The Use of Antimicrobials in Italian Heavy Pig Fattening Farms
Abstract
1. Introduction
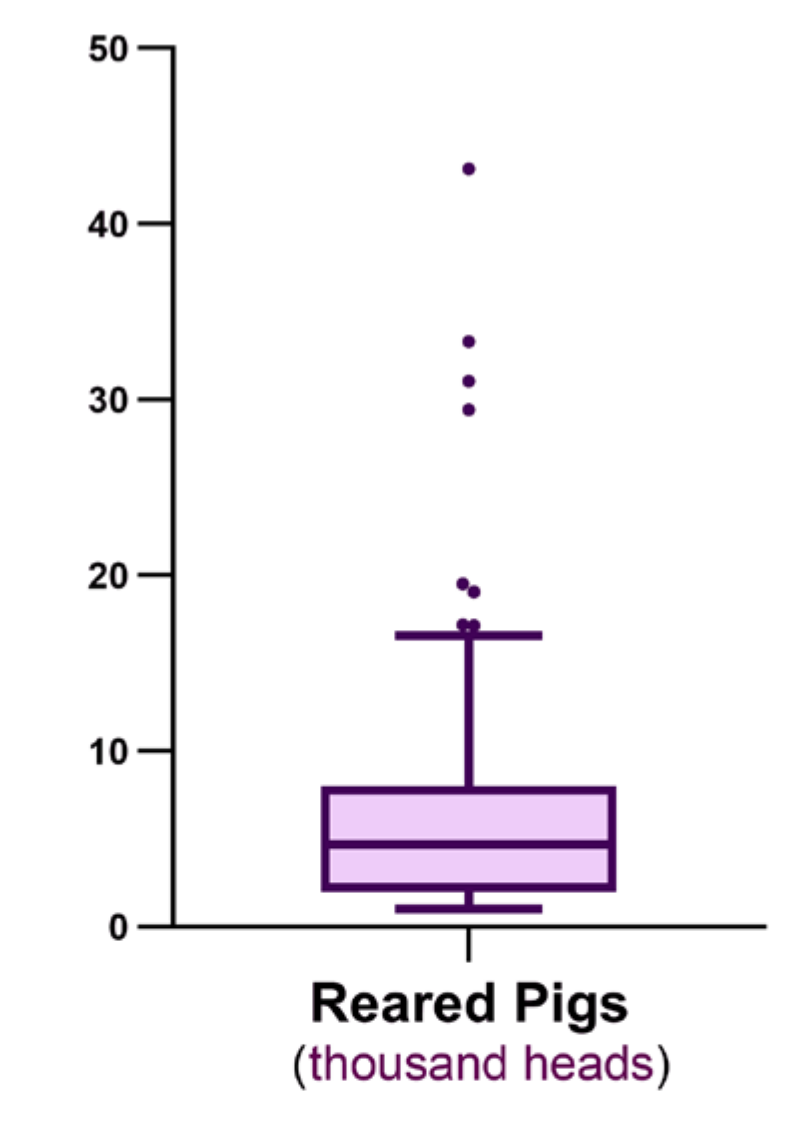
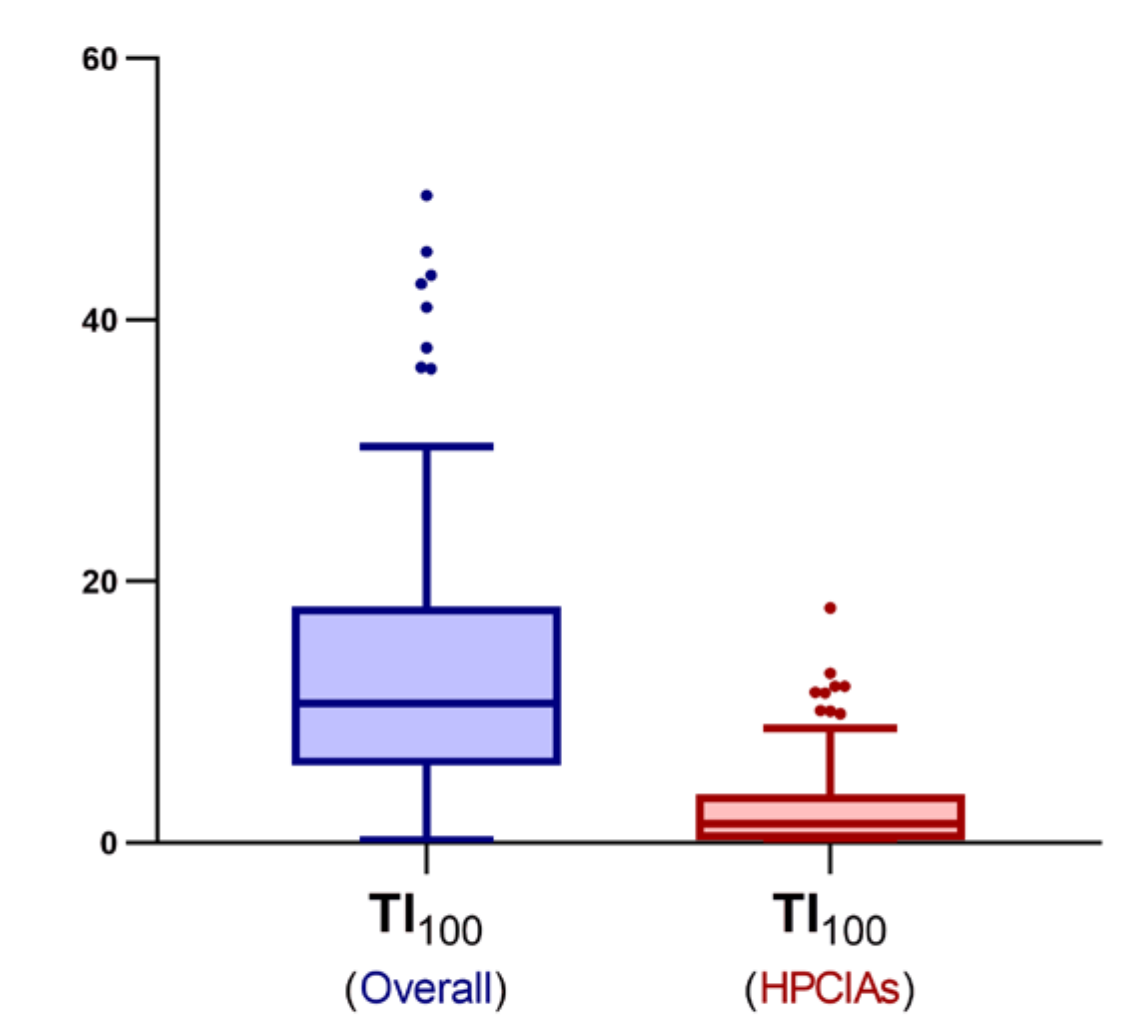
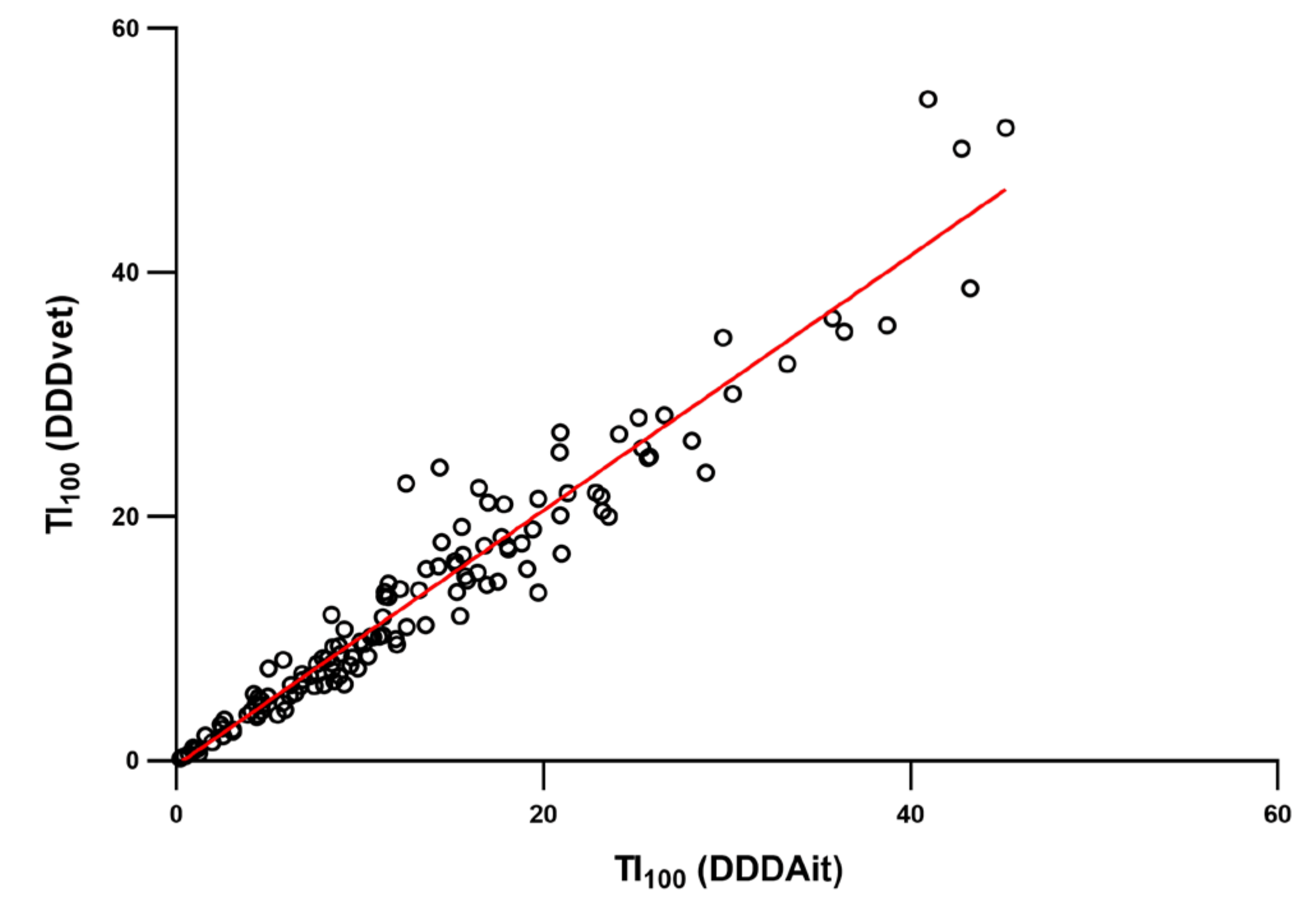
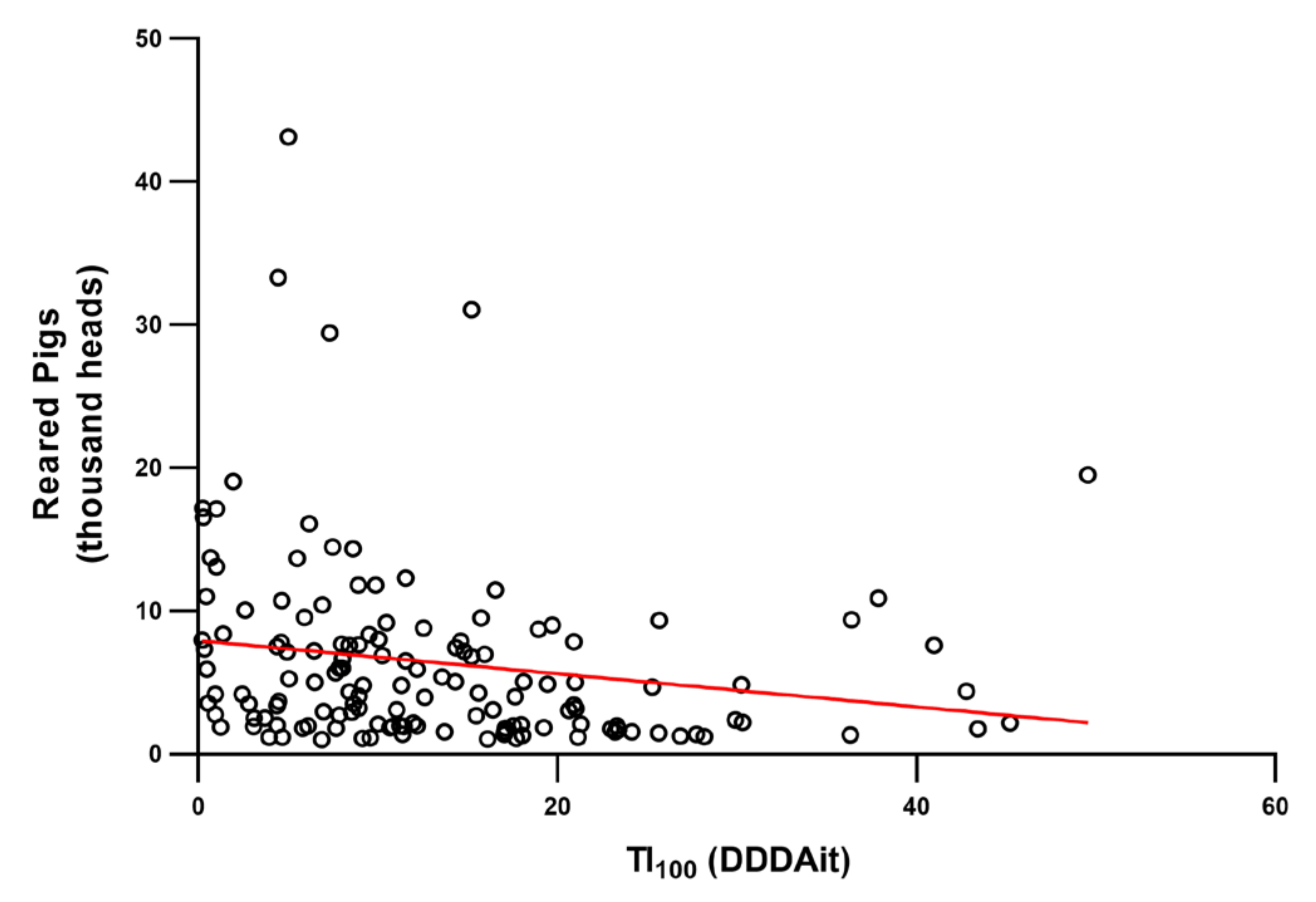
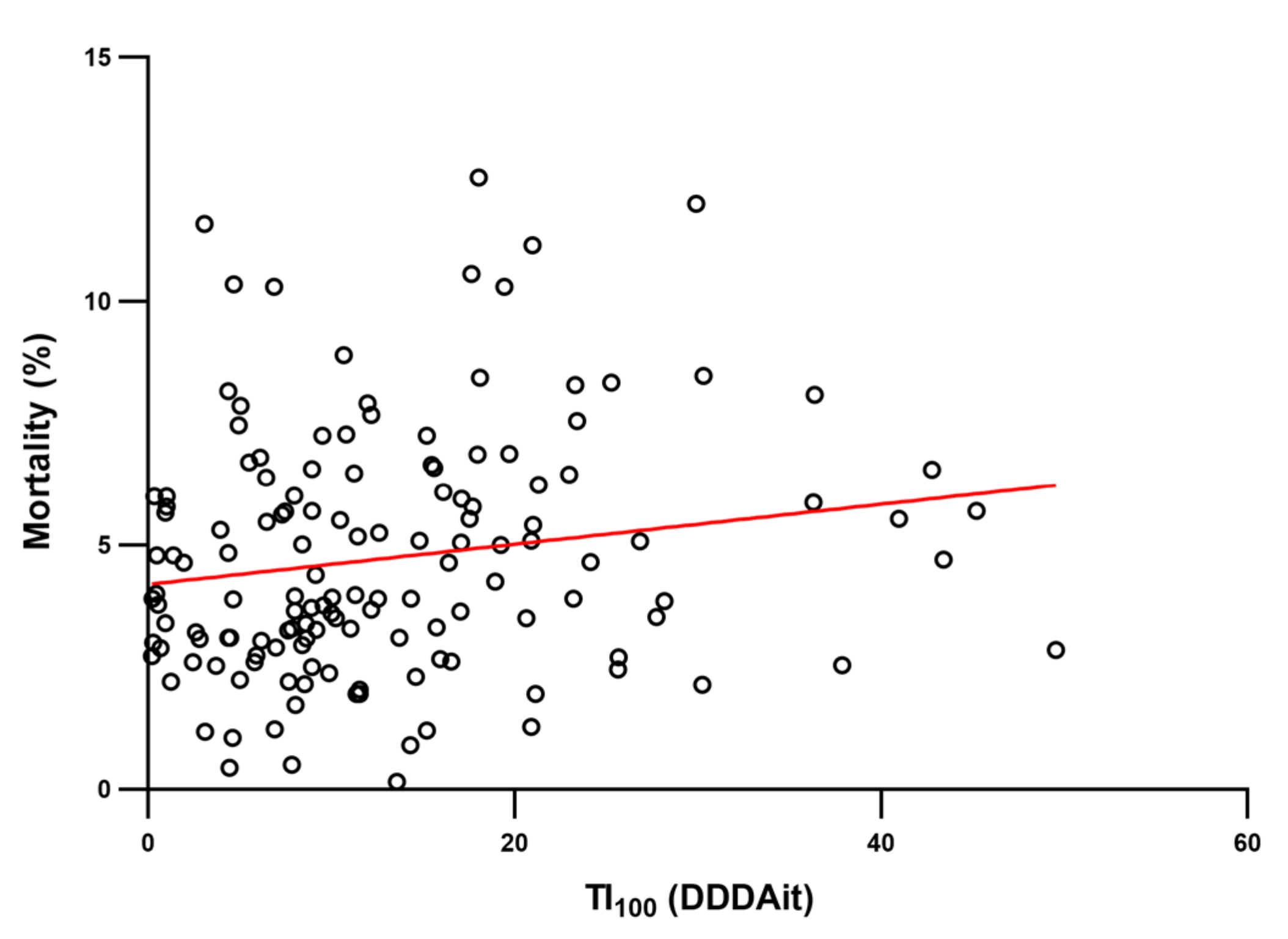
2. Results
3. Discussion
4. Materials and Methods
4.1. Sample Selection and Data Collection
4.2. Estimation of Antimicrobial Use
4.3. Data Management and Statistical Analysis
4.4. Data Availability
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aarestrup, F.M. Association between the consumption of antimicrobial agents in animal husbandry and the occurrence of resistant bacteria among food animals. Int. J. Antimicrob. Agents 1999, 12, 279–285. [Google Scholar] [CrossRef]
- Marshall, B.M.; Levy, S.B. Food Animals and Antimicrobials: Impacts on Human Health. Clin. Microbiol. Rev. 2011, 24, 718–733. [Google Scholar] [CrossRef]
- WHO. Critically Important Antimicrobials for Human Medicine, 1th Revision. Available online: https://apps.who.int/iris/bitstream/handle/10665/43765/9789241595742_eng.pdf (accessed on 2 June 2020).
- WHO. Critically Important Antimicrobials for Human Medicine, 6th Revision. Available online: https://apps.who.int/iris/bitstream/handle/10665/312266/9789241515528-eng.pdf (accessed on 2 June 2020).
- European Surveillance of Veterinary Antimicrobial Consumption (ESVAC) Project. Sales of Veterinary Antimicrobial Agents in 31 European Countries in 2018 (EMA/24309/2020). Available online: https://www.ema.europa.eu/en/documents/report/sales-veterinary-antimicrobial-agents-31-european-countries-2018-trends-2010-2018-tenth-esvac-report_en.pdf (accessed on 6 November 2020).
- EUROSTAT. Slaughtering in Slaughterhouses: Annual Data (Pigmeat, Thousand Heads (Animal)). Available online: https://appsso.eurostat.ec.europa.eu/nui/submitViewTableAction.do (accessed on 6 June 2020).
- Luppi, A.; Bonilauri, P.; Dottori, M.; Gherpelli, Y.; Biasi, G.; Merialdi, G.; Maioli, G.; Martelli, P. Antimicrobial Resistance of F4+ Escherichia Coli Isolated from Swine in Italy. Transbound. Emerg. Dis. 2015, 62, 67–71. [Google Scholar] [CrossRef]
- Normanno, G.; Dambrosio, A.; Lorusso, V.; Samoilis, G.; Di Taranto, P.; Parisi, A. Methicillin-resistant Staphylococcus aureus (MRSA) in slaughtered pigs and abattoir workers in Italy. Food Microbiol. 2015, 51, 51–56. [Google Scholar] [CrossRef]
- Curcio, L.; Luppi, A.; Bonilauri, P.; Gherpelli, Y.; Pezzotti, G.; Pesciaroli, M.; Magistrali, C.F. Detection of the colistin resistance gene mcr-1 in pathogenic Escherichia coli from pigs affected by post-weaning diarrhoea in Italy. J. Glob. Antimicrob. Resist. 2017, 10, 80–83. [Google Scholar] [CrossRef]
- Bava, L.; Zucali, M.; Sandrucci, A.; Tamburini, A. Environmental impact of the typical heavy pig production in Italy. J. Clean. Prod. 2017, 140, 685–691. [Google Scholar] [CrossRef]
- Callens, B.; Persoons, D.; Maes, D.; Laanen, M.; Postma, M.; Boyen, F.; Haesebrouck, F.; Butaye, P.; Catry, B.; Dewulf, J. Prophylactic and metaphylactic antimicrobial use in Belgian fattening pig herds. Prev. Vet. Med. 2012, 106, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Bos, M.E.H.; Taverne, F.J.; van Geijlswijk, I.M.; Mouton, J.W.; Mevius, D.J.; Heederik, D.J.J. Consumption of Antimicrobials in Pigs, Veal Calves, and Broilers in The Netherlands: Quantitative Results of Nationwide Collection of Data in 2011. PLoS ONE 2013, 8, e77525. [Google Scholar] [CrossRef] [PubMed]
- Trauffler, M.; Griesbacher, A.; Fuchs, K.; Koefer, J. Antimicrobial drug use in Austrian pig farms: Plausibility check of electronic on-farm records and estimation of consumption. Vet. Rec. 2014, 175, 402. [Google Scholar] [CrossRef]
- Sjolund, M.; Postma, M.; Collineau, L.; Losken, S.; Backhans, A.; Belloc, C.; Emanuelson, U.; Beilage, E.G.; Stark, K.; Dewulf, J.; et al. Quantitative and qualitative antimicrobial usage patterns in farrow-to-finish pig herds in Belgium, France, Germany and Sweden. Prev. Vet. Med. 2016, 130, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Hemonic, A.; Chauvin, C.; Delzescaux, D.; Verliat, F.; Correge, I.; French Working Group ‘antimicrobials in the Swine Industry. Reliable estimation of antimicrobial use and its evolution between 2010 and 2013 in French swine farms. Porc. Health Manag. 2018, 4, 8. [Google Scholar] [CrossRef] [PubMed]
- Kruse, A.B.; Kristensen, C.S.; Nielsen, L.R.; Alban, L. A register-based study on associations between vaccination, antimicrobial use and productivity in conventional Danish finisher pig herds during 2011 to 2014. Prev. Vet. Med. 2019, 164, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Kummerlen, D.; Echtermann, T.; von Gerlach, F.; Muntener, C.; Sidler, X. Analyses of antimicrobial usage in 598 pig farms in Switzerland in 2017. Schweiz. Arch. Tierheilkd. 2019, 161, 809–820. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.H.; Takagi, H.; Yamane, I.; Yamazaki, H.; Naito, M.; Kure, K.; Sugiura, K. Antimicrobial usage on 72 farrow-to-finish pig farms in Japan from 2015 to 2017. Prev. Vet. Med. 2019, 173, 104802. [Google Scholar] [CrossRef] [PubMed]
- Sarrazin, S.; Joosten, P.; Van Gompel, L.; Luiken, R.E.C.; Mevius, D.J.; Wagenaar, J.A.; Heederik, D.J.J.; Dewulf, J.; Wagenaar, J.; Graveland, H.; et al. Quantitative and qualitative analysis of antimicrobial usage patterns in 180 selected farrow-to-finish pig farms from nine European countries based on single batch and purchase data. J. Antimicrob. Chemother. 2019, 74, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Vieira, A.R.; Pires, S.M.; Houe, H.; Emborg, H.D. Trends in slaughter pig production and antimicrobial consumption in Danish slaughter pig herds, 2002–2008. Epidemiol. Infect. 2011, 139, 1601–1609. [Google Scholar] [CrossRef] [PubMed]
- AACTING Consortium. Guidelines for Collection, Analysis and Reporting of Farm-Level Antimicrobial Use, in the Scope of Antimicrobial Stewardship (Version 1.2). Available online: https://aacting.org/swfiles/files/AACTING_Guidelines_V1.2_2019.07.02_54.pdf (accessed on 6 June 2020).
- Collineau, L.; Belloc, C.; Stark, K.D.C.; Hemonic, A.; Postma, M.; Dewulf, J.; Chauvin, C. Guidance on the Selection of Appropriate Indicators for Quantification of Antimicrobial Usage in Humans and Animals. Zoonoses Public Health 2017, 64, 165–184. [Google Scholar] [CrossRef]
- Sanders, P.; Vanderhaeghen, W.; Fertner, M.; Fuchs, K.; Obritzhauser, W.; Agunos, A.; Carson, C.; Hog, B.B.; Andersen, V.D.; Chauvin, C.; et al. Monitoring of Farm-Level Antimicrobial Use to Guide Stewardship: Overview of Existing Systems and Analysis of Key Components and Processes. Front. Vet. Sci. 2020, 7, 540. [Google Scholar] [CrossRef]
- European Surveillance of Veterinary Antimicrobial Consumption (ESVAC) Project. Defined Daily Doses for Animals (DDDvet) and Defined Course Doses for Animals (DCDvet) (EMA/224954/2016). Available online: https://www.ema.europa.eu/en/documents/other/defined-daily-doses-animals-dddvet-defined-course-doses-animals-dcdvet-european-surveillance_en.pdf (accessed on 2 June 2020).
- Italian Ministry of Health. Piano Nazionale di Contrasto dell’Antimicrobico-Resistenza (PNCAR) 2017–2020. Available online: http://www.salute.gov.it/imgs/C_17_pubblicazioni_2660_allegato.pdf (accessed on 6 June 2020).
- Bortolaia, V.; Hendriksen, R.S.; Høg, B.B.; Ellis-Iversen, J.; Korsgaard, H.B.; Petersen, C.K.; Boel, J.; Dalby, T.; Hammerum, A.M.; Hansen, F. Danmap 2018: Use of Antimicrobial Agents and Occurrence of Antimicrobial Resistance in Bacteria From Food Animals, Food and Humans in Denmark; Statens Serum Institut: Copenhagen, Denmark, 2019. [Google Scholar]
- Belgian Veterinary Surveillance of Antibiotic Consumption (BelVet-SAC). National Consumption Report. 2018. Available online: https://belvetsac.ugent.be/BelvetSAC_report_2018.pdf (accessed on 5 November 2020).
- Kruse, A.B.; de Knegt, L.V.; Nielsen, L.R.; Alban, L. No Clear Effect of Initiating Vaccination against Common Endemic Infections on the Amounts of Prescribed Antimicrobials for Danish Weaner and Finishing Pigs during 2007–2013. Front. Vet. Sci. 2017, 3, 120. [Google Scholar] [CrossRef]
- Netherlands Veterinary Medicines Institute (SDa). Usage of Antibiotics in Agricultural Livestock in the Netherlands in 2018. Trends and Benchmarking of Livestock Farms and Veterinarians. Available online: https://cdn.i-pulse.nl/autoriteitdiergeneesmiddelen/userfiles/Publications/2018-def-rapport1.pdf (accessed on 5 November 2020).
- Kruse, A.B.; Nielsen, L.R.; Alban, L. Herd typologies based on multivariate analysis of biosecurity, productivity, antimicrobial and vaccine use data from Danish sow herds. Prev. Vet. Med. 2020, 181, 104487. [Google Scholar] [CrossRef]
- EMA. Answer to the Request from the European Commission for Updating the Scientific Advice on the Impact on Public Health and Animal Health of the Use of Antibiotics in Animals—Categorisation of Antimicrobials (EMA/CVMP/CHMP/682198/2017). Available online: https://www.ema.europa.eu/en/documents/other/answer-request-european-commission-updating-scientific-advice-impact-public-health-animal-health-use_en.pdf (accessed on 5 June 2020).
- Bolinger, H.; Kathariou, S. The Current State of Macrolide Resistance in Campylobacter spp.: Trends and Impacts of Resistance Mechanisms. Appl. Environ. Microbiol. 2017, 83, e00416-17. [Google Scholar] [CrossRef] [PubMed]
- Heredia, N.; Garcia, S. Animals as sources of food-borne pathogens: A review. Anim. Nutr. 2018, 4, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Alban, L.; Nielsen, E.O.; Dahl, J. A human health risk assessment for macrolide-resistant Campylobacter associated with the use of macrolides in Danish pig production. Prev. Vet. Med. 2008, 83, 115–129. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Y.; Wang, Y.; Walsh, T.R.; Yi, L.X.; Zhang, R.; Spencer, J.; Doi, Y.; Tian, G.B.; Dong, B.L.; Huang, X.H.; et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: A microbiological and molecular biological study. Lancet Infect. Dis. 2016, 16, 161–168. [Google Scholar] [CrossRef]
- WHO. Critically Important Antimicrobials for Human Medicine, 4th Revision. 2013. Available online: http://apps.who.int/iris/bitstream/10665/251715/1/9789241511469-eng.pdf (accessed on 2 June 2020).
- Postma, M.; Sjolund, M.; Collineau, L.; Losken, S.; Stark, K.D.C.; Dewulf, J. Assigning defined daily doses animal: A European multi-country experience for antimicrobial products authorized for usage in pigs. J. Antimicrob. Chemother. 2015, 70, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Diana, A.; Santinello, M.; Penasa, M.; Scali, F.; Magni, E.; Alborali, G.L.; Bertocchi, L.; De Marchi, M. Use of antimicrobials in beef cattle: An observational study in the north of Italy. Prev. Vet. Med. 2020, 181, 105032. [Google Scholar] [CrossRef] [PubMed]
- Laanen, M.; Persoons, D.; Ribbens, S.; de Jong, E.; Callens, B.; Strubbe, M.; Maes, D.; Dewulf, J. Relationship between biosecurity and production/antimicrobial treatment characteristics in pig herds. Vet. J. 2013, 198, 508–512. [Google Scholar] [CrossRef]
- Postma, M.; Vanderhaeghen, W.; Sarrazin, S.; Maes, D.; Dewulf, J. Reducing Antimicrobial Usage in Pig Production without Jeopardizing Production Parameters. Zoonoses Public Health 2017, 64, 63–74. [Google Scholar] [CrossRef]
- Raasch, S.; Collineau, L.; Postma, M.; Backhans, A.; Sjolund, M.; Belloc, C.; Emanuelson, U.; Beilage, E.G.; Stark, K.; Dewulf, J.; et al. Effectiveness of alternative measures to reduce antimicrobial usage in pig production in four European countries. Porc. Health Manag. 2020, 6, 6. [Google Scholar] [CrossRef]
- Stygar, A.H.; Chantziaras, I.; Toppari, I.; Maes, D.; Niemi, J.K. High biosecurity and welfare standards in fattening pig farms are associated with reduced antimicrobial use. Anim. Int. J. Anim. Biosci. 2020, 1–9. [Google Scholar] [CrossRef]
- Tarakdjian, J.; Capello, K.; Pasqualin, D.; Santini, A.; Cunial, G.; Scollo, A.; Mannelli, A.; Tomao, P.; Vonesch, N.; Martino, G.D. Antimicrobial use on Italian Pig Farms and its Relationship with Husbandry Practices. Animals (Basel) 2020, 10, 417. [Google Scholar] [CrossRef] [PubMed]
- Statistiche Anagrafe Nazionale Zootecnica. Consistenza Allevamenti e Capi Suini. Available online: https://www.vetinfo.it/j6_statistiche/#/report-pbi/31 (accessed on 2 June 2020).
- European Surveillance of Veterinary Antimicrobial Consumption (ESVAC) Project. Principles on Assignment of Defined Daily Dose for Animals (DDDvet) and Defined Course Dose for Animals (DCDvet) (EMA/710019/2014). Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2015/06/WC500188890.pdf (accessed on 14 June 2020).
- Timmerman, T.; Dewulf, J.; Catry, B.; Feyen, B.; Opsomer, G.; de Kruif, A.; Maes, D. Quantification and evaluation of antimicrobial drug use in group treatments for fattening pigs in Belgium. Prev. Vet. Med. 2006, 74, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Hosmer, D.W.; Lemeshow, S. Applied Logistic Regression; Wiley: New York, NY, USA, 1989; 307p. [Google Scholar]
| Class | Total AMU (%) | % of farms with use> 0 | % of AMU at Farm-Level:Median (Range) |
|---|---|---|---|
| Aminocyclitols | 0.4 | 45.5 | 0 (0–5.4) |
| Aminoglycosides | 3.4 | 11.2 | 0 (0–32.6) |
| Amphenicols | 2.2 | 82.5 | 1.1 (0–100) |
| Cephalosporins (III and IV gen.) | 0.1 | 9.8 | 0 (0–6.7) |
| Lincosamides | 22.1 | 69.2 | 3.2 (0–95.8) |
| Macrolides | 8.8 | 63.6 | 2.1 (0–82.3) |
| Penicillins | 13.4 | 91.6 | 11.9 (0–74.4) |
| Penicillins (antistaphylococcal) | 0.2 | 51.7 | < 0.1 (0–5.8) |
| Pleuromutilins | 9.2 | 55.9 | 1.9 (0–89.3) |
| Polymyxins | 5.3 | 51.7 | 0.1 (0–55.3) |
| Quinolones | 2.6 | 58.7 | 0.1 (0–54.6) |
| Sulphonamides | 5.6 | 26.6 | 0 (0–93.3) |
| Tetracyclines | 26.6 | 83.2 | 24.5 (0–97.9) |
| Formulation | |||
| Injectables | 5.4 | 96.5 | 3.1 (0–100) |
| Premixes | 42.9 | 72.7 | 42.9 (0–99.9) |
| Oral (powder and solutions) | 51.7 | 85.3 | 40.2 (0–100) |
| Active Ingredient | Class | Form |
|---|---|---|
| Dicloxacillin | Penicillins (antistaphylococcal) | Injectable |
| Sulfadiazine | Sulphonamides | Injectable |
| Sulfadimidine | Sulphonamides | Injectable |
| Sulfamerazine | Sulphonamides | Injectable |
| Tildipirosin | Macrolides | Injectable |
| Tulathromycin | Macrolides | Injectable |
| Ampicillin | Penicillins | Premix |
| Flumequine | Quinolones | Premix |
| Gentamicin | Aminoglycosides | Premix |
| Sulfamerazine | Sulphonamides | Premix |
| Thiamphenicol | Amphenicols | Premix |
| Dicloxacillin | Penicillins (antistaphylococcal) | Injectable |
| Sulfadiazine | Sulphonamides | Injectable |
| Sulfadimidine | Sulphonamides | Injectable |
| Sulfamerazine | Sulphonamides | Injectable |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scali, F.; Santucci, G.; Maisano, A.M.; Giudici, F.; Guadagno, F.; Tonni, M.; Amicabile, A.; Formenti, N.; Giacomini, E.; Lazzaro, M.; et al. The Use of Antimicrobials in Italian Heavy Pig Fattening Farms. Antibiotics 2020, 9, 892. https://doi.org/10.3390/antibiotics9120892
Scali F, Santucci G, Maisano AM, Giudici F, Guadagno F, Tonni M, Amicabile A, Formenti N, Giacomini E, Lazzaro M, et al. The Use of Antimicrobials in Italian Heavy Pig Fattening Farms. Antibiotics. 2020; 9(12):892. https://doi.org/10.3390/antibiotics9120892
Chicago/Turabian StyleScali, Federico, Giovanni Santucci, Antonio M. Maisano, Francesca Giudici, Federica Guadagno, Matteo Tonni, Alberto Amicabile, Nicoletta Formenti, Enrico Giacomini, Massimiliano Lazzaro, and et al. 2020. "The Use of Antimicrobials in Italian Heavy Pig Fattening Farms" Antibiotics 9, no. 12: 892. https://doi.org/10.3390/antibiotics9120892
APA StyleScali, F., Santucci, G., Maisano, A. M., Giudici, F., Guadagno, F., Tonni, M., Amicabile, A., Formenti, N., Giacomini, E., Lazzaro, M., Bontempi, G., Vitale, N., Alban, L., Dewulf, J., Ianieri, A., Ghidini, S., Belluzzi, G., Candela, L., Maggio, A., ... Alborali, G. L. (2020). The Use of Antimicrobials in Italian Heavy Pig Fattening Farms. Antibiotics, 9(12), 892. https://doi.org/10.3390/antibiotics9120892








