Cephalosporins’ Cross-Reactivity and the High Degree of Required Knowledge. Case Report and Review of the Literature
Abstract
1. Introduction
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Johansson, S.G.; Bieber, T.; Dahl, R.; Friedmann, P.S.; Lanier, B.Q.; Lockey, R.F.; Motala, C.; Ortega Martell, J.A.; Platts-Mills, T.A.; Ring, J.; et al. Revised nomenclature for allergy for global use: Report of the nomenclature review committee of the World Allergy Organization, October 2003. J. Allergy Clin. Immunol. 2004, 113, 832–836. [Google Scholar] [CrossRef]
- Thong, B.Y.; Tan, T.C. Epidemiology and risk factors for drug allergy. Br. J. Clin. Pharmacol. 2011, 71, 684–700. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, T.; Gandhi, T.K.; Seger, A.C.; Hsieh, T.C.; Bates, D.W. Adverse drug events and medication errors: Detection and classification methods. Qual. Saf. Health Care 2004, 13, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Kohn, L.T.; Corrigan, J.M.; Donaldson, M.S. To Err is Human: Building a Safer Health System; National Academy Press: Washington, DC, USA, 2000. [Google Scholar]
- Di Sanzo, M.; Cipolloni, L.; Borro, M.; La Russa, R.; Santurro, A.; Scopetti, M.; Simmaco, M.; Frati, P. Clinical Applications of Personalized Medicine: A New Paradigm and Challenge. Curr. Pharm. Biotechnol. 2017, 18, 194–203. [Google Scholar] [CrossRef] [PubMed]
- La Russa, R.; Fineschi, V.; Di Sanzo, M.; Gatto, V.; Santurro, A.; Martini, G.; Scopetti, M.; Frati, P. Personalized medicine and adverse drug reactions: The Experience of an Italian teaching hospital. Curr. Pharm. Biotechnol. 2017, 18, 274–281. [Google Scholar] [CrossRef]
- Trubiano, J.A.; Stone, C.A.; Grayson, M.L.; Urbancic, K.; Slavin, M.A.; Thursky, K.A.; Phillips, E.J. The 3 Cs of Antibiotic Allergy-Classification, Cross-Reactivity, and Collaboration. J. Allergy Clin. Immunol. Pract. 2017, 5, 1532–1542. [Google Scholar] [CrossRef]
- Blumenthal, K.G.; Peter, J.G.; Trubiano, J.A.; Phillips, E.J. Antibiotic allergy. Lancet 2019, 393, 183–198. [Google Scholar] [CrossRef]
- Zhou, L.; Dhopeshwarkar, N.; Blumenthal, K.G.; Goss, F.; Topaz, M.; Slight, S.P.; Bates, D.W. Drug allergies documented in electronic health records of a large healthcare system. Allergy 2016, 71, 1305–1313. [Google Scholar] [CrossRef]
- Giraldi, G.; Montesano, M.; Napoli, C.; Frati, P.; La Russa, R.; Santurro, A.; Scopetti, M.; Orsi, G.B. Healthcare-associated infections due to multidrug-resistant organisms: A surveillance study on extra hospital stay and direct costs. Curr. Pharm. Biotechnol. 2019, 20, 643–652. [Google Scholar] [CrossRef]
- Pichichero, M.E. Cephalosporins can be prescribed safely for penicillin allergic patients. J. Fam. Pract. 2006, 55, 106–112. [Google Scholar]
- Thoburn, R.; Johnson, J.E., 3rd; Cluff, L.E. Studies on the epidemiology of adverse drug reactions. IV. The relationship of cephalothin and penicillin allergy. JAMA 1966, 198, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Yuson, C.; Kumar, K.; Le, A.; Ahmadie, A.; Banovic, T.; Heddle, R.; Kette, F.; Smith, W.; Hissaria, P. Immediate cephalosporin allergy. Intern. Med. J. 2019, 49, 985–993. [Google Scholar] [CrossRef] [PubMed]
- Pichichero, M.E. A review of evidence supporting the American Academy of Pediatrics recommendation for prescribing cephalosporin antibiotics for penicillin-allergic patients. Pediatrics 2005, 115, 1048–1057. [Google Scholar] [CrossRef] [PubMed]
- Romano, A.; Gaeta, F.; Arribas Poves, M.F.; Valluzzi, R.L. Cross-Reactivity among Beta-Lactams. Curr. Allergy Asthma Rep. 2016, 16, 24. [Google Scholar] [CrossRef]
- Madaan, A.; Li, J.T. Cephalosporin allergy. Immunol. Allergy Clin. N. Am. 2004, 24, 463–476. [Google Scholar] [CrossRef]
- Pichichero, M.E. Use of selected cephalosporins in penicillin-allergic patients: A paradigm shift. Diagn. Microbiol. Infect. Dis. 2007, 57 (Suppl. 3), 13S–18S. [Google Scholar] [CrossRef]
- Mirakian, R.; Leech, S.C.; Krishna, M.T.; Richter, A.G.; Huber, P.A.; Farooque, S.; Khan, N.; Pirmohamed, M.; Clark, A.T.; Nasser, S.M.; et al. Management of allergy to penicillins and other beta-lactams. Clin. Exp. Allergy 2015, 45, 300–327. [Google Scholar] [CrossRef]
- Blumenthal, K.G.; Parker, R.A.; Shenoy, E.S.; Walensky, R.P. Improving clinical outcomes in patients with methicillin-sensitive Staphylococcus aureus bacteremia and reported penicillin allergy. Clin. Infect. Dis. 2015, 61, 741–749. [Google Scholar] [CrossRef]
- Blumenthal, K.G.; Shenoy, E.S.; Huang, M.; Kuhlen, J.L.; Ware, W.A.; Parker, R.A.; Walensky, R.P. The impact of reporting a prior penicillin allergy on the treatment of methicillin sensitive Staphylococcus aureus bacteremia. PLoS ONE 2016, 11, e0159406. [Google Scholar] [CrossRef]
- Macy, E.; Contreras, R. Health care use and serious infection prevalence associated with penicillin “allergy” in hospitalized patients: A cohort study. J. Allergy Clin. Immunol. 2014, 133, 790–796. [Google Scholar] [CrossRef]
- Jeffres, M.N.; Narayanan, P.P.; Shuster, J.E.; Schramm, G.E. Consequences of avoiding beta-lactams in patients with beta-lactam allergies. J. Allergy Clin. Immunol. 2016, 137, 1148–1153. [Google Scholar] [CrossRef] [PubMed]
- Pichichero, M.E.; Zagursky, R. Penicillin and cephalosporin allergy. Ann. Allergy Asthma Immunol. 2014, 112, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Joint Task Force on Practice Parameters; American Academy of Allergy Asthma and Immunology; American College of Allergy Asthma and Immunology; Joint Council of Allergy Asthma and Immunology. Drug allergy: An updated practice parameter. Ann. Allergy Asthma Immunol. 2010, 105, 259–273. [Google Scholar] [CrossRef] [PubMed]
- Woydt, L.; Bernhard, M.; Kirsten, H.; Burkhardt, R.; Hammer, N.; Gries, A.; Dreßler, J.; Ondruschka, B. Intra-individual alterations of serum markers routinely used in forensic pathology depending on increasing post-mortem interval. Sci. Rep. 2018, 8, 12811. [Google Scholar] [CrossRef]
- Kelkar, S.P.; Li, J.T. Cephalosporin allergy. N. Engl. J. Med. 2001, 345, 804–809. [Google Scholar] [CrossRef]
- Chaudhry, S.B.; Veve, M.P.; Wagner, J.L. Cephalosporins: A focus study on side chains and beta-lactam cross-reactivity. Pharmacy 2019, 7, 103. [Google Scholar] [CrossRef]
- Kanny, G.; Guenard, L.; Demoly, P.; Ponvert, C.; Grand, J.; Gallen, C.; Chalmet, P.; Croizier, A.; Jacquier, J.; Morisset, M.; et al. Severe drug allergy: The first 100 cases declared to Allergy Vigilance Network. J. Allergy Clin. Immunol. 2005, 115, S183. [Google Scholar] [CrossRef]
- Novalbos, A.; Sastre, J.; Cuesta, J.; De Las Heras, M.; Lluch-Bernal, M.; Bombín, C.; Quirce, S. Lack of allergic cross-reactivity to cephalosporins among patients allergic to penicillins. Clin. Exp. Allergy 2001, 31, 438–443. [Google Scholar] [CrossRef]
- Macy, E.; Contreras, R. Adverse reactions associated with oral and parenteral use of cephalosporins: A retrospective population-based analysis. J. Allergy Clin. Immunol. 2015, 135, 745–752. [Google Scholar] [CrossRef]
- Dickson, S.D.; Salazar, K.C. Diagnosis and management of immediate hypersensitivity reactions to cephalosporins. Clin. Rev. Allergy Immunol. 2013, 45, 131–142. [Google Scholar] [CrossRef]
- Baldo, B.A.; Pham, N.H. Immunoglobulin E binding determinants on b-lactam drugs. Curr. Opin. Allergy Clin. Immunol. 2002, 2, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Harle, D.G.; Baldo, B.A. Drugs as allergens: An immunoassay for detecting IgE antibodies to cephalosporins. Int. Arch. Allergy Appl. Immunol. 1990, 92, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Baldo, B.A.; Rimmer, J. Beta-lactam allergenic determinants: Fine structural recognition of a cross-reacting determinant on benzylpenicillin and cephalotin. Clin. Exp. Allergy 2002, 32, 1644–1650. [Google Scholar] [CrossRef] [PubMed]
- Montanez, M.I.; Mayorga, C.; Torres, M.J.; Ariza, A.; Blanca, M.; Perez-Inestrosa, E. Synthetic approach to gain insight into antigenic determinants of cephalosporins: In vitro studies of chemical structure-IgE molecular recognition relationships. Chem. Res. Toxicol. 2011, 24, 706–717. [Google Scholar] [CrossRef]
- Poston, S.A.; Jennings, H.R.; Poe, K.L. Cefazolin tolerance does not predict ceftriaxone hypersensitivity: Unique side chains precipitate anaphylaxis. Pharmacotherapy 2004, 24, 668–672. [Google Scholar] [CrossRef]
- Romano, A.; Gaeta, F.; Valluzzi, R.L.; Maggioletti, M.; Zaffiro, A.; Caruso, C.; Quarantino, D. IgE-mediated hypersensitivity to cephalosporins: Cross-reactivity and tolerability of alternative cephalosporins. J. Allergy Clin. Immunol. 2015, 136, 685–691. [Google Scholar] [CrossRef]
- Orhan, F.; Odemis, E.; Yaris, N.; Okten, A.; Erduran, E.; Durmaz, M.; Yayla, S. A case of IgE mediated hypersensitivity to cefepime. Allergy 2004, 59, 239–241. [Google Scholar] [CrossRef]
- Guéant, J.L.; Guéant-Rodriguez, R.M.; Viola, M.; Valluzzi, R.L.; Romano, A. IgE-mediated hypersensitivity to cephalosporins. Curr. Pharm. Des. 2006, 12, 3335–3345. [Google Scholar] [CrossRef]
- Pham, N.H.; Baldo, B.A. Beta-lactam drug allergens: Fine structural recognition patterns of cephalosporin-reactive IgE antibodies. J. Mol. Recognit. 1996, 9, 287–296. [Google Scholar] [CrossRef]
- Baldo, B.A.; Pham, N.H. Allergenic significance of cephalosporin side chains. J. Allergy Clin. Immunol. 2015, 136, 1426–1428. [Google Scholar] [CrossRef][Green Version]
- Sánchez-Sancho, F.; Perez-Inestrosa, E.; Suau, R.; Montañez, M.I.; Mayorga, C.; Torres, M.J.; Romano, A.; Blanca, M. Synthesis, characterization and immunochemical evaluation of cephalosporin antigenic determinants. J. Mol. Recognit. 2003, 16, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Romano, A.; Viola, M.; Guéant-Rodriguez, R.M.; Valluzzi, R.L.; Guéant, J.L. Selective immediate hypersensitivity to cefodizime. Allergy 2005, 60, 1545–1546. [Google Scholar] [CrossRef] [PubMed]
- Pipet, A.; Veyrac, G.; Wessel, F.; Jolliet, P.; Magnan, A.; Demoly, P.; Bousquet, P.J. A statement on cefazolin immediate hypersensitivity: Data from a large database, and focus on the cross-reactivities. Clin. Exp. Allergy 2011, 41, 1602–1608. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.W.; Castells, M.C. Perioperative anaphylaxis to cefazolin. Allergy Asthma Proc. 2004, 25, 23–26. [Google Scholar] [PubMed]
- Atanasković-Marković, M.; Gavrović-Jankulović, M.; Cirković Velicković, T.; Vucković, O.; Todorić, D. Type-I hypersensitivity to ceftriaxone and cross-reactivity with cephalexin and ampicillin. Allergy 2003, 58, 537–538. [Google Scholar] [CrossRef]
- Antunez, C.; Blanca-Lopez, N.; Torres, M.J.; Mayorga, C.; Perez-Inestrosa, E.; Montañez, M.I.; Fernandez, T.; Blanca, M. Evaluation of cross-reactivity with a panel of penicillins and cephalosporins. J. Allergy Clin. Immunol. 2006, 117, 404–410. [Google Scholar] [CrossRef]
- Poetker, D.M.; Smith, T.L. What rhinologists and allergists should know about the medico-legal implications of antibiotic use: A review of the literature. Int. Forum Allergy Rhinol. 2015, 5, 104–110. [Google Scholar] [CrossRef]
- Jeffres, M.N.; Hall-Lipsy, E.A.; Travis-King, S.; Cleary, J.D. Systematic review of professional liability when prescribing beta-lactams for patients with a known penicillin allergy. Ann. Allergy Asthma Immunol. 2018, 121, 530–536. [Google Scholar] [CrossRef]
- Gatto, V.; Scopetti, M.; La Russa, R.; Santurro, A.; Cipolloni, L.; Viola, R.V.; Di Sanzo, M.; Frati, P.; Fineschi, V. Advanced Loss Eventuality Assessment and Technical Estimates: An Integrated Approach for Management of Healthcare-Associated Infections. Curr. Pharm. Biotechnol. 2019, 20, 625–634. [Google Scholar] [CrossRef]
- Solensky, R. Allergy to beta-lactam antibiotics. J. Allergy Clin. Immunol. 2012, 130, 1442. [Google Scholar] [CrossRef]
- Romano, A.; Mayorga, C.; Torres, M.J.; Artesani, M.C.; Suau, R.; Sánchez, F.; Pérez, E.; Venuti, A.; Blanca, M. Immediate allergic reactions to cephalosporins: Cross-reactivity and selective responses. J. Allergy Clin. Immunol. 2000, 106, 1177–1183. [Google Scholar] [CrossRef] [PubMed]
- Romano, A.; Guéant-Rodriguez, R.M.; Viola, M.; Amoghly, F.; Gaeta, F.; Nicolas, J.P.; Guéant, J.L. Diagnosing immediate reactions to cephalosporins. Clin. Exp. Allergy 2005, 35, 1234–1242. [Google Scholar] [CrossRef] [PubMed]
- Romano, A.; Guéant-Rodriguez, R.M.; Viola, M.; Pettinato, R.; Guéant, J.L. Cross-reactivity and tolerability of cephalosporins in patients with immediate hypersensitivity to penicillins. Ann. Intern. Med. 2004, 141, 16–22. [Google Scholar] [CrossRef]
- Yang, M.S.; Kang, D.Y.; Seo, B.; Park, H.J.; Park, S.Y.; Kim, M.Y.; Park, K.H.; Koo, S.M.; Nam, Y.H.; Kim, S.; et al. Incidence of cephalosporin-induced anaphylaxis and clinical efficacy of screening intradermal tests with cephalosporins: A large multicentre retrospective cohort study. Allergy 2018, 73, 1833–1841. [Google Scholar] [CrossRef]
- Riezzo, I.; Bello, S.; Neri, M.; Turillazzi, E.; Fineschi, V. Ceftriaxone intradermal test-related fatal anaphylactic shock: A medico-legal nightmare. Allergy 2010, 65, 130–131. [Google Scholar] [CrossRef]
- Borro, M.; Gentile, G.; Cipolloni, L.; Foldes-Papp, Z.; Frati, P.; Santurro, A.; Lionetto, L.; Simmaco, M. Personalised Healthcare: The DiMA Clinical Model. Curr. Pharm. Biotechnol. 2017, 18, 242–252. [Google Scholar] [CrossRef]
- Santurro, A.; Vullo, A.M.; Borro, M.; Gentile, G.; La Russa, R.; Simmaco, M.; Frati, P.; Fineschi, V. Personalized Medicine Applied to Forensic Sciences: New Advances and Perspectives for a Tailored Forensic Approach. Curr. Pharm. Biotechnol. 2017, 18, 263–273. [Google Scholar] [CrossRef]
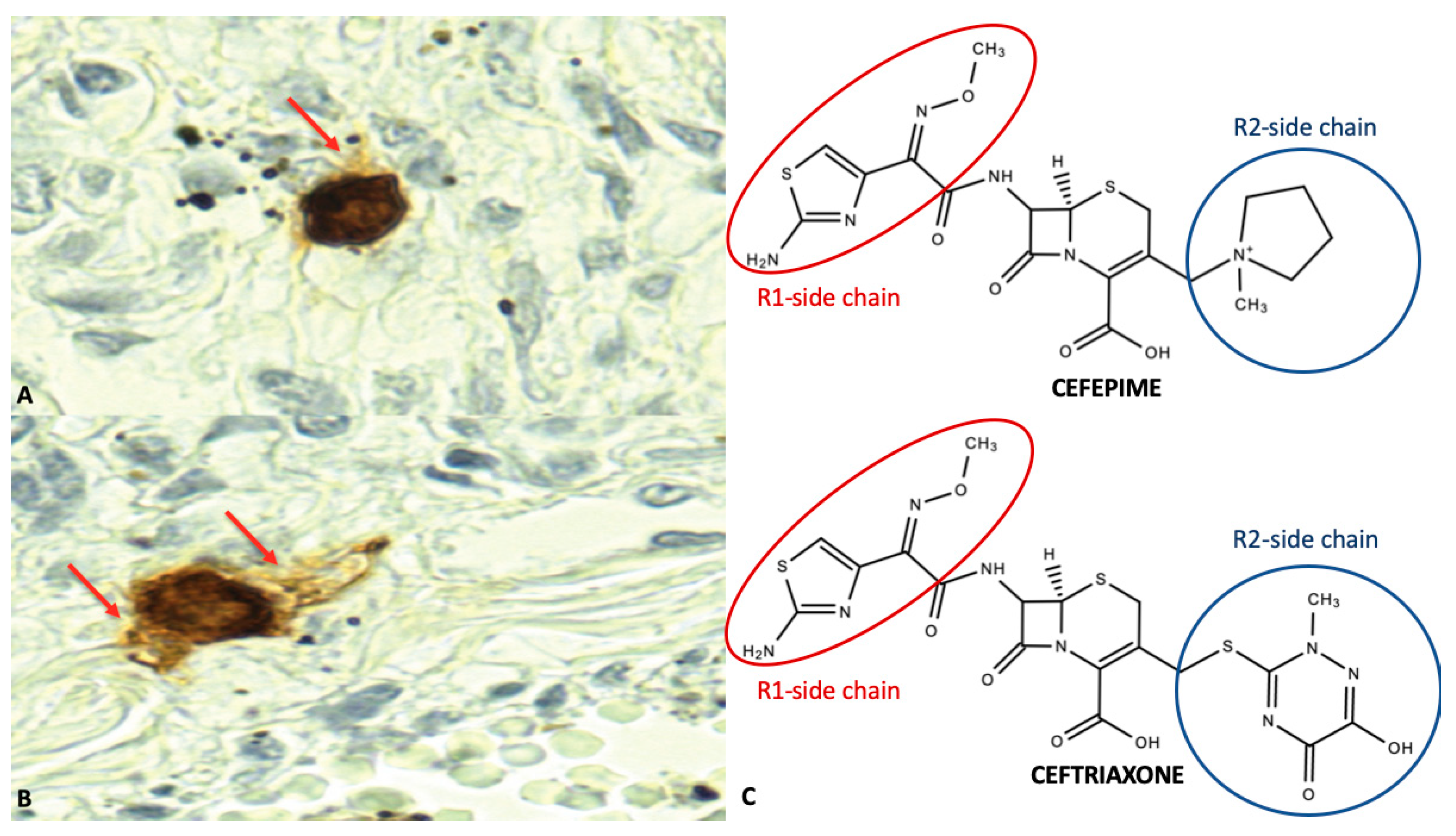
| DOP 1 | ROA 2 | Antibiotic |
|---|---|---|
| 05.02.2003 | i.m. 1 fl 1 gr | Ceftazidime |
| 15.09.2006 | cpr 500 mg | Ciprofloxacin |
| 03.11.2006 | i.m. 1 gr | Ceftriaxone |
| 24.11.2006 | os 875 mg + 125 mg | Amoxicillin + Clavulanic acid |
| 05.05.2008 | cps 400 mg | Ceftibuten |
| 09.06.2008 | i.m. 1 fl 2 gr | Piperacillin + Tazobactam |
| 09.06.2008 | cpr 500 mg | Levofloxacin |
| 15.12.2010 | im 1 fl 1 gr | Ceftriaxone |
| 17.12.2010 | im 1 fl 1 gr | Ceftriaxone |
| 30.12.2010 | cpr 750 mg | Ciprofloxacin |
| 31.01.2011 | cpr 750 mg | Ciprofloxacin |
| 23.02.2011 | im 1 fl 1 gr | Cefepime |
| 04.04.2011 | cpr 875 mg | Amoxicillin + Clavulanic acid |
| 10.11.2011 | cpr 750 mg | Ciprofloxacin |
| 09.02.2012 | cpr riv 500 mg | Ciprofloxacin |
| 20.02.2012 | cpr 500 mg | Levofloxacin |
| 06.04.2012 | i.m. 1 fl 1 gr | Cefepime * |
| 06.04.2012 | cpr 750 mg | Ciprofloxacin |
| 19.04.2012 | cpr 875 mg | Amoxicillin + Clavulanic acid |
| 19.04.2012 | cpr 500 mg | Levofloxacin |
| 06.11.2012 | i.m. 1 fl 2 gr | Piperacillin + Tazobactam |
| 06.11.2012 | cpr 750 mg | Ciprofloxacin |
| 12.11.2012 | cpr 750 mg | Ciprofloxacin |
| 06.12.2012 | cpr 750 mg | Ciprofloxacin |
| 03.01.2013 | i.m. 1 fl 2 gr | Piperacillin + Tazobactam |
| 03.01.2013 | cpr 750 mg | Ciprofloxacin |
| 18.02.2013 | i.m. 1 fl 1 gr | Ceftriaxone |
| 18.02.2013 | cpr 875 mg | Amoxicillin + Clavulanic acid |
| Allergens | Blood—3 h After Death * | Blood—24 h After Death * |
|---|---|---|
| c1 (Penicillin G) | 1.08 | 0.26 |
| c2 (Penicillin V) | 3.47 | 1.57 |
| c5 (Ampicillin) | 1.33 | 0.45 |
| c6 (Amoxicillin) | 1.26 | 0.26 |
| c7 (Cefaclor) | 1.36 | 0.53 |
| g6 (Timothy grass-Phleum pratense) | 1.75 | 0.55 |
| t9 (Olive-Olea europaea) | 1.20 | 0.27 |
| t23 (Cypress-Cupressus sempervirens) | 1.28 | 0.28 |
| f1 (Egg) | 1.97 | 0.83 |
| f2 (Milk) | 1.67 | 0.46 |
| d1 (Dermatophagoides pteronyssinus) | 1.31 | 0.33 |
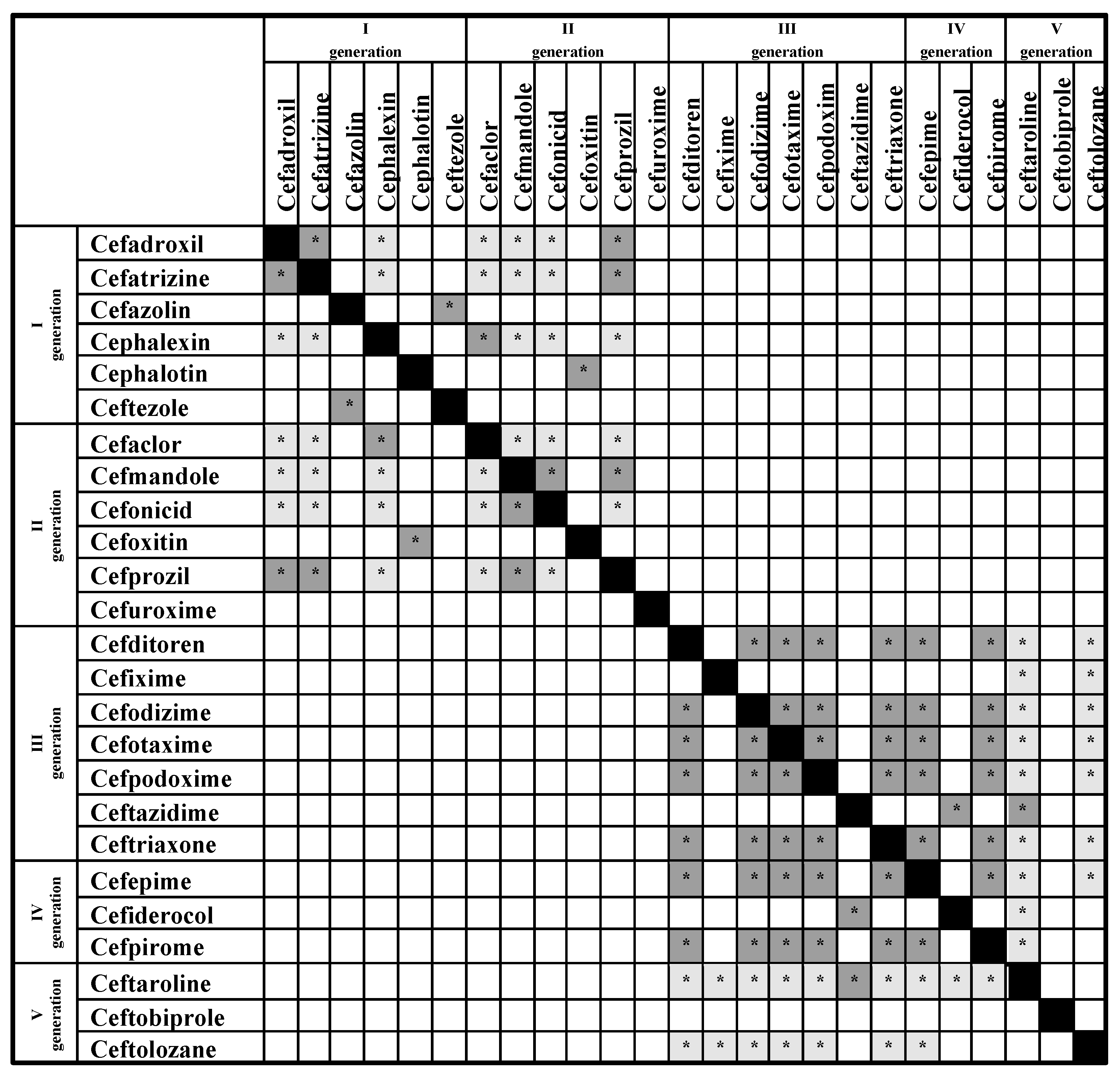
| Exact R2-Side Chains | Similar R2-Side Chains | |
|---|---|---|
| Cefazolin | Ceftezole | |
| Cefepime | Cefiderocol | |
| Cefiderocol | Cefepime | |
| Cefixime | Cefdinir | |
| Cefmandole | Cefoperazone, Cephapirin | Cefuroxime |
| Cefonicid | Cefmandole, Cefoperazone, Cefotetan | |
| Cefotaxime | Cephalotin, Cephapirin | Cefuroxime |
| Cefoxitin | Cefuroxime | Cefotaxime, Cefoxitin, Cephapirin |
| Cefpirone | Ceftazidime | |
| Ceftezole | Cefazolin | |
| Cefuroxime | Cefoxitin | Cefotaxime, Cephalotin, Cephapirin |
| Cephalotin | Cephapirin | Cefoxitin, Cefuroxime |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Errico, S.; Frati, P.; Zanon, M.; Valentinuz, E.; Manetti, F.; Scopetti, M.; Santurro, A.; Fineschi, V. Cephalosporins’ Cross-Reactivity and the High Degree of Required Knowledge. Case Report and Review of the Literature. Antibiotics 2020, 9, 209. https://doi.org/10.3390/antibiotics9050209
D’Errico S, Frati P, Zanon M, Valentinuz E, Manetti F, Scopetti M, Santurro A, Fineschi V. Cephalosporins’ Cross-Reactivity and the High Degree of Required Knowledge. Case Report and Review of the Literature. Antibiotics. 2020; 9(5):209. https://doi.org/10.3390/antibiotics9050209
Chicago/Turabian StyleD’Errico, Stefano, Paola Frati, Martina Zanon, Eleonora Valentinuz, Federico Manetti, Matteo Scopetti, Alessandro Santurro, and Vittorio Fineschi. 2020. "Cephalosporins’ Cross-Reactivity and the High Degree of Required Knowledge. Case Report and Review of the Literature" Antibiotics 9, no. 5: 209. https://doi.org/10.3390/antibiotics9050209
APA StyleD’Errico, S., Frati, P., Zanon, M., Valentinuz, E., Manetti, F., Scopetti, M., Santurro, A., & Fineschi, V. (2020). Cephalosporins’ Cross-Reactivity and the High Degree of Required Knowledge. Case Report and Review of the Literature. Antibiotics, 9(5), 209. https://doi.org/10.3390/antibiotics9050209





