Declining Prevalence of Methicillin-Resistant Staphylococcus aureus Septic Arthritis and Osteomyelitis in Children: Implications for Treatment
Abstract
1. Introduction
2. Methods
2.1. Study Design and Participants
2.2. Data Collected
2.3. Statistical Analysis
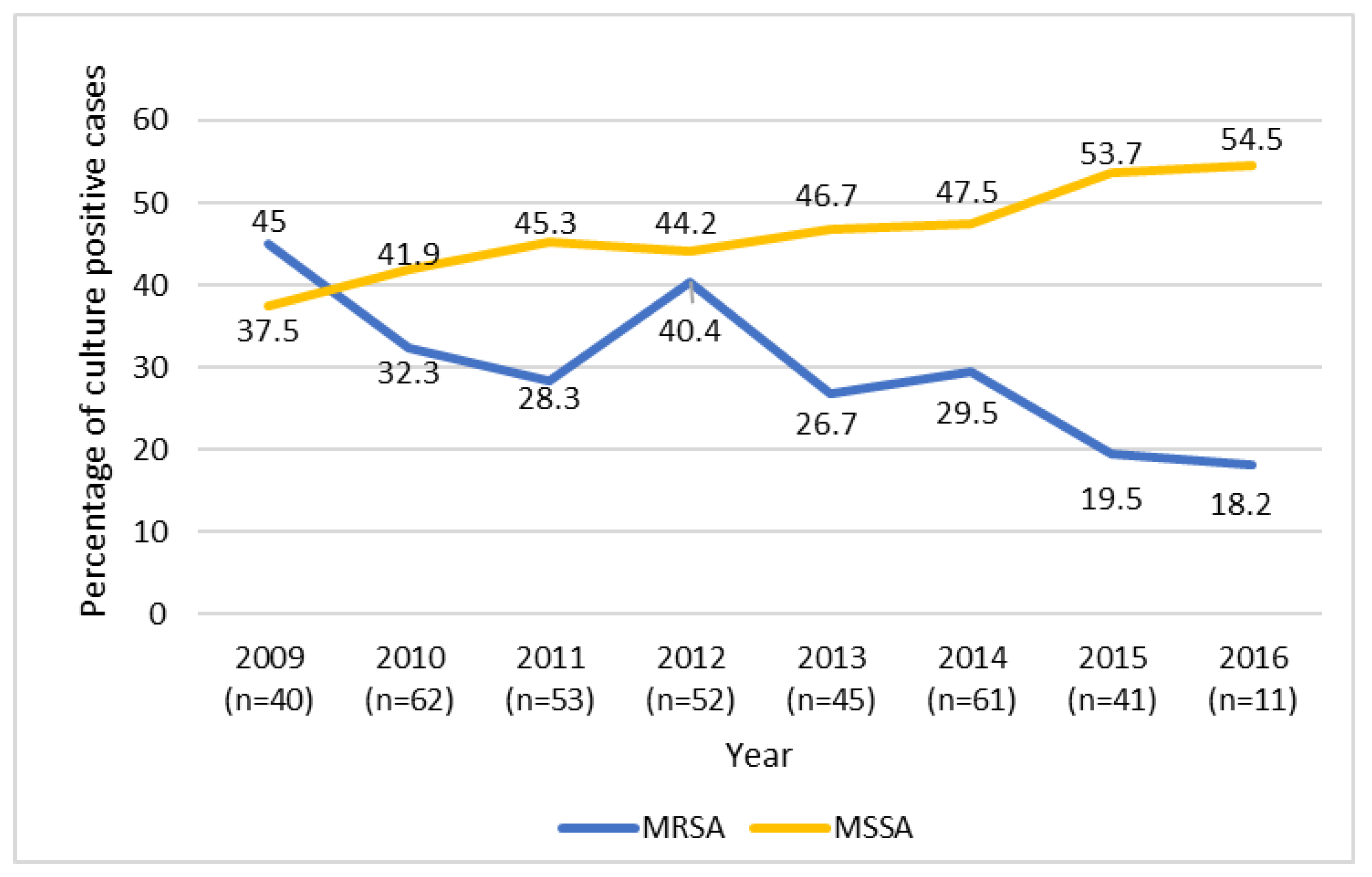
3. Results
3.1. Microbiology of Infections
3.2. Antimicrobial Regimen and Susceptibility
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Arnold, J.C.; Bradley, J.S. Osteoarticular Infections in Children. Infect. Dis. Clin. North Am. 2015, 29, 557–574. [Google Scholar] [CrossRef]
- Copley, L.A. Pediatric musculoskeletal infection: Trends and antibiotic recommendations. J. Am. Acad. Orthop. Surg. 2009, 17, 618–626. [Google Scholar] [CrossRef]
- Saavedra-Lozano, J.; Falup-Pecurariu, O.; Faust, S.N.; Girschick, H.; Hartwig, N.; Kaplan, S.; Lorrot, M.; Mantadakis, M.; Peltola, E.; Rojo, H.; et al. Bone and Joint Infections. Pediatric Infect. Dis. J. 2017, 36, 788–799. [Google Scholar] [CrossRef]
- Darville, T.; Jacobs, R.F. Management of acute hematogenous osteomyelitis in children. Pediatric Infect. Dis. J. 2004, 23, 255–257. [Google Scholar] [CrossRef]
- Branson, J.; Vallejo, J.G.; Flores, A.R.; Hulten, K.G.; Mason, E.O.; Kaplan, S.L.; McNeil, J.C. The contemporary microbiology and rates of concomitant osteomyelitis in acute septic arthritis. Pediatric Infect. Dis. J. 2017, 36, 267–273. [Google Scholar] [CrossRef]
- Kaplan, S.L. Recent lessons for the management of bone and joint infections. J. Infect. 2014, 68 (Suppl. 1), S51–S56. [Google Scholar] [CrossRef]
- Arnold, S.R.; Elias, D.; Buckingham, S.C.; Thomas, E.D.; Novais, E.; Arkader, A.; Howard, C. Changing patterns of acute hematogenous osteomyelitis and septic arthritis: Emergence of community-associated methicillin-resistant Staphylococcus aureus. J. Pediatric Orthop. 2006, 26, 703–708. [Google Scholar] [CrossRef]
- Sarkissian, E.J.; Gans, I.; Gunderson, M.A.; Myers, S.H.; Spiegel, D.A.; Flynn, J.M. Community-acquired methicillin-resistant Staphylococcus aureus musculoskeletal infections: Emerging trends over the past decade. J. Pediatric Orthop. 2016, 36, 323–327. [Google Scholar] [CrossRef]
- Gerber, J.S.; Coffin, S.E.; Smathers, S.A.; Zaoutis, T.E. Trends in the incidence of methicillin-resistant Staphylococcus aureus infection in children’s hospitals in the United States. Clin. Infect. Dis. 2009, 49, 65–71. [Google Scholar] [CrossRef]
- Ju, K.L.; Zurakowski, D.; Kocher, M.S. Differentiating between methicillin-resistant and methicillin-sensitive Staphylococcus aureus osteomyelitis in children: An evidence-based clinical prediction algorithm. J. Bone Jt. Surg. 2011, 93, 1693–1701. [Google Scholar] [CrossRef]
- Dietrich, L.N.; Reid, D.; Doo, D.; Fineberg, N.S.; Khoury, J.G.; Gilbert, S.R. Predicting MSSA in acute hematogenous osteomyelitis in a setting with MRSA prevalence. J. Pediatric Orthop. 2015, 35, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Kini, A.R.; Shetty, V.; Kumar, A.M.; Shetty, S.M.; Shetty, A. Community-associated, methicillin-susceptible, and methicillin-resistant Staphylococcus aureus bone and joint infections in children: Experience from India. J. Pediatric Orthop. Part B 2013, 22, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Davis, W.T.; Gilbert, S.R. Comparison of methicillin-resistant versus susceptible Staphylococcus aureus pediatric osteomyelitis. J. Pediatric Orthop. 2018, 38, e285–e291. [Google Scholar] [CrossRef]
- McNeil, J.C.; Forbes, A.R.; Vallejo, J.G.; Flores, A.R.; Hulten, K.G.; Mason, E.O.; Kaplan, S.L. Role of operative or interventional radiology-guided cultures for osteomyelitis. Pediatrics 2016, 137, e20154616. [Google Scholar] [CrossRef]
- Kok, E.Y.; Vallejo, J.G.; Sommer, L.M.; Rosas, L.; Kaplan, S.L.; Hulten, K.G.; McNeil, J.C. Association of vancomycin MIC and molecular characteristics with clinical outcomes in methicillin-susceptible Staphylococcus aureus acute hematogenous osteoarticular infections in children. Antimicrob. Agents Chemother. 2018, 62, e00084-18. [Google Scholar] [CrossRef]
- Sutter, D.E.; Milburn, E.; Chukwuma, U.; Dzialowy, N.; Maranich, A.M.; Hospenthal, D.R. Changing susceptibility of Staphylococcus aureus in a US pediatric population. Pediatrics 2016, 137, e20153099. [Google Scholar] [CrossRef]
- Hulten, K.G.; Mason, E.O.; Lamberth, L.B.; Forbes, A.R.; Revell, P.A.; Kaplan, S.L. Analysis of invasive community-acquired methicillin-susceptible Staphylococcus aureus infections during a period of declining community acquired methicillin-resistant Staphylococcus aureus infections at a large children’s hospital. Pediatric Infect. Dis. J. 2018, 37, 235–241. [Google Scholar] [CrossRef]
- Liu, C.; Bayer, A.; Cosgrove, S.E.; Daum, R.S.; Fridkin, S.K.; Gorwitz, R.J.; Kaplan, S.L.; Karchmer, A.W.; Levine, D.P.; Murray, B.E.; et al. Clinical practice guidelines by the Infectious Disease Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin. Infect. Dis. 2011, 52, e18–e55. [Google Scholar] [CrossRef]
- Harik, N.S.; Smeltzer, M.S. Management of acute hematogenous osteomyelitis in children. Expert Rev. Anti-Infect. Ther. 2010, 8, 175–181. [Google Scholar] [CrossRef]
- McBride, S.; Thurm, C.; Gouripeddi, R.; Stone, B.; Jaggard, P.; Shah, S.S.; Tieder, J.S.; Butcher, R.; Weiser, J.; Hall, M.; et al. Comparison of empiric antibiotics for acute osteomyelitis in children. Hosp. Pediatrics 2018, 8, 280–287. [Google Scholar] [CrossRef]
- Lansell, A.V.Y.; Suchdev, P.; Figueroa, J.; Kirpalani, A. Impact of antibiotic pretreatment on cultures in children with osteomyelitis and septic arthritis 2019. Manuscript submitted for publication.
- Nan, T.J.; Benvenuti, M.A.; Mignemi, M.E.; Martus, J.; Wood, J.B.; Thomsen, I.P.; Schoenecker, J.G. Similar clinical severity and outcomes for methicillin-resistant and methicillin-susceptible Staphylococcus aureus pediatric musculoskeletal infections. Open Forum Infect. Dis. 2017, 4, ofx013. [Google Scholar]
- Bocchini, C.E.; Hulten, K.G.; Mason, E.O.; Gonzalez, B.E., Jr.; Hammerman, W.A.; Kaplan, S.L. Panton-Valentine leukocidin genes are associated with enhanced inflammatory response and local disease in acute hematogenous Staphylococcus aureus osteomyelitis in children. Pediatrics 2006, 117, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Aguilar, G.; Avalos-Mishaan, A.; Hulten, K.; Hammerman, W.; Mason, E.O.; Kaplan, S.L., Jr. Community-acquired, methicillin-resistant and methicillin-susceptible Staphylococcus aureus musculoskeletal infections in children. Pediatric Infect. Dis. J. 2004, 23, 701–706. [Google Scholar] [CrossRef]
- Panzer, J.D.; Brown, D.C.; Epstein, W.L.; Lipson, R.L.; Mahaffey, H.W.; Atkinson, W.H. Clindamycin levels in various body tissues and fluids. J. Clin. Pharmacol. New Drugs 1972, 12, 259–262. [Google Scholar] [CrossRef]
- Peltola, H.; Paakkonen, M.; Kallio, P.; Kallio, M.J. Clindamycin vs. first-generation cephalosporins for acute osteoarticular infections of childhood--a prospective quasi-randomized controlled trial. Clin. Microbiol. Infect. 2012, 18, 582–589. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Aguilar, G.; Hammerman, W.A.; Mason, E.O.; Kaplan, S.L., Jr. Clindamycin treatment of invasive infections caused by community-acquired, methicillin-resistant and methicillin-susceptible Staphylococcus aureus in children. Pediatric Infect. Dis. J. 2003, 22, 593–598. [Google Scholar] [CrossRef]
| Median (IQR) or % | All Culture Positive Cases n = 365 | MRSA n = 114 | MSSA n = 166 | p-value 1 |
|---|---|---|---|---|
| Patient Characteristics | ||||
| Age, years | 7.9 (2.7–11.7) | 6.0 (3.2–10.9) | 10.7 (6.3–12.6) | <0.001 |
| Male Sex | 60.6 | 61.4 | 65.7 | 0.47 |
| Race/Ethnicity | <0.001 | |||
| African American | 37.8 | 51.8 | 24.7 | <0.001 |
| Asian | 4.1 | 0.9 | 6.6 | 0.031 |
| Caucasian | 50.4 | 43.0 | 60.8 | 0.003 |
| Hispanic | 1.6 | 1.8 | 1.8 | 1.00 |
| Other/Unknown | 6.0 | 2.6 | 6.0 | 0.19 |
| Type of Infection | 0.040 | |||
| AHO only | 51.2 | 53.5 | 59.0 | 0.36 |
| SA only | 18.1 | 7.0 | 13.9 | 0.07 |
| Both AHO and SA | 30.7 | 39.5 | 27.1 | 0.030 |
| Initial WBC count, × 103/mm3 | 11.4 (8.5–15.4) | 12.0 (8.5–16.0) | 10.5 (7.7–13.8) | 0.031 |
| Initial CRP, mg/dL | 9.3 (4.3–19.5) | 17.3 (6.9–25.0) | 7.8 (4.1–17.1) | <0.001 |
| Initial ESR, mm/h | 47.0 (29.5–66.5) | 51.0 (32.0–71.0) | 44.0 (28.0–60.0) | 0.048 |
| Duration of symptoms at presentation, days | 4.0 (3.0–7.0) | 4.0 (3.0–7.0) | 5.0 (3.0–7.0) | 0.27 |
| Fever at presentation | 86.6 | 85.0 | 91.6 | 0.09 |
| Antibiotic regimen | 0.31 | |||
| Regimen on clindamycin without vancomycin | 46.7 | 41.3 | 47.7 | |
| On vancomycin 2 | 53.3 | 58.7 | 52.4 | |
| Outcomes | ||||
| Length of stay, days | 6.7 (4.8–9.8) | 8.3 (5.9–13.5) | 6.1 (4.7–8.3) | <0.001 |
| Re-admitted ≤60 days | 5.8 | 8.8 | 3.6 | 0.07 |
| Required PICU admission | 11.8 | 20.2 | 9.6 | 0.012 |
| Subperiosteal Abscess | 32.3 | 46.5 | 29.5 | 0.004 |
| Outcome | Estimate (95% CI)- Odds Ratio or Ratio of Geometric LS-Means for MRSA (vs. MSSA-ref) 2 | p-value |
|---|---|---|
| Length of stay 1, days | 1.27 (1.09–1.48) | 0.003 |
| Re-admitted ≤60 days (vs. not) | 2.21 (0.65–7.56) | 0.21 |
| Required PICU admission (vs. none) | 1.34 (0.54–3.33) | 0.53 |
| Subperiosteal abscess (vs. none) | 1.14 (0.60–2.15) | 0.69 |
| Comparison | Estimate | ||
|---|---|---|---|
| Length of stay 1, days | Pathogen Isolated | Ratio of Geometric LS-Means (95% CI) | p-value 2 |
| MRSA (vs. none) | 1.35 (1.11–1.65) | <0.001 | |
| MRSA (vs. MSSA) | 1.26 (1.05–1.52) | <0.001 | |
| MRSA (vs. non-S. aureus) | 1.14 (0.91–1.42) | 0.43 | |
| MSSA (vs. non-S. aureus) | 0.90 (0.77–1.05) | 0.56 | |
| MSSA (vs. none) | 1.07 (0.90–1.27) | 0.73 | |
| Non-S. aureus (vs. none) | 1.19 (0.98–1.44) | 0.09 | |
| Re-admitted ≤60 days | Pathogen Isolated | Odds Ratio (95% CI) | p-value 2 |
| MRSA (vs. none) | 1.35 (0.26–7.09) | 0.97 | |
| MRSA (vs. MSSA) | 2.13 (0.48–9.51) | 0.56 | |
| MRSA (vs. non-S. aureus) | 0.83 (0.16–4.45) | 0.99 | |
| MSSA (vs. non-S. aureus) | 0.39 (0.07–2.27) | 0.52 | |
| MSSA (vs. none) | 0.63 (0.12–3.36) | 0.89 | |
| Non-S. aureus (vs. none) | 1.62 (0.45–4.98) | 0.92 | |
| Required PICU admission | Pathogen Isolated | Odds Ratio (95% CI) | p-value 2 |
| MRSA (vs. none) | 1.53 (0.32–7.32) | 0.90 | |
| MRSA (vs. MSSA) | 1.31 (0.43–4.01) | 0.92 | |
| MRSA (vs. non-S. aureus) | 1.10 (0.20–5.96) | 1.00 | |
| MSSA (vs. non-S. aureus) | 0.84 (0.15–4.75) | 0.99 | |
| MSSA (vs. none) | 1.16 (0.26–5.23) | 0.99 | |
| Non-S. aureus (vs. none) | 1.39 (0.20–9.64) | 0.97 | |
| Subperiosteal abscess | Pathogen Isolated | Odds Ratio (95% CI) | p-value 2 |
| MRSA (vs. none) | 6.40 (2.09–19.66) | <0.001 | |
| MRSA (vs. MSSA) | 1.26 (0.58–2.75) | 0.87 | |
| MRSA (vs. non-S. aureus) | 1.44 (0.52–3.97) | 0.80 | |
| MSSA (vs. non-S. aureus) | 1.14 (0.41–3.18) | 0.99 | |
| MSSA (vs. none) | 5.08 (1.72–15.05) | <0.001 | |
| Non-S. aureus (vs. none) | 4.46 (1.29–15.42) | 0.011 |
| N = 525 Total Median (IQR) or % | Regimen Containing Clindamycin w/o Vancomycin n = 279 | Regimen Containing Vancomycin 1 n = 246 | p-value 2 | |
|---|---|---|---|---|
| Patient Characteristics | Age | 6.2 (1.7–10.7) | 5.9 (2.5–11.3) | 0.40 |
| Male Sex | 57.3 | 64.6 | 0.09 | |
| Race/Ethnicity | 0.95 | |||
| African American | 33.3 | 34.1 | 0.85 | |
| Asian | 4.3 | 4.1 | 1.00 | |
| Caucasian | 52.0 | 53.3 | 0.79 | |
| Hispanic | 1.1 | 1.2 | 1.00 | |
| Other | 9.3 | 7.3 | 0.43 | |
| Type of infection | 0.004 | |||
| AHO | 45.5 | 43.5 | 0.64 | |
| SA | 34.8 | 25.2 | 0.017 | |
| Both AHO and SA | 19.7 | 31.3 | 0.002 | |
| Initial WBC count, x 103/mm3 | 12.2 (9.0–15.5) | 11.0 (8.8–15.4) | 0.28 | |
| Initial CRP, mg/dL | 6.0 (2.6–12.6) | 8.9 (3.6–20.9) | <0.001 | |
| Initial ESR, mm/h | 43 (28–65) | 48 (26–68) | 0.24 | |
| Duration of symptoms at presentation, days | 4 (2–6) | 4 (3–7) | 0.025 | |
| Fever at presentation | 77.8 | 79.3 | 0.68 | |
| Outcomes | Length of stay, days | 5.5 (4.0–7.1) | 6.7 (4.9–11.0) | <0.001 |
| Re-admitted ≤60 days | 3.6 | 6.1 | 0.18 | |
| Outcome | Predictor | Ratio of Geometric LS-Means (95% CI) 2 | p-value |
| Length of stay 1, days * | Clindamycin (vs. vancomycin) | 0.84 (0.76–0.92) | <0.001 |
| Odds Ratio (95% CI) | |||
| Re-admitted ≤60 days | Clindamycin (vs. vancomycin) | 0.88 (0.36–2.16) | 0.77 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weiss, L.; Lansell, A.; Figueroa, J.; Suchdev, P.S.; Kirpalani, A. Declining Prevalence of Methicillin-Resistant Staphylococcus aureus Septic Arthritis and Osteomyelitis in Children: Implications for Treatment. Antibiotics 2020, 9, 101. https://doi.org/10.3390/antibiotics9030101
Weiss L, Lansell A, Figueroa J, Suchdev PS, Kirpalani A. Declining Prevalence of Methicillin-Resistant Staphylococcus aureus Septic Arthritis and Osteomyelitis in Children: Implications for Treatment. Antibiotics. 2020; 9(3):101. https://doi.org/10.3390/antibiotics9030101
Chicago/Turabian StyleWeiss, Lindsay, Amanda Lansell, Janet Figueroa, Parminder S. Suchdev, and Anjali Kirpalani. 2020. "Declining Prevalence of Methicillin-Resistant Staphylococcus aureus Septic Arthritis and Osteomyelitis in Children: Implications for Treatment" Antibiotics 9, no. 3: 101. https://doi.org/10.3390/antibiotics9030101
APA StyleWeiss, L., Lansell, A., Figueroa, J., Suchdev, P. S., & Kirpalani, A. (2020). Declining Prevalence of Methicillin-Resistant Staphylococcus aureus Septic Arthritis and Osteomyelitis in Children: Implications for Treatment. Antibiotics, 9(3), 101. https://doi.org/10.3390/antibiotics9030101






