Antimicrobial Activity of Bee Venom and Melittin against Borrelia burgdorferi
Abstract
:1. Introduction
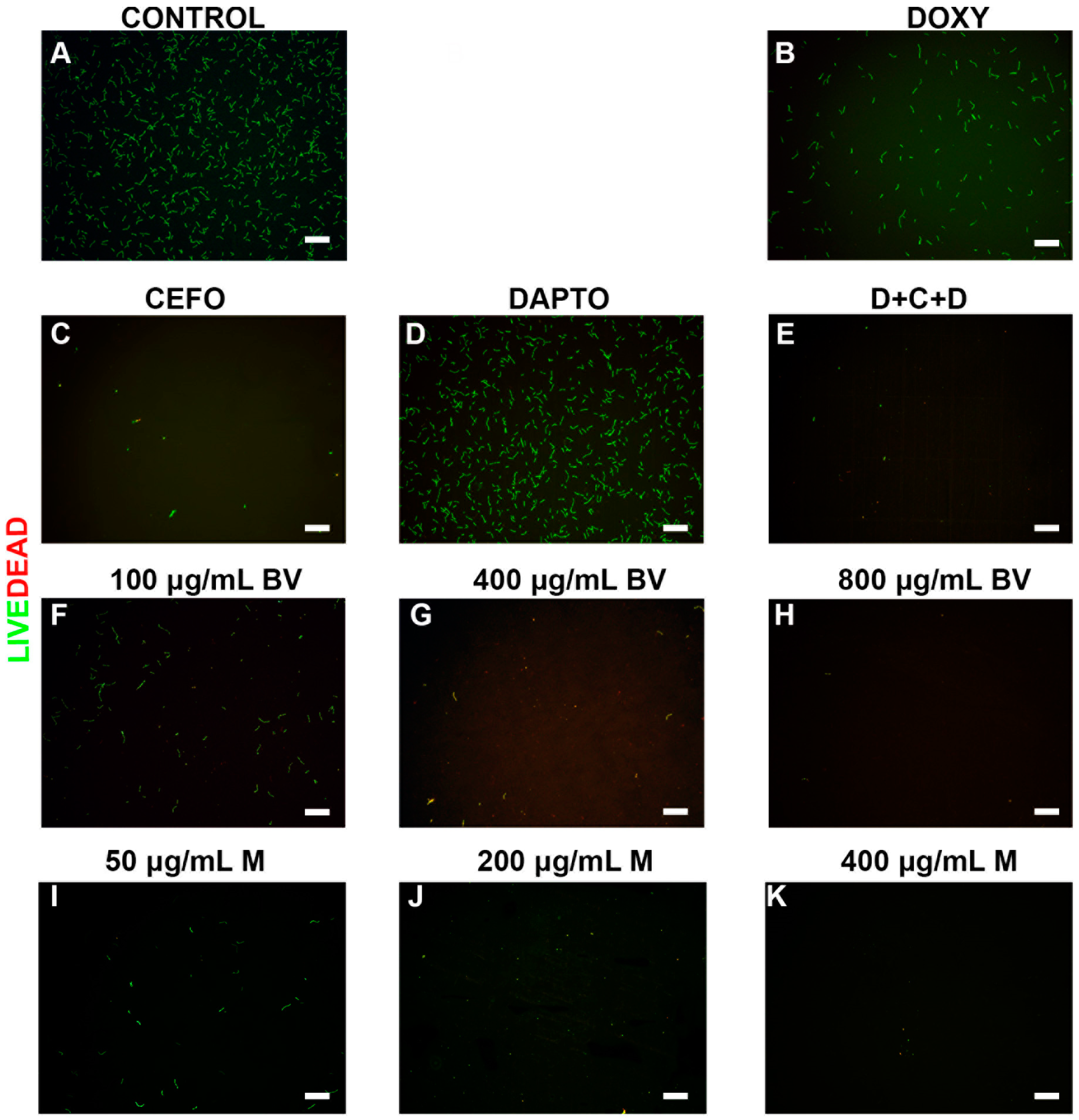
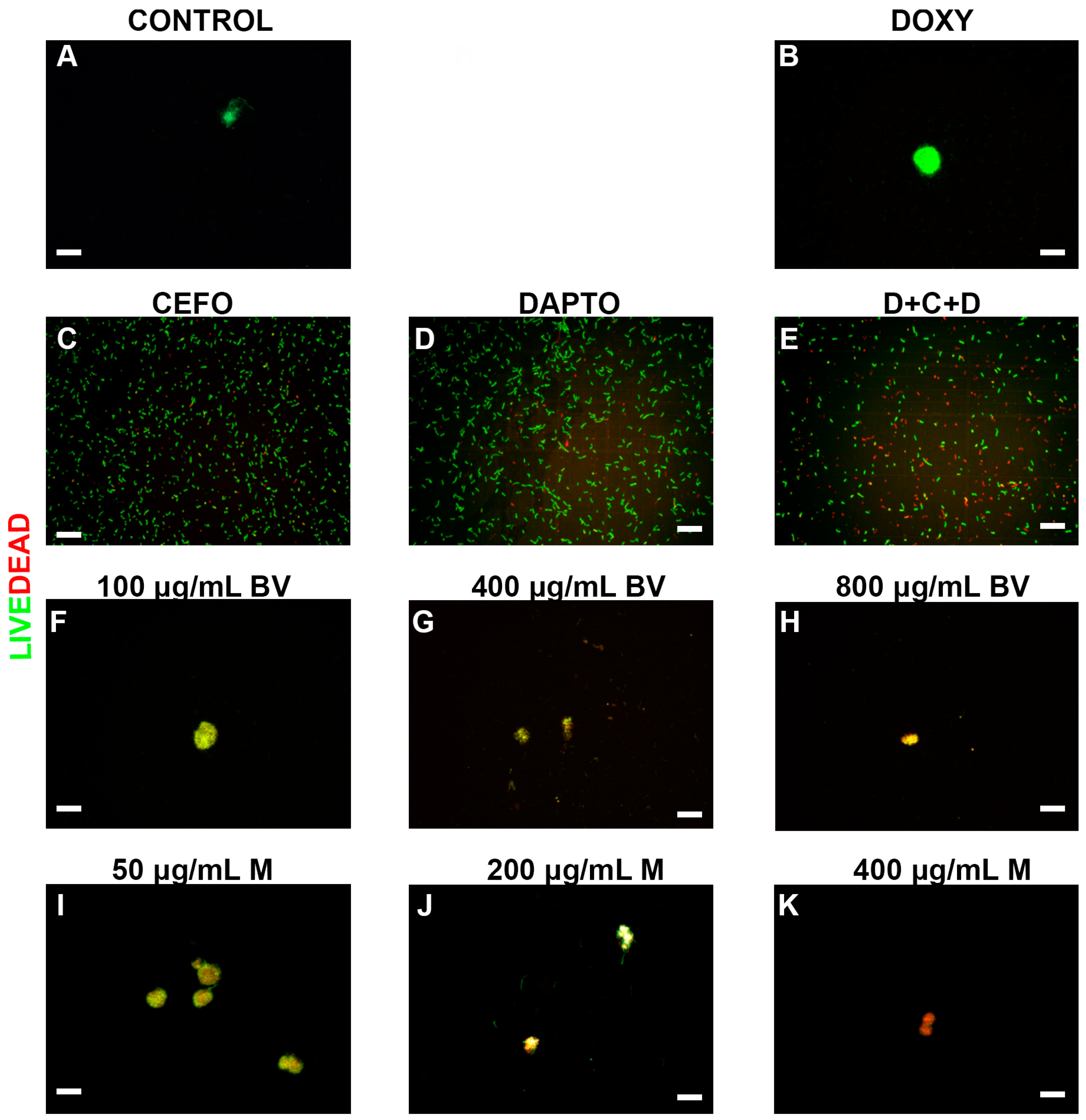
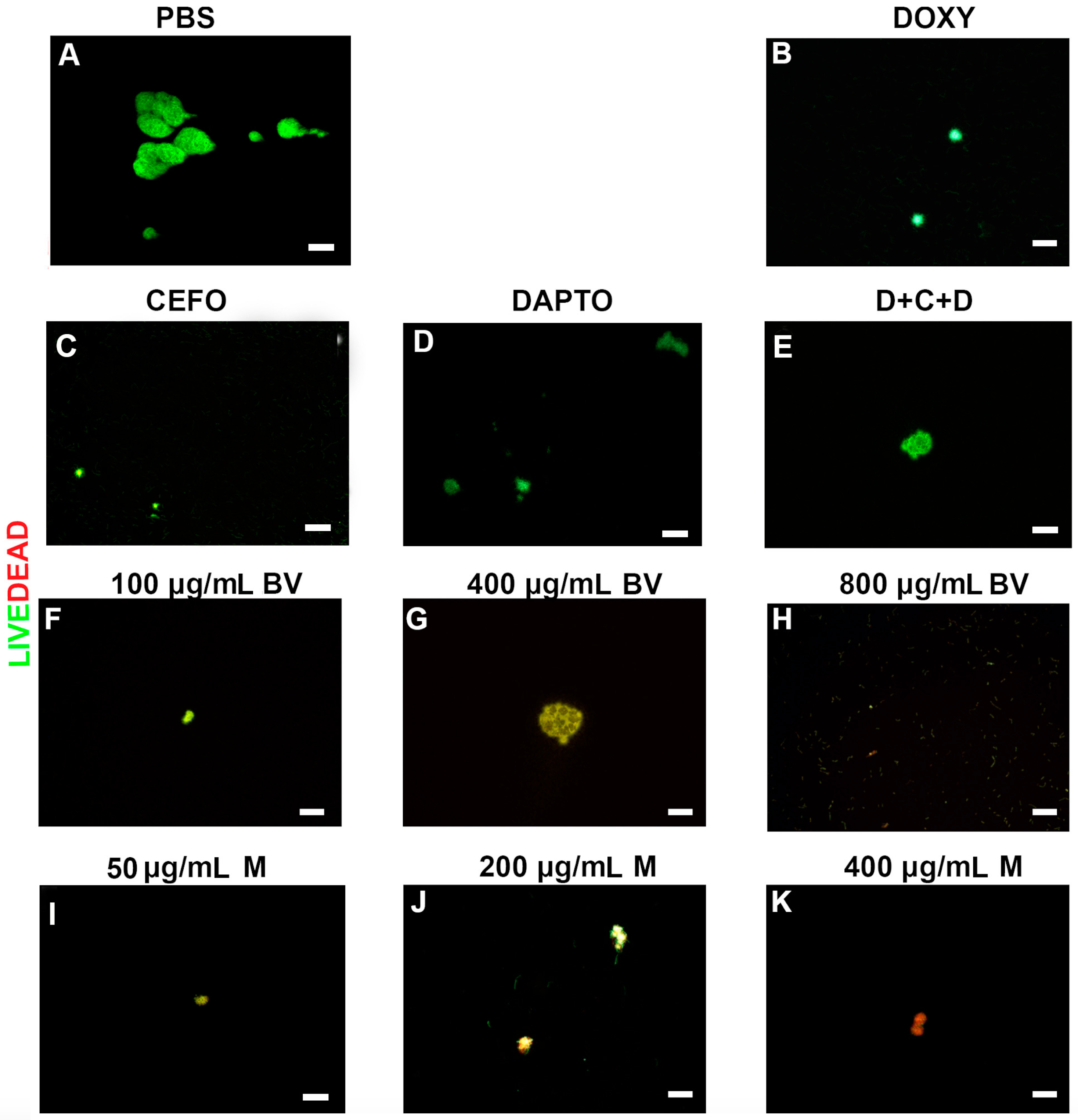
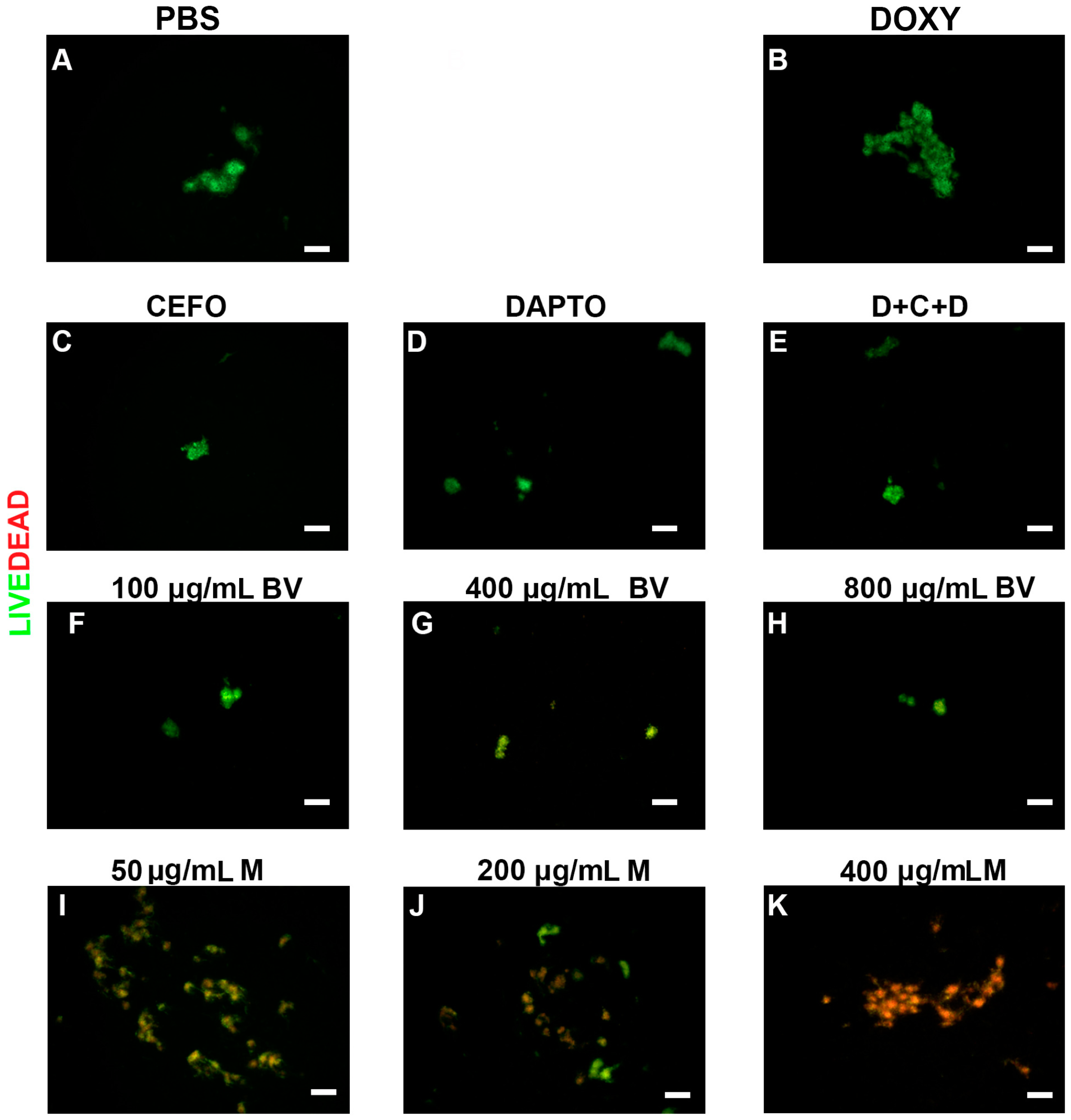
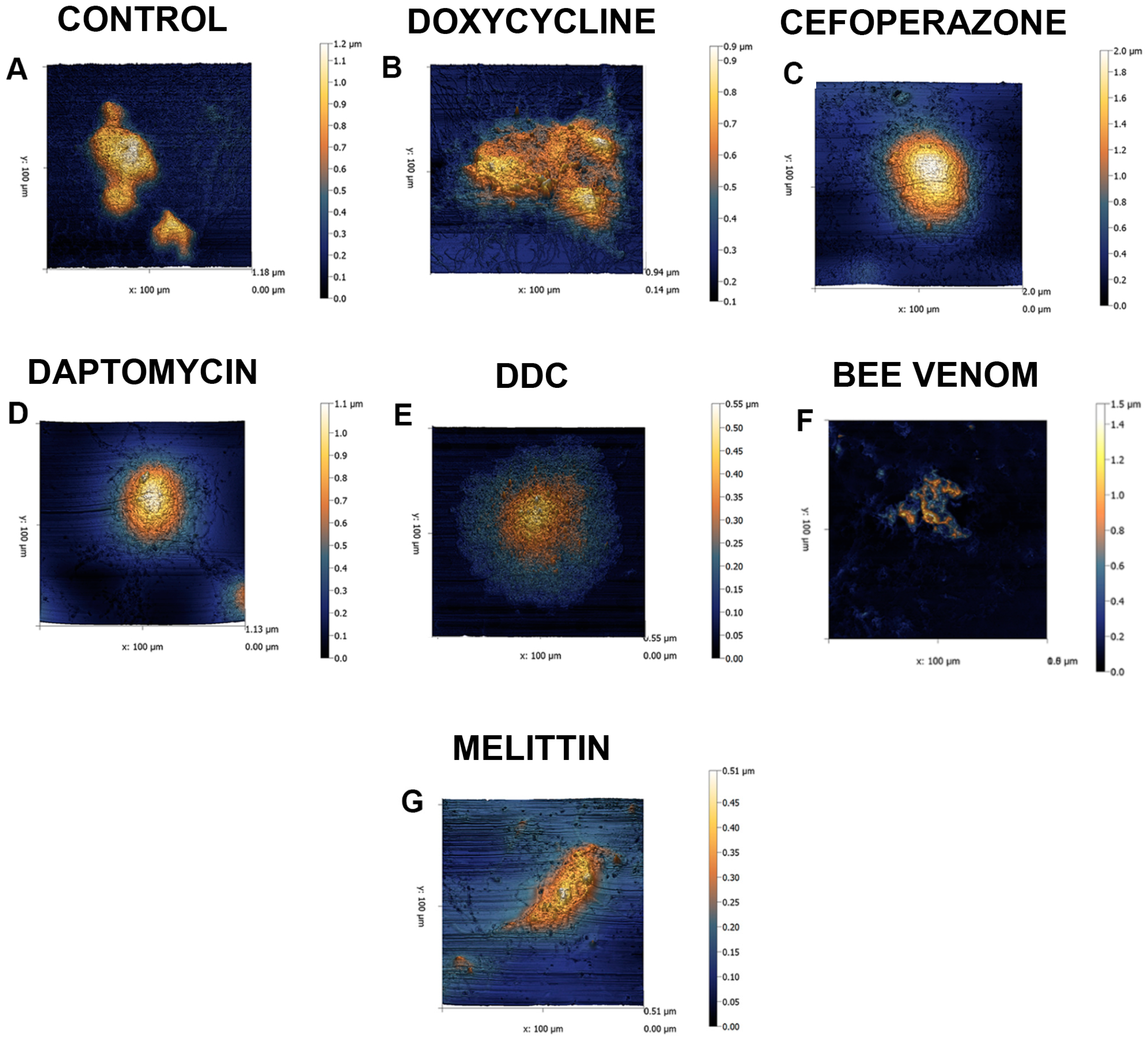
2. Results
3. Discussion
4. Materials and Methods
4.1. Bacterial Culture
4.2. Antimicrobial Agent Preparation
4.3. Antimicrobial Testing
4.3.1. Bacterial Preparation
4.3.2. Subculture Experiments
4.3.3. SYBR Green I/Propidium Iodide Assay
4.3.4. Direct Viable Cell Counts of B. burgdorferi
4.3.5. Autofluorescence of Antimicrobials
4.3.6. Quantitative Assay for Attached Biofilms.
4.4. Atomic Force Microscopy
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Funding
Author Contributions
Conflicts of Interest
References
- Barbour, A.G.; Hayes, S.F. Biology of Borrelia species. Microbiol. Rev. 1986, 50, 381–400. [Google Scholar] [PubMed]
- Rudenko, N.; Golovchenko, M.; Grubhoffer, L.; Oiver, J.H., Jr. Updates on Borrelia burgdorferi sensu lato complex with respect to public health. Ticks Tick-borne Dis. 2011, 2, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Brisson, D.; Vandermause, M.F.; Meece, J.K.; Reed, K.D.; Dykhyizen, D.E. Evolution of Northeastern and Midwestern Borrelia burgdorferi, United States. Emerg. Infect. Dis. 2010, 16, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Center of Disease Control and Prevention. Lyme Disease. 2016. Available online: http://www.cdc.gov/lyme/ (accessed on 13 September 2017).
- Wormser, G.P.; Nadelman, R.B.; Dattwyler, R.J.; Dennis, D.T.; Shapiro, E.D.; Steere, A.C.; Rush, T.J.; Rahn, D.W.; Coyle, P.K.; Persing, D.H.; et al. Practice guidelines for the treatment of Lyme disease. Clin. Infect. Dis. 2000, 31, S1–S14. [Google Scholar] [CrossRef] [PubMed]
- Sapi, E.; Kaur, N.; Anyanwu, S.; Leuke, D.F.; Data, A.; Patel, S.; Rossi, M.; Stricker, R.B. Evaluation of in vitro antibiotic susceptibility of different morphological forms of Borrelia burgdorferi. Infect. Drug Resist. 2011, 4, 97–113. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Wang, T.; Shi, W.; Zhang, S.; Sullivan, D.; Auwaerter, P.G.; Zhang, Y. Identification of novel activity against Borrelia burgdorferi persisters using an FDA approved drug library. Emerg. Microbes. Infect. 2014, 3. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Auwaerter, P.G.; Zhang, Y. Drug combinations against Borrelia burgdorferi persisters in vitro: Eradication achieved by using Daptomycin, Cefoperazone and Doxycycline. PLoS ONE 2015, 10, E0117207. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Shi, W.; Zhang, S.; Sullivan, D.; Auwaerter, P.G.; Zhang, Y. A drug combination screen identifies drugs active against amoxicillin-induced round bodies of in vitro Borrelia burgdorferi persisters from an FDA drug library. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Weitner, M.; Shi, W.; Zhang, S.; Zhang, Y. Eradication of biofilm-like microcolony structures of Borrelia burgdorferi by daunomycin and daptomycin but not mitomycin C in combination with doxycycline and cefuroxime. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Hodzic, E.; Fen, S.; Holden, K.; Freet, K.J.; Barthold, S.W. Persistence of Borrelia burgdorferi following antibiotic treatment in mice. Antimicrob. Agents Chemother. 2008, 52, 1728–1736. [Google Scholar] [CrossRef] [PubMed]
- Barthold, S.W.; Hodzic, E.; Imai, D.M.; Feng, S.; Yang, X.; Luft, B.J. Ineffectiveness of tigecycline against persistent Borrelia burgdorferi. Antimicrob. Agents Chemother. 2010, 54, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Embers, M.E.; Barthold, S.W.; Borda, J.T.; Bowers, L.; Doyle, L.; Hodzic, E.; Jacobs, M.B.; Hasenkampf, N.R.; Martin, D.S.; Narasimhan, S.; et al. Persistence of Borrelia burgdorferi in Rhesus macaques following antibiotic treatment of disseminated infection. PLoS ONE 2012, 7, E29914. [Google Scholar] [CrossRef]
- Motaleb, M.A.; Corum, L.; Bono, J.L.; Elias, A.F.; Rosa, P.; Samuels, D.S.; Charon, N.W. Borrelia burgdorferi periplasmic flagella have both skeletal and motility functions. Proc. Natl. Acad. Sci. USA 2000, 97, 10899–10904. [Google Scholar] [CrossRef] [PubMed]
- Sal, M.S.; Li, C.; Motalab, M.A.; Shibata, S.; Aizawa, S.; Charon, N.W. Borrelia burgdorferi uniquely regulates its motility genes and has an intricate flagellar hook-basal body structure. J. Bacteriol. 2008, 190, 1912–1921. [Google Scholar] [CrossRef] [PubMed]
- Brorson, Ø.; Brorson, S.H. Transformation of cystic forms of Borrelia burgdorferi to normal, mobile spirochetes. Infection 1997, 25, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Alban, P.S.; Johnson, P.W.; Nelson, D.R. Serum-starvation-induced changes in protein synthesis and morphology of Borrelia burgdorferi. Microbiology 2000, 146, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Murgia, R.; Cinco, M. Induction of cystic forms by different stress conditions in Borrelia burgdorferi. APMIS 2004, 112, 57–62. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, A.B. Spirochetal cyst forms in neurodegenerative disorders, hiding in plain sight. J. Med. Hypotheses 2006, 67, 819–832. [Google Scholar] [CrossRef] [PubMed]
- Miklossy, J.; Kasas, S.; Zurn, A.D.; McCall, S.; Yu, S.; McGeer, P.L. Persisting atypical and cystic forms of Borrelia burgdorferi and local inflammation in Lyme neuroborreliosis. J. Neuroinflamm. 2008, 5. [Google Scholar] [CrossRef] [PubMed]
- Brorson, Ø.; Brorson, S.H.; Scythes, J.; MacAllister, J.; Wier, A.; Margulis, L. Destruction of spirochete Borrelia burgdorferi round-body propagules (RBs) by the antibiotic tigecycline. Proc. Natl. Acad. Sci. USA 2009, 106, 18656–18661. [Google Scholar] [CrossRef] [PubMed]
- Sapi, E.; Bastian, S.L.; Mpoy, C.M.; Scott, S.; Rattelle, A.; Pabbati, N.; Poruri, A.; Burugu, D.; Theophilus, P.A.; Pham, T.V.; et al. Characterization of biofilm formation by Borrelia burgdorferi in vitro. PLoS ONE 2012, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Brorson, Ø.; Brorson, S.H. In vitro conversion of Borrelia burgdorferi to cystic forms in spinal fluid, and transformation to mobile spirochetes by incubation in BSK-H medium. Infection 1998, 26, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Vancova, M.; Rudenko, N.; Vanecek, J.M.; Golovchenko, M.; Stand, M. Pleomorphism and viability of the Lyme disease pathogen Borrelia burgdorferi exposed to physiological stress conditions: A correlative cryo-fluorescence and cryo-scanning electron microscopy study. Front. Microbiol. 2017, 8, 596. [Google Scholar] [CrossRef] [PubMed]
- Sapi, E.; Theophilus, P.A.S.; Burugu, D.; Leuke, D.F. Effect of Rpon, Rpos and Luxs pathways on the biofilm formation and antibiotic sensitivity of Borrelia burgdorferi. Eur. J. Microbiol. Immunol. 2016, 6, 272–286. [Google Scholar] [CrossRef] [PubMed]
- Theophilus, P.A.; Victoria, M.J.; Socarras, K.M.; Filush, K.R.; Gupta, K.; Luecke, D.F.; Sapo, E. Effectiveness of Stevia rebaudiana whole leaf extract against the various morphological forms of Borrelia burgdorferi in vitro. Eur. Microbiol. Immunol. 2015, 5, 268–280. [Google Scholar] [CrossRef] [PubMed]
- Raghuraman, H.; Chattopadhyay, A. Melittin: Membrane-active peptide with diverse functions. Biosci. Rep. 2007, 27, 189–223. [Google Scholar] [CrossRef] [PubMed]
- Carter, V.; Underhill, A.; Baber, I.; Sylla, L.; Baby, M.; Larget-Thiery, I.; Zettor, A.; Bourgouin, C.; Langel, U.; Faye, I.; et al. Killer bee molecules: Antimicrobial peptides as effector molecules to target sporogonic stages of Plasmodium. PLoS Pathog. 2013, 9, E1003790. [Google Scholar] [CrossRef] [PubMed]
- Lubke, L.L.; Garon, C.F. The antimicrobial agent melittin exhibits powerful in vitro inhibitory effects on the Lyme disease spirochete. Clin. Infect. Dis. 1997, 25, S48–S51. [Google Scholar] [CrossRef] [PubMed]
- Alia, O.; Laila, M.; Antonious, A. Antimicrobial effect of melittin isolated from Syrian honeybee (Apis mellifera) venom and its wound healing potential. Int. J. Pharm. Sci. Rev. Res. 2013, 21, 318–324. [Google Scholar]
- Feng, J.; Wang, T.; Zhang, S.; Shi, W.; Zhang, Y. An optimized SYBR Green I/PI assay for rapid viability assessment and antibiotic susceptibility testing for Borrelia burgdorferi. PLoS ONE 2014, 9, E111809. [Google Scholar] [CrossRef] [PubMed]
- Renggli, S.; Keck, W.; Jenal, U.; Ritz, D. Role of autofluorescence in flow cytometric analyses of Escherichia coli treated with bactericidal antibiotics. J. Bacteriol. 2013, 195. [Google Scholar] [CrossRef] [PubMed]
- Sapi, E.; Balasubramanian, K.; Poruri, A.; Maghsoudlou, J.S.; Socarras, K.M.; Timmaraju, A.V.; Filush, K.R.; Gupta, K.; Shaikh, S.; Theophilus, P.A.; et al. Evidence of in vivo existence of Borrelia biofilm in Borrelial lymphocytomas. Eur. J. Microbiol. Immunol. 2016, 6, 9–24. [Google Scholar] [CrossRef] [PubMed]
- Stricker, R.B.; Johnson, L. Lyme disease: The next decade. Infect. Drug Resist. 2011, 4, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zamai, L.; Bareggi, R.; Santavenere, E.; Vitale, M. Subtraction of autofluorescent dead cells from lymphocyte flow cytometric binding assay. Cytometry 1993, 14, 951–954. [Google Scholar] [CrossRef] [PubMed]
- Schadow, K.H.; Simpson, W.A.; Christensen, G.D. Characteristics of adherence to plastic tissue culture plates of coagulase-negatice staphylococci exposed to subinhibitory concentrations of antimicrobial agents. J. Infect. Dis. 1998, 157, 71–77. [Google Scholar] [CrossRef]
- Hoffman, L.R.; D’Argenio, L.A.; MacCoss, M.J.; Zhang, Z.; Jones, R.A.; Miller, S.I. Aminoglycoside antibiotics induce bacterial biofilm formation. Nature 2005, 436, 1171–1175. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, J.B. Antibiotic-induced biofilm formation. Int. J. Artif. Organs 2011, 34, 737–751. [Google Scholar] [CrossRef] [PubMed]
- Song, N.; Duperthuy, M.; Wai, S. Sub-optimal treatment of bacterial biofilms (Review). Antibiotics 2016, 5. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.B.; Lee, H.J.; Han, H.J.; Mar, W.C.; Kang, S.K.; Yoon, O.B.; Beitz, A.J.; Lee, J.H. The water-soluble fraction of bee venom produces antinociceptive and anti-inflammatory effects on rheumatoid arthritis in rats. Life Sci. 2002, 71, 191–204. [Google Scholar] [CrossRef]
- Jo, M.; Hee Park, M.H.; Kollipara, P.S.; An, B.J.; Song, H.S.; Han, S.B.; Kim, J.H.; Song, M.J.; Hong, J.T. Anti-cancer effect of bee venom toxin and melittin in ovarian cancer cells through induction of death receptors and inhibition of JAK2/STAT3 pathway. Toxicol. Appl. Pharmacol. 2012, 258, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Adade, C.M.; Olivera, I.R.S.; Pais, J.A.R.; Souto-Padrón, T. Melittin peptide kills Trypanosoma cruzi parasites by inducing different cell pathways. Toxicon 2013, 69, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Hood, J.L.; Jallouck, A.P.; Campbell, N.; Ratner, L.; Wickline, S.A. Cytolytic nanoparticles attenuate HIV-1 infectivity. Antivir. Ther. 2013, 9, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Nitecka-Buchta, A.; Buchta, P.; Tabeńska-Bosakowska, E.; Walcyńska-Dragoń, K.; Baron, S. Myorelaxant Effect of bee venom topical skin application in patients with RDC/TMD Ia and RDC/TMD Ib: A randomized, double blinded study. BioMed Res. Intern. 2014, 2014, 296053. [Google Scholar] [CrossRef] [PubMed]
- Hancock, R.E.; Sahl, H.G. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol. 2006, 24, 1551–1557. [Google Scholar] [CrossRef] [PubMed]
- Mataraci, E.; Dosler, S. In vitro activities of antibiotics and antimicrobial cationic peptides alone and in combination against Methicillin-Resistant Staphylococcus aureus biofilms. Antimicrob. Agents Chemother. 2012, 56, 6366–6371. [Google Scholar] [CrossRef] [PubMed]
- Dobson, A.J.; Purves, J.; Kamysz, W.; Rolff, J. Comparing selection on S. aureus between antimicrobial peptides and common antibiotics. PLoS Pathog. 2013, 8, E76512. [Google Scholar] [CrossRef] [PubMed]
- Jamasbi, E.; Mularski, A.; Separovic, F. Model membrane and cell studies of antimicrobial activity of Melittin analogues. Curr. Top. Med. Chem. 2016, 16, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Singh, A.K.; Wu, X.; Lyu, Y.; Bhunia, A.K.; Narsimhan, G. Characterization of antimicrobial activity against Listeria and cytotoxicity of native melittin and its mutant variants. Colloids Surf. B Biointerfaces 2016, 143, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Zhu, X.; Tan, T.; Weizhong, L.; Shan, A. Design of embedded-hybrid antimicrobial peptides with enhanced cell selectivity and anti-biofilm activity. PLoS ONE 2014, 9, E98935. [Google Scholar] [CrossRef] [PubMed]
- Hancock, R.E.W. Alteration in outer membrane permeability. Ann. Rev. Microbiol. 1984, 38, 237–264. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.; Ulmschneider, J.P.; Ulmschneider, M.B.; White, S.H. Conformational states of melittin at a bilayer interface. Biophys. J. 2013, 104, L12–L14. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Nomura, F.; Yokoyama, Y.; Tanaka-Takiguchi, Y.; Homma, M.; Takiguchi, K. Multiple membrane interactions and versatile vesicle deformations elicited by Melittin. Toxins 2013, 5, 637–664. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, F.M.; Hammac, G.K.; Guptill, L.; Seleem, M.N. Antibacterial activity of novel cationic peptides against clinical isolates of multi-drug resistant Staphylococcus pseudintermedius from infected dogs. PLoS ONE 2014. [Google Scholar] [CrossRef] [PubMed]
- Brogen, K.A. Antimicrobial peptides: Pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005, 3, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Batoni, G.; Maisetta, G.; Brancatisano, F.L.; Esin, S.; Campa, M. Use of antimicrobial peptides against microbial biofilms: Advantages and limits. Curr. Med. Chem. 2011, 18, 256–279. [Google Scholar] [CrossRef] [PubMed]
- Leandro, L.F.; Mendes, C.A.; Casemiro, L.A.; Vinholis, A.H.; Cunha, W.R.; de Almeida, R.; Martins, C.H. Antimicrobial activity of apitoxin, melittin and phospholipase A2 of honey bee (Apis mellifera) venom against oral pathogens. An. Acad. Bras. Cienc. 2015, 87, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Strempel, N.; Strehmel, J.; Overhage, J. Potential application of antimicrobial peptides in the treatment of bacterial biofilm infections. Curr. Pharm. Des. 2015, 21, 67–84. [Google Scholar] [CrossRef] [PubMed]
- Lewis, K. Riddle of biofilm resistance. Antimicrob. Agents Chemother. 2001, 45, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- Le, C.F.; Fang, C.M.; Sekaran, S.D. Intracellular targeting mechanisms by antimicrobial peptides. Antimicrob. Agents Chemother. 2017, 61, 12. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Ling, C.; Huang, X. Study on purification of melittin and its effect on anti-tumor in vitro. Chin. J. Biochem. Pharm. 2003, 24, 163–166. [Google Scholar]
- Liu, M.; Wang, H.; Liu, L.; Wang, B.; Sun, G. Melittin-MIL-2 fusion protein as a candidate for cancer immunotherapy. J. Transl. Med. 2016, 14. [Google Scholar] [CrossRef] [PubMed]
- Maher, S.; Mcclean, S. Melittin exhibits necrotic cytotoxicity in gastrointestinal cells which is attenuated by cholesterol. Biochem. Pharmacol. 2008, 75, 1104–1114. [Google Scholar] [CrossRef] [PubMed]
- Jallouk, A.P.; Moley, K.H.; Omurtag, K.; Hu, G.; Lanza, G.M.; Wickline, S.A.; Hood, J.L. Nanoparticle incorporation of Melittin reduces sperm and vaginal epithelium cytotoxicity. PLoS ONE 2014, 9, E95411. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.B.; Lee, B.H.; Nikapitiya, C.; Kim, J.H.; Kim, T.H.; Lee, H.C.; Kim, C.G.; Lee, J.S.; Kim, C.J. Inhibitory effects of bee venom and its components against viruses in vitro and in vivo. J. Microbiol. 2016, 54, 853–866. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.M.; Jeong, Y.-J.; Cho, H.J.; Park, K.K.; Chung, I.K.; Lee, I.K.; Kwak, J.Y.; Chang, H.W.; Kim, C.H.; Moon, S.K.; et al. Melittin suppresses HIF-1α/VEGF expression through inhibition of ERK and mTOR/p70S6K pathway in human cervical carcinoma cells. PLoS ONE 2013, 8, E69380. [Google Scholar] [CrossRef] [PubMed]
- Necas, D.; Klapetek, P. Gwyddion: An open-source software for SPM data analysis. Cent. Europ. J. Phys. 2012. [Google Scholar] [CrossRef]
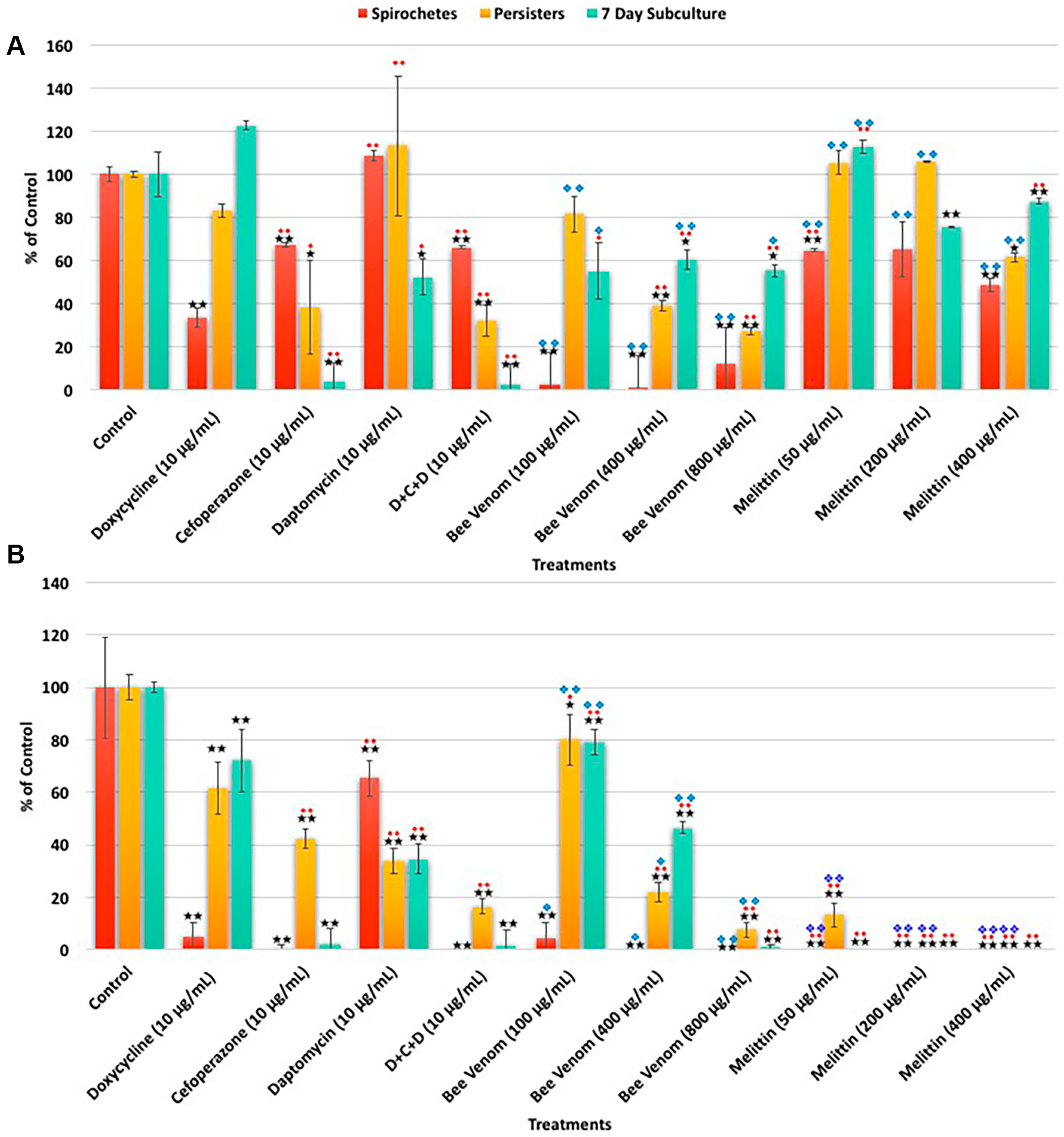
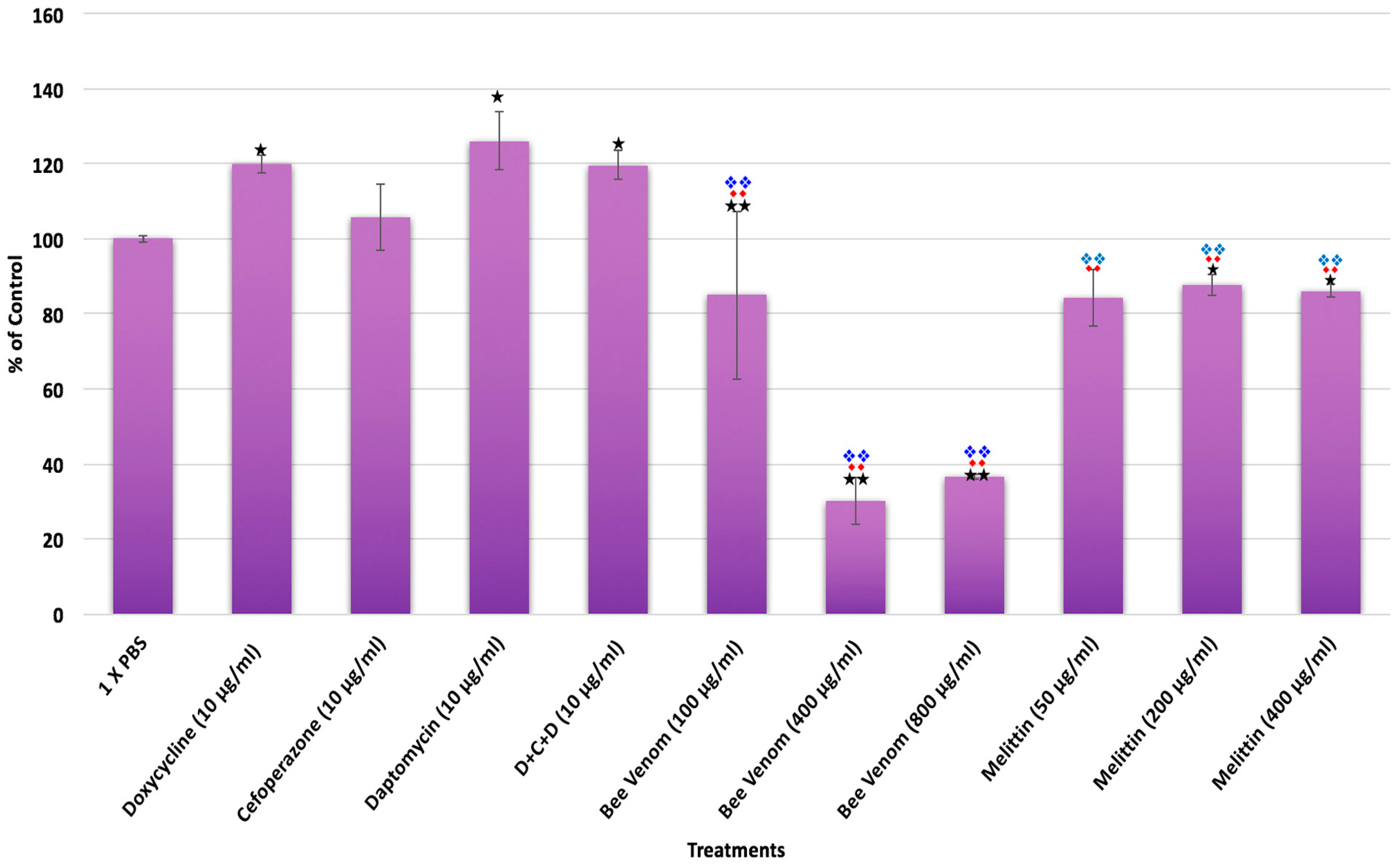
| A. SYBR Green I/PI Assay | Spirochetes | Persisters | 7 Day Subculture | ||||||
| Treatments | % Control | % SD | % Median | % Control | % SD | % Median | % Control | % SD | % Median |
| Control | 100 | 11 | 100 | 100 | 12 | 100 | 100 | 16 | 100 |
| Doxycycline (10 μg/mL) | 33 | 4 | 33 | 83 | 3 | 86 | 122 | 2 | 83 |
| Cefoperazone (10 μg/mL) | 67 | 1 | 66 | 38 | 22 | 41 | 4 | 9 | 3 |
| Daptomycin (10 μg/mL) | 108 | 2 | 107 | 113 | 32 | 120 | 52 | 8 | 43 |
| D + C + D (10 μg/mL) | 66 | 1 | 65 | 32 | 7 | 34 | 3 | 9 | 2 |
| Bee venom (100 μg/mL) | 61 | 11 | 63 | 62 | 7 | 74 | 88 | 29 | 141 |
| Bee venom (400 μg/mL) | 45 | 11 | 37 | 44 | 25 | 43 | 59 | 19 | 115 |
| Bee venom (800 μg/mL) | 33 | 8 | 32 | 32 | 2 | 35 | 95 | 31 | 55 |
| Melittin (50 μg/mL) | 65 | 1 | 67 | 105 | 6 | 91 | 113 | 3 | 103 |
| Melittin (200 μg/mL) | 65 | 24 | 67 | 106 | 0 | 94 | 75 | 0 | 69 |
| Melittin (400 μg/mL) | 49 | 6 | 54 | 61 | 2 | 57 | 87 | 1 | 80 |
| B. Direct Counting Assay | Spirochetes | Persisters | 7 Day Subculture | ||||||
| Treatments | % Control | % SD | % Median | % Control | % SD | % Median | % Control | % SD | % Median |
| Control | 100 | 19 | 100 | 100 | 5 | 100 | 100 | 8 | 100 |
| Doxycycline (10 μg/mL) | 5 | 6 | 5 | 62 | 10 | 67 | 72 | 12 | 60 |
| Cefoperazone (10 μg/mL) | 1 | 1 | 0 | 43 | 4 | 43 | 2 | 6 | 2 |
| Daptomycin (10 μg/mL) | 65 | 7 | 73 | 34 | 5 | 36 | 35 | 6 | 175 |
| D + C + D (10 μg/mL) | 0 | 0 | 0 | 17 | 3 | 17 | 2 | 6 | 0 |
| Bee venom (100 μg/mL) | 5 | 6 | 4 | 80 | 10 | 79 | 79 | 5 | 65 |
| Bee venom (400 μg/mL) | 0 | 0 | 0 | 22 | 4 | 21 | 47 | 2 | 29 |
| Bee venom (800 μg/mL) | 0 | 0 | 0 | 8 | 3 | 7 | 1 | 1 | 0 |
| Melittin (50 μg/mL) | 0 | 0 | 0 | 13 | 5 | 13 | 0 | 0 | 0 |
| Melittin (200 μg/mL) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Melittin (400 μg/mL) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Socarras, K.M.; Theophilus, P.A.S.; Torres, J.P.; Gupta, K.; Sapi, E. Antimicrobial Activity of Bee Venom and Melittin against Borrelia burgdorferi. Antibiotics 2017, 6, 31. https://doi.org/10.3390/antibiotics6040031
Socarras KM, Theophilus PAS, Torres JP, Gupta K, Sapi E. Antimicrobial Activity of Bee Venom and Melittin against Borrelia burgdorferi. Antibiotics. 2017; 6(4):31. https://doi.org/10.3390/antibiotics6040031
Chicago/Turabian StyleSocarras, Kayla M., Priyanka A. S. Theophilus, Jason P. Torres, Khusali Gupta, and Eva Sapi. 2017. "Antimicrobial Activity of Bee Venom and Melittin against Borrelia burgdorferi" Antibiotics 6, no. 4: 31. https://doi.org/10.3390/antibiotics6040031
APA StyleSocarras, K. M., Theophilus, P. A. S., Torres, J. P., Gupta, K., & Sapi, E. (2017). Antimicrobial Activity of Bee Venom and Melittin against Borrelia burgdorferi. Antibiotics, 6(4), 31. https://doi.org/10.3390/antibiotics6040031







