Nisin in Combination with Cinnamaldehyde and EDTA to Control Growth of Escherichia coli Strains of Swine Origin
Abstract
1. Introduction
2. Results
2.1. Bacterial Susceptibility to Antimicrobial Compounds
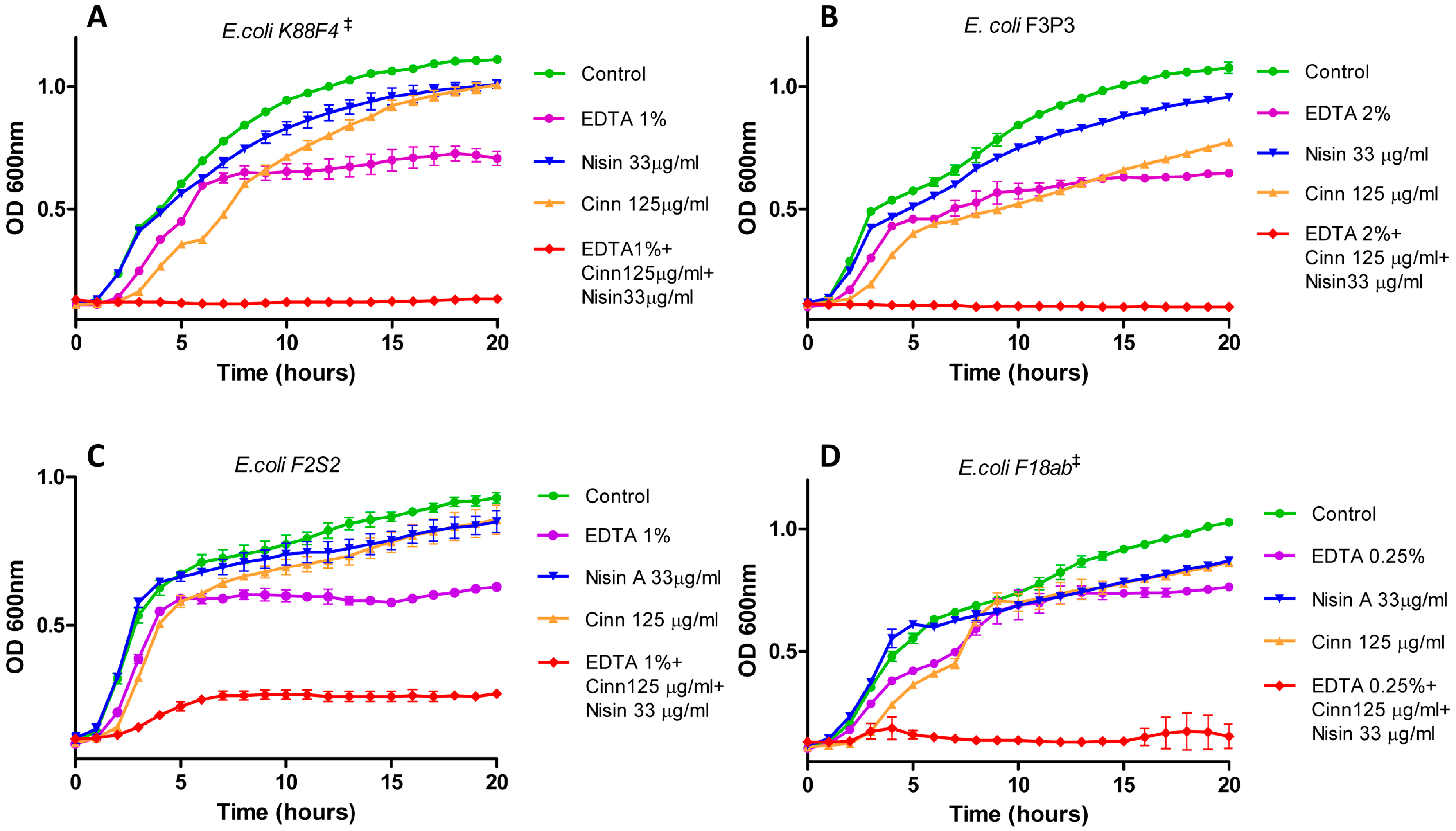
2.2. Growth Curve-Based Comparisons of the Activity of Nisin A and Natural Antimicrobial Combinations
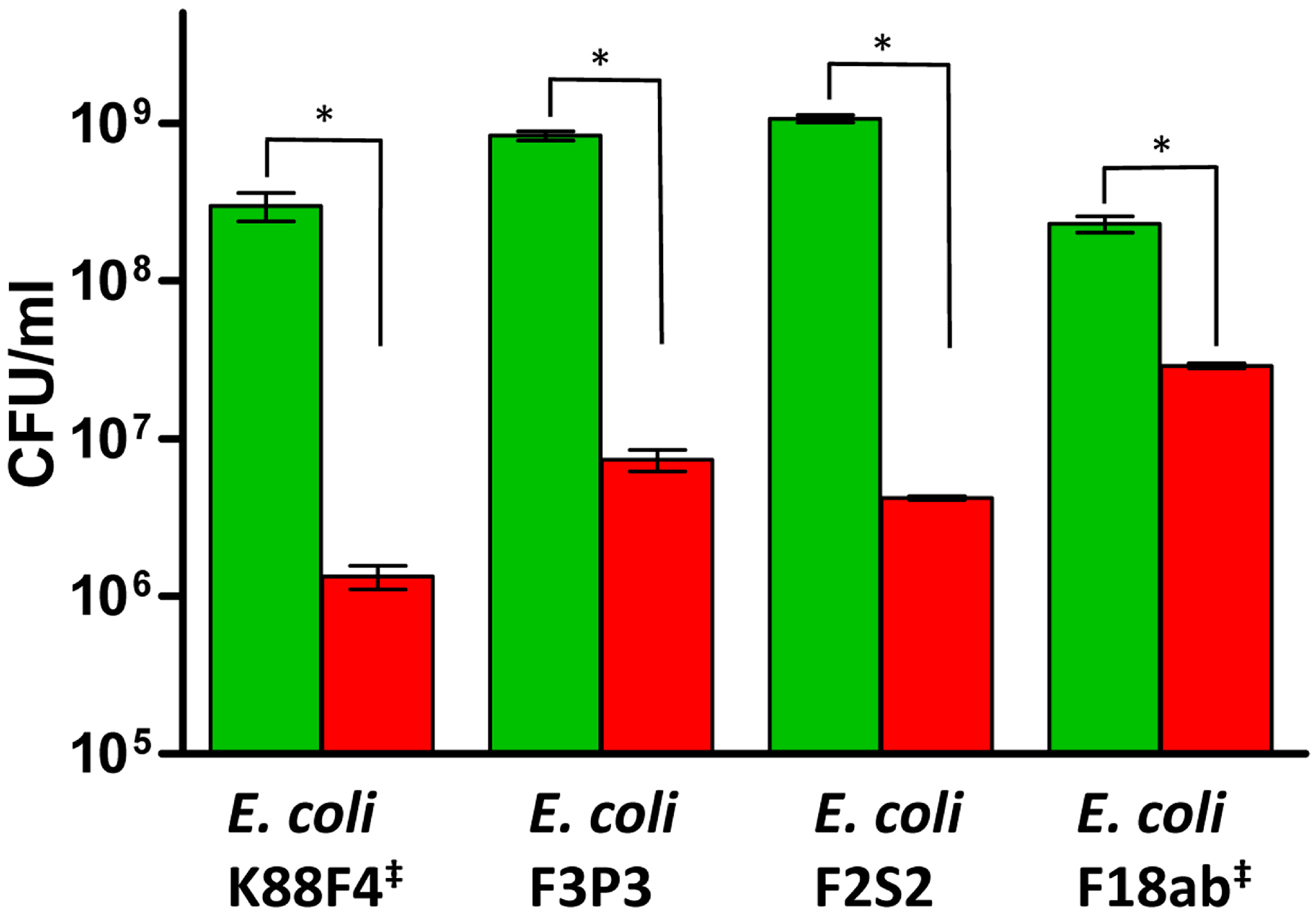
2.3. Kill Assay Determination of the Activity of Nisin A and Natural Antimicrobial Combinations
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains and Growth Conditions
4.2. Minimum Inhibitory Concentration Assays
4.3. Nisin Purification
4.4. Growth Curve Experiments
4.5. Kill Assay Analysis
4.6. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rhouma, M.; Letellier, A.; Beaudry, F.; Fairbrother, J.M. Post weaning diarrhea in pigs: Risk factors and non-colistin-based control strategies. Acta Vet. Scand. 2017, 59. [Google Scholar] [CrossRef] [PubMed]
- De Briyne, N.; Atkinson, J.; Pokludová, L.; Borriello, S.P. Antibiotics used most commonly to treat animals in Europe. Vet. Rec. 2014, 175. [Google Scholar] [CrossRef] [PubMed]
- Rolain, J.-M. Food and human gut as reservoirs of transferable antibiotic resistance encoding genes. Front. Microbiol. 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-Y.; Wang, Y.; Walsh, T.R.; Yi, L.X.; Zhang, R.; Spencer, J.; Yu, L.F. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: A microbiological and molecular biological study. Lancet Infect. Dis. 2016, 16, 161–168. [Google Scholar] [CrossRef]
- Haenni, M.; Poirel, L.; Kieffer, N.; Châtre, P.; Saras, E.; Métayer, V.; Madec, J.Y. Co-occurrence of extended spectrum β lactamase and MCR-1 encoding genes on plasmids. Lancet Infect. Dis. 2016, 16, 281–282. [Google Scholar] [CrossRef]
- Delves-Broughton, J.; Blackburn, P.; Evans, R.J.; Hugenholtz, J. Applications of the bacteriocin, nisin. Antonie Van Leeuwenhoek 1996, 69, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.T.; Wu, J.Q.; Xie, F.; Hu, S.H.; Mo, Y. Efficacy of nisin in treatment of clinical mastitis in lactating dairy cows. J. Dairy Sci. 2007, 90, 3980–3985. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Hu, S.; Cao, L. Therapeutic effect of nisin Z on subclinical mastitis in lactating cows. Antimicrob. Agents Chemother. 2007, 51, 3131–3135. [Google Scholar] [CrossRef] [PubMed]
- Breukink, E.; de Kruijff, B. Lipid II as a target for antibiotics. Nat. Rev. Drug Discov. 2006, 5, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Wiedemann, I.; Breukink, E.; van Kraaij, C.; Kuipers, O.P.; Bierbaum, G.; de Kruijff, B.; Sahl, H.G. Specific binding of nisin to the peptidoglycan precursor lipid II combines pore formation and inhibition of cell wall biosynthesis for potent antibiotic activity. J. Biol. Chem. 2001, 276, 1772–1779. [Google Scholar] [CrossRef] [PubMed]
- Bonelli, R.R.; Schneider, T.; Sahl, H.G.; Wiedemann, I. Insights into in vivo activities of lantibiotics from gallidermin and epidermin mode-of-action studies. Antimicrob. Agents Chemother. 2006, 50, 1449–1457. [Google Scholar] [CrossRef] [PubMed]
- Breukink, E.; de Kruijff, B. The lantibiotic nisin, a special case or not? Biochim. Biophys. Acta 1999, 1462, 223–234. [Google Scholar] [CrossRef]
- Ettayebi, K.; El Yamani, J.; Rossi-Hassani, B. Synergistic effects of nisin and thymol on antimicrobial activities in Listeria monocytogenes and Bacillus subtilis. FEMS Microbiol. Lett. 2000, 183, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Pol, I.E.; Smid, E.J. Combined action of nisin and carvacrol on Bacillus cereus and Listeria monocytogenes. Lett. Appl. Microbiol. 1999, 29, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Periago, P.M.; Moezelaar, R. Combined effect of nisin and carvacrol at different pH and temperature levels on the viability of different strains of Bacillus cereus. Int. J. Food Microbiol. 2001, 68, 141–148. [Google Scholar] [CrossRef]
- Field, D.; Daly, K.; O’Connor, P.M.; Cotter, P.D.; Hill, C.; Ross, R.P. Efficacies of nisin A and nisin V semipurified preparations alone and in combination with plant essential oils for controlling Listeria monocytogenes. Appl. Environ. Microbiol. 2015, 81, 2762–2769. [Google Scholar] [CrossRef] [PubMed]
- Branen, J.K.; Davidson, P.M. Enhancement of nisin, lysozyme, and monolaurin antimicrobial activities by ethylenediaminetetraacetic acid and lactoferrin. Int. J. Food Microbiol. 2004, 90, 63–74. [Google Scholar] [CrossRef]
- Boziaris, I.S.; Adams, M.R. Effect of chelators and nisin produced in situ on inhibition and inactivation of gram negatives. Int. J. Food Microbiol. 1999, 53, 105–113. [Google Scholar] [CrossRef]
- Al Atya, A.K.; Abriouel, H.; Kempf, I.; Jouy, E.; Auclair, E.; Vachée, A.; Drider, D. Effects of colistin and bacteriocins combinations on the in vitro growth of Escherichia coli strains from swine origin. Probiot. Antimicrob. Proteins 2016, 8, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Naghmouchi, K.; Baah, J.; Hober, D.; Jouy, E.; Rubrecht, C.; Sané, F.; Drider, D. Synergistic effect between colistin and bacteriocins in controlling gram-negative pathogens and their potential to reduce antibiotic toxicity in mammalian epithelial cells. Antimicrob. Agents Chemother. 2013, 57, 2719–2725. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Bai, X.; Zhao, A.; Lan, R.; Du, H.; Wang, T.; Jin, D. Characterization of Shiga toxin-producing Escherichia coli isolated from healthy pigs in China. BMC Microbiol. 2014, 14. [Google Scholar] [CrossRef] [PubMed]
- Corvec, S.; Tafin, U.F.; Betrisey, B.; Borens, O.; Trampuz, A. Activities of fosfomycin, tigecycline, colistin, and gentamicin against extended-spectrum-β-lactamase-producing Escherichia coli in a foreign-body infection model. Antimicrob. Agents Chemother. 2013, 57, 1421–1427. [Google Scholar] [CrossRef] [PubMed]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential oils in food preservation: Mode of action, synergies, and interactions with food matrix components. Front. Microbiol. 2012, 3. [Google Scholar] [CrossRef] [PubMed]
- Nagy, B.; Fekete, P.Z. Enterotoxigenic Escherichia coli in veterinary medicine. Int. J. Med. Microbiol. 2005, 295, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.; Salaheen, S.; Biswas, D. Animal health: Global antibiotic issues A2. In Encyclopedia of Agriculture and Food Systems; Van Alfen, N.K., Ed.; Academic Press: Oxford, UK, 2014; pp. 346–357. [Google Scholar]
- Alves, F.C.; Barbosa, L.N.; Andrade, B.F.; Albano, M.; Furtado, F.B.; Pereira, A.F.M.; Júnior, A.F. Short communication: Inhibitory activities of the lantibiotic nisin combined with phenolic compounds against Staphylococcus aureus and Listeria monocytogenes in cow milk. J. Dairy Sci. 2016, 99, 1831–1836. [Google Scholar] [CrossRef] [PubMed]
- Campion, A.; Morrissey, R.; Field, D.; Cotter, P.D.; Hill, C.; Ross, R.P. Use of enhanced nisin derivatives in combination with food-grade oils or citric acid to control Cronobacter sakazakii and Escherichia coli O157:H7. Food Microbiol. 2017, 65, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; van Heel, A.J.; Montalban-Lopez, M.; Kuipers, O.P. Potentiating the activity of nisin against Escherichia coli. Front. Cell Dev. Biol. 2016, 4. [Google Scholar] [CrossRef] [PubMed]
- Aquilina, G.; Azimonti, G.; Bampidis, V.; Bastos, M.D.L.; Bories, G.; Chesson, A.; Kouba, M. Products or Substances used in Animal, Safety and efficacy of Diarr-Stop S Plus® (Na2EDTA, tannin-rich extract of Castanea sativa, thyme oil and oregano oil) as a feed additive for pigs for fattening. EFSA J. 2016, 14. [Google Scholar] [CrossRef]
- Wynn, J.E.; Riet, B.V.; Borzelleca, J.F. The toxicity and pharmacodynamics of EGTA: Oral administration to rats and comparisons with EDTA. Toxicol. Appl. Pharmacol. 1970, 16, 807–817. [Google Scholar] [CrossRef]
- Yang, F.Y.; Lin, Z.H.; Li, S.G. Reversal of mitochondrial swelling by ethylenediaminetetraacetate. Sci. Sin. 1964, 13, 1518–1522. [Google Scholar] [PubMed]
- Khan, A.; Vu, K.D.; Riedl, B.; Lacroix, M. Optimization of the antimicrobial activity of nisin, Na-EDTA and pH against gram-negative and gram-positive bacteria. LWT Food Sci. Technol. 2015, 61, 124–129. [Google Scholar] [CrossRef]
- Partanen, K.H.; Mroz, Z. Organic acids for performance enhancement in pig diets. Nutr. Res. Rev. 1999, 12, 117–145. [Google Scholar] [CrossRef] [PubMed]
- Tsiloyiannis, V.K.; Kyriakis, S.C.; Vlemmas, J.; Sarris, K. The effect of organic acids on the control of post-weaning oedema disease of piglets. Res. Vet. Sci. 2001, 70, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Suiryanrayna, M.; Ramana, J.V. A review of the effects of dietary organic acids fed to swine. J. Anim. Sci. Biotechnol. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhen, Z.; Wang, X.; Guo, N. Synergy of a combination of nisin and citric acid against Staphylococcus aureus and Listeria monocytogenes. Food Addit. Contam. Part A 2017, 34, 2058–2068. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Zhang, S.; Wang, H.; Piao, X. Essential oil and aromatic plants as feed additives in non-ruminant nutrition: A review. J. Anim. Sci. Biotechnol. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Li, S.Y.; Ru, Y.J.; Liu, M.; Xu, B.; Péron, A.; Shi, X.G. The effect of essential oils on performance, immunity and gut microbial population in weaner pigs. Livest. Sci. 2012, 145, 119–123. [Google Scholar] [CrossRef]
- Michiels, J.; Missotten, J.; Rasschaert, G.; Dierick, N.; Heyndrickx, M.; De Smet, S. Effect of organic acids on Salmonella colonization and shedding in weaned piglets in a seeder model. J. Food Prot. 2012, 75, 1974–1983. [Google Scholar] [CrossRef] [PubMed]
- Walia, K.; Argüello, H.; Lynch, H.; Leonard, F.C.; Grant, J.; Yearsley, D.; Lawlor, P.G. Effect of strategic administration of an encapsulated blend of formic acid, citric acid, and essential oils on Salmonella carriage, seroprevalence, and growth of finishing pigs. Prev. Vet. Med. 2017, 137, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Stensland, I.; Kim, J.C.; Bowring, B.; Collins, A.M.; Mansfield, J.P.; Pluske, J.R.A. Comparison of diets supplemented with a feed additive containing organic acids, cinnamaldehyde and a permeabilizing complex, or zinc oxide, on post-weaning diarrhoea, selected bacterial populations, blood measures and performance in weaned pigs experimentally infected with enterotoxigenic E. coli. Animals 2015, 5, 1147–1168. [Google Scholar] [CrossRef]
- Józefiak, D.; Kierończyk, B.; Juśkiewicz, J.; Zduńczyk, Z.; Rawski, M.; Długosz, J.; Højberg, O. Dietary nisin modulates the gastrointestinal microbial ecology and enhances growth performance of the broiler chickens. PLoS ONE 2013, 8, e85347. [Google Scholar] [CrossRef] [PubMed]
- Laukova, A.; Chrastinová, Ľ.; Plachá, I.; Kandričáková, A.; Szabóová, R.; Strompfová, V.; Žitňan, R. Beneficial effect of lantibiotic nisin in rabbit husbandry. Probiot. Antimicrob. Proteins 2014, 6, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Field, D.; Quigley, L.; O’Connor, P.M.; Rea, M.C.; Daly, K.; Cotter, P.D.; Ross, R.P. Studies with bioengineered nisin peptides highlight the broad-spectrum potency of nisin V. Microb. Biotechnol. 2010, 3, 473–486. [Google Scholar] [CrossRef] [PubMed]
- Field, D.; Begley, M.; O’Connor, P.M.; Daly, K.M.; Hugenholtz, F.; Cotter, P.D.; Ross, R.P. Bioengineered nisin A derivatives with enhanced activity against both gram positive and gram negative pathogens. PLoS ONE 2012, 7, e46884. [Google Scholar] [CrossRef] [PubMed]
- Healy, B.; Field, D.; O’Connor, P.M.; Hill, C.; Cotter, P.D.; Ross, R.P. Intensive mutagenesis of the nisin hinge leads to the rational design of enhanced derivatives. PLoS ONE 2013, 8, e79563. [Google Scholar] [CrossRef] [PubMed]
| Antibiotic | E. coli Strain | ||||
|---|---|---|---|---|---|
| F150F3 | F2S2 | K88F4 ‡ | F3P3 | F18ab ‡ | |
| Chloramphenicol | >50 | 12.5 | >50 | >50 | 12.5 |
| Tetracycline | >50 | 1.56 | 50 | 25 | 50 |
| Penicillin G | >50 | 25 | 25 | 25 | 12.5 |
| Streptomycin | >50 | 25 | >50 | 12.5 | 1.56 |
| Cefoxitin | >50 | >50 | >50 | >50 | >50 |
| Erythromycin | >50 | >50 | >50 | >50 | 50 |
| Lincomycin | >50 | >50 | >50 | >50 | >50 |
| Ceftazidine | <0.4 | <0.4 | <0.4 | <0.4 | <0.4 |
| Cefuroxime | 6.25 | 3.12 | 6.25 | 6.25 | 6.25 |
| Cefradine | 25 | 12.5 | 25 | 25 | 12.5 |
| Cefsulodin | >50 | 50 | 50 | 50 | 50 |
| Colistin | 0.39 | 0.39 | <0.2 | <0.2 | 25 |
| Polymyxin B | 0.39 | 0.39 | <0.2 | <0.2 | 25 |
| Carvacrol | >1250 | >1250 | >1250 | >1250 | >1250 |
| Cinnamaldehyde | 1250 | 1250 | 1250 | >1250 | 1250 |
| Thymol | >1250 | >1250 | >1250 | >1250 | >1250 |
| Nisin | 200 | 200 | 200 | 200 | 200 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Field, D.; Baghou, I.; Rea, M.C.; Gardiner, G.E.; Ross, R.P.; Hill, C. Nisin in Combination with Cinnamaldehyde and EDTA to Control Growth of Escherichia coli Strains of Swine Origin. Antibiotics 2017, 6, 35. https://doi.org/10.3390/antibiotics6040035
Field D, Baghou I, Rea MC, Gardiner GE, Ross RP, Hill C. Nisin in Combination with Cinnamaldehyde and EDTA to Control Growth of Escherichia coli Strains of Swine Origin. Antibiotics. 2017; 6(4):35. https://doi.org/10.3390/antibiotics6040035
Chicago/Turabian StyleField, Des, Inès Baghou, Mary C. Rea, Gillian E. Gardiner, R. Paul Ross, and Colin Hill. 2017. "Nisin in Combination with Cinnamaldehyde and EDTA to Control Growth of Escherichia coli Strains of Swine Origin" Antibiotics 6, no. 4: 35. https://doi.org/10.3390/antibiotics6040035
APA StyleField, D., Baghou, I., Rea, M. C., Gardiner, G. E., Ross, R. P., & Hill, C. (2017). Nisin in Combination with Cinnamaldehyde and EDTA to Control Growth of Escherichia coli Strains of Swine Origin. Antibiotics, 6(4), 35. https://doi.org/10.3390/antibiotics6040035






