L. and Matricaria chamomilla L. essential oils. J. Med. Plants Res. 2013, 7, 2197–2207. [Google Scholar]
- Ali, E.M. Contributions of some biological activities of honey bee venom. J. Apic. Res. 2014, 53, 441–451. [Google Scholar] [CrossRef]
- Huang, D.T.; Thomas, M.A.; Christopherson, R.I. Divalent metal derivatives of the hamster dihydroorotase domain. Biochemistry 1999, 38, 9964–9970. [Google Scholar] [CrossRef]
- Porter, T.N.; Li, Y.; Raushel, F.M. Mechanism of the dihydroorotase reaction. Biochemistry 2004, 43, 16285–16292. [Google Scholar] [CrossRef]
- Manoharan, R.K.; Lee, J.H.; Kim, Y.G.; Lee, J. Alizarin and Chrysazin Inhibit Biofilm and Hyphal Formation by Candida albicans. Front. Cell. Infect. Microbiol. 2017, 7, 447. [Google Scholar] [CrossRef]
- Khalil, N.M.; Abd El-Ghany, M.N.; Rodriguez-Couto, S. Antifungal and anti-mycotoxin efficacy of biogenic silver nanoparticles produced by Fusarium chlamydosporum and Penicillium chrysogenum at non-cytotoxic doses. Chemosphere 2019, 218, 477–486. [Google Scholar] [CrossRef] [PubMed]
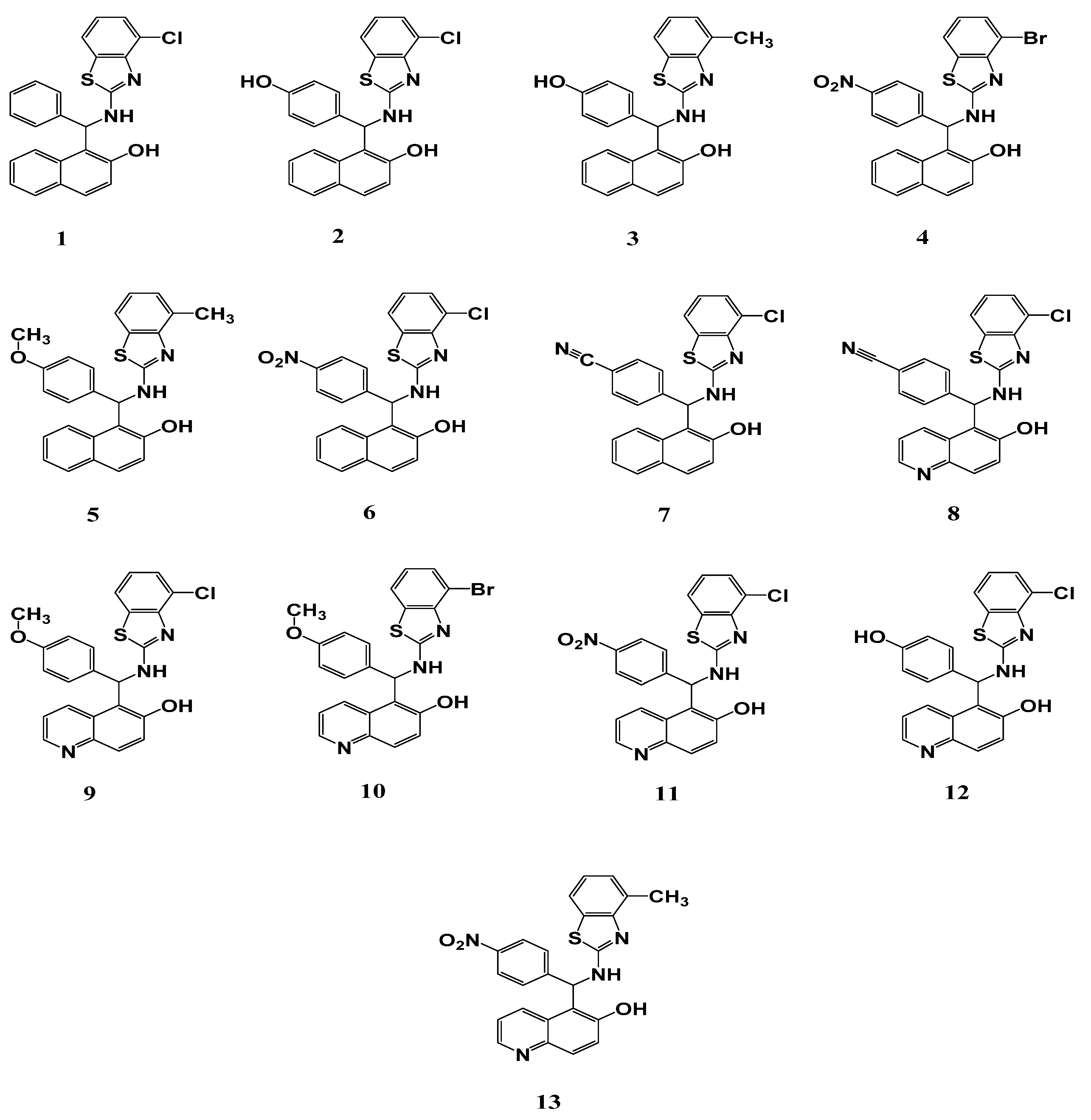

| Compound | Fungi | Gram-Positive Bacteria | Gram-Negative Bacteria | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Candida albicans | Aspergillus niger | Staphylococcus aureus | Bacillus subtilis | Escherichia coli | |||||||||||
| IZD (mm) | MIC (µg/mL) | MFC (µg/mL) | IZD (mm) | MIC (µg/mL) | MFC (µg/mL) | IZD (mm) | MIC (µg/mL) | MBC (µg/mL) | IZD (mm) | MIC (µg/mL) | MBC (µg/mL) | IZD (mm) | MIC (µg/mL) | MBC (µg/mL) | |
| 1 | 0 f ± 0.0 | - | - | 9 e ± 0.12 | 200 a | 400 a | 13 e ± 0.53 | 100 b | 200 b | 15 d ± 0.32 | 200 a | 400 a | 0 h ± 0.0 | - | - |
| 2 | 9 e ± 0.27 | 100 a | 200 a | 7 f ± 0.15 | 200 a | 400 a | 15 d ± 0.31 | 50 c | 100 c | 14 e ± 0.12 | 100 b | 200 b | 6 f ± 0.11 | 100 a | 200 a |
| 3 | 15 c ± 0.38 | 25 c | 50 c | 18 b ± 0.91 | 25 d | 50 d | 25 b ± 0.24 | 50 c | 100 c | 21 c ± 0.31 | 25 d | 50 d | 27 b ± 0.25 | 25 c | 50 c |
| 4 | 18 b ± 0.54 | 50 b | 100 b | 12 d ± 0.23 | 50 c | 100 c | 19 c ± 0.61 | 50 c | 100 c | 22 b ± 0.26 | 50 c | 100 c | 25c ± 0.31 | 50 b | 100 b |
| 5 | 0 f ± 0.0 | - | - | 0 g ± 0.0 | - | - | 10 a ± 0.52 | 200 a | 400 a | 14 e ± 0.57 | 200 a | 400 a | 18 d ± 0.51 | 100 a | 200 a |
| 6 | 0 f ± 0.0 | - | - | 0 g ± 0.0 | - | - | 0 f ± 0.0 | - | - | 0 f ± 0.0 | - | - | 0 g ± 0.0 | - | - |
| 7 | 0 f ± 0.0 | - | - | 0 g ± 0.0 | - | - | 0 f ± 0.0 | - | - | 0 f ± 0.0 | - | - | 0 g ± 0.0 | - | - |
| 8 | 0 f ± 0.0 | - | - | 0 g ± 0.0 | - | - | 0 f ± 0.0 | - | - | 0 f ± 0.0 | - | - | 0 g ± 0.0 | - | - |
| 9 | 0 f ± 0.0 | - | - | 0 g ± 0.0 | - | - | 0 f ± 0.0 | - | - | 0 f ± 0.0 | - | - | 0 g ± 0.0 | - | - |
| 10 | 13 d ± 0.11 | 100 a | 200 a | 11 b ± 0.02 | 100 b | 200 b | 15 d ± 0.21 | 50 c | 100 c | 15 d ± 0.23 | 50 c | 100 c | 12 e ± 0.61 | 25 c | 50 c |
| 11 | 0 f ± 0.0 | - | - | 0 g ± 0.0 | - | - | 0 f ± 0.0 | - | - | 0 f ± 0.0 | - | - | 0 g ± 0.0 | - | - |
| 12 | 16 c ± 0.21 | 50 b | 100 b | 14 c ± 0.36 | 100 b | 200 b | 20 c ± 0.06 | 100 b | 200 b | 14 e ± 0.15 | 100 b | 200 b | 19 d ± 0.19 | 50 b | 100 b |
| 13 | 0 f ± 0.0 | - | - | 0 g ± 0.0 | - | - | 0 f ± 0.0 | - | - | 0 a ± 0.0 | - | - | 0 g ± 0.0 | - | - |
| DMSO | 0 f ± 0.0 | - | - | 0 g ± 0.0 | - | - | 0 f ± 0.0 | - | - | 0 f ± 0.0 | - | - | 0 g ± 0.0 | - | - |
| Fluconazole | 28 a ± 0.62 | 6.25 d | 12.5 d | 26 a ± 0.82 | 3.13 e | 6.25 e | 0 f ± 0.0 | - | - | 0 f ± 0.0 | - | - | 0 g ± 0.0 | - | - |
| Kanamycin | 0 f ± 0.0 | - | - | 0 g ± 0.0 | - | - | 29 a ± 0.84 | 6.25 d | 12.5 d | 28 a ± 0.62 | 3.13 d | 6.25 e | 31 a ± 0.81 | 6.25 d | 12.5 d |
| Compound | Specific Activity of Escherichia coli Dihydroorotase (nmol/min/mg protein) |
|---|---|
| DMSO | 119 a ± 1.7 |
| Kanamycin | 26 l ± 0.38 |
| 1 | 85 f ± 0.91 |
| 2 | 100 c ± 1.1 |
| 3 | 45 k ± 0.62 |
| 4 | 60 j ± 0.015 |
| 5 | 82 g ± 1.15 |
| 6 | 92 e ± 1.51 |
| 7 | 111 b ± 0.98 |
| 8 | 98 d ± 0.82 |
| 9 | 102 c ± 0.91 |
| 10 | 65 i ± 0.43 |
| 11 | 73 h ± 0.24 |
| 12 | 75 h ± 0.31 |
| 13 | 104 c ± 0.73 |
| Compound | Yeast Form Count (cell/mL) | Filamentous Form Count (cell/mL) | % of Dimorphism |
|---|---|---|---|
| Control (DMSO) | 50 k ± 4.0 | 1730 e ± 4.0 | 97.1 |
| Fluconazole | 350 | 400 k ± 0.5 | 12.5 |
| 1 | 410 d ± 5.0 | 1810 d ± 1.0 | 77.3 |
| 2 | 450 b ± 2.5 | 1178 i ± 1.5 | 61.7 |
| 3 | 306 e ± 1.0 | 393 l ± 1.5 | 22 |
| 4 | 10 l ± 2.5 | 12 o ± 0.76 | 16.66 |
| 5 | 448 c ± 5.0 | 1320 h ± 5.0 | 66 |
| 6 | 162 i ± 2.5 | 2033 a ± 1.5 | 92 |
| 7 | 186 g ± 4.5 | 1691 f ± 0.5 | 89 |
| 8 | 162 i ± 5.0 | 1620 g ± 2.5 | 90 |
| 9 | 92 j ± 7.5 | 1840 c ± 2.0 | 95 |
| 10 | 243 f ± 5.0 | 363 m ± 1.5 | 33 |
| 11 | 10 l ± 2.5 | 144 n ± 2.0 | 93 |
| 12 | 497 a ± 6.0 | 904 j ± 2.0 | 45 |
| 13 | 164 h ± 7.5 | 1844 b ± 2.0 | 92.10 |
| Compound | Docking Score | Glide Ligand Efficiency | Glide Lipo | Glide H-Bond |
|---|---|---|---|---|
| HDDP | −7.37 | −0.57 | −0.15 | −0.80 |
| 3 | −5.02 | −0.17 | −1.70 | −0.47 |
| 4 | −4.57 | −0.16 | −1.84 | −0.32 |
| 10 | −4.87 | −0.16 | −1.61 | −0.52 |
| 12 | −2.54 | −0.08 | −0.97 | −0.16 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morsy, M.A.; Ali, E.M.; Kandeel, M.; Venugopala, K.N.; Nair, A.B.; Greish, K.; El-Daly, M. Screening and Molecular Docking of Novel Benzothiazole Derivatives as Potential Antimicrobial Agents. Antibiotics 2020, 9, 221. https://doi.org/10.3390/antibiotics9050221
Morsy MA, Ali EM, Kandeel M, Venugopala KN, Nair AB, Greish K, El-Daly M. Screening and Molecular Docking of Novel Benzothiazole Derivatives as Potential Antimicrobial Agents. Antibiotics. 2020; 9(5):221. https://doi.org/10.3390/antibiotics9050221
Chicago/Turabian StyleMorsy, Mohamed A., Enas M. Ali, Mahmoud Kandeel, Katharigatta N. Venugopala, Anroop B. Nair, Khaled Greish, and Mahmoud El-Daly. 2020. "Screening and Molecular Docking of Novel Benzothiazole Derivatives as Potential Antimicrobial Agents" Antibiotics 9, no. 5: 221. https://doi.org/10.3390/antibiotics9050221
APA StyleMorsy, M. A., Ali, E. M., Kandeel, M., Venugopala, K. N., Nair, A. B., Greish, K., & El-Daly, M. (2020). Screening and Molecular Docking of Novel Benzothiazole Derivatives as Potential Antimicrobial Agents. Antibiotics, 9(5), 221. https://doi.org/10.3390/antibiotics9050221









