A Risk Assessment of Antibiotic Pan-Drug-Resistance in the UK: Bayesian Analysis of an Expert Elicitation Study
Abstract
:1. Introduction
2. Results
2.1. Probability of Occurrence
2.2. Impact on Patients
2.2.1. Number of Bacteraemias
2.2.2. Mortality
2.2.3. Additional Length of Stay
2.2.4. High-Risk Patients
3. Discussion
4. Materials and Methods
4.1. Expert Panel and Remit
4.2. Risk Assessment
4.3. Expert Elicitation
4.4. Likelihood of the Scenario
4.5. Impact Assessment
4.6. Affected Patient Groups
4.7. Hospital Length of Stay
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- De Kraker, M.E.A.; Davey, P.G.; Grundmann, H. on behalf of the BURDEN study group. Mortality and hospital stay associated with resistant Staphylococcus aureus and Escherichia coli bacteremia: Estimating the burden of antibiotic resistance in Europe. PLoS Med. 2011, 8, e1001104. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control. Antimicrobial Resistance Surveillance in Europe 2014. Available online: http://ecdc.europa.eu/en/publications/_layouts/forms/Publication_DispForm.aspx?List=4f55ad51–4aed-4d32-b960-af70113dbb90&ID=1400 (accessed on 18 November 2016).
- Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. Available online: http://www.cdc.gov/drugresistance/threat-report-2013 (accessed on 18 November 2016).
- Australian Government. Responding to the Threat of Antimicrobial Resistance. Commonwealth of Australia, 2015. Available online: http://www.health.gov.au/internet/main/publishing.nsf/content/1803C433C71415CACA257C8400121B1F/$File/amr-strategy-2015–2019.pdf (accessed on 18 November 2016).
- WHO. The Evolving Threat of Antimicrobial Resistance. Options for Action. Available online: http://apps.who.int/iris/bitstream/10665/44812/1/9789241503181_eng.pdf (accessed on 18 November 2016).
- Davies, S.C. Annual Report of the Chief Medical Officer, Volume Two, 2011. Available online: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/138331/CMO_Annual_Report_Volume_2_2011.pdf (accessed on 18 November 2016).
- Department of Health. UK Five Year Antimicrobial Resistance Strategy 2013 to 2018. Available online: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/244058/20130902_UK_5_year_AMR_strategy.pdf (accessed on 18 November 2016).
- O’Hagan, A.; Buck, C.E.; Daneshkhah, A.; Eiser, J.R.; Garthwaite, P.H.; Jenkinson, D.J.; Oakley, J.E.; Rakow, T. Uncertain Judgements: Eliciting Experts’ Probabilities; John Wiley & Sons: Chichester, UK, 2006. [Google Scholar]
- Bernardo, J.M.; Smith, A.F.M. Bayesian Theory, 2nd ed.; John Wiley & Sons: Chicester, UK, 2000. [Google Scholar]
- Gelman, A.; Carlin, J.B.; Stern, H.S.; Dunson, D.B.; Vehtari, A.; Rubin, D.B. Bayesian Data Analysis, 3rd ed.; Chapman & Hall/CRC: Boca Raton, FL, USA, 2014. [Google Scholar]
- Kennedy, M.C.; Clough, H.E.; Turner, J. Case studies in Bayesian microbial risk assessment. Environ. Health 2009, 8. [Google Scholar] [CrossRef] [PubMed]
- The Global Economic Impact of Anti-Microbial Resistance. Available online: https://www.kpmg.com/UK/en/IssuesAndInsights/ArticlesPublications/Documents/PDF/Issues%20and%20Insights/amr-report-final.pdf (accessed on 18 November 2016).
- Estimating the Economic Costs of Antimicrobial Resistance. Model and Results. Available online: http://www.rand.org/content/dam/rand/pubs/research_reports/RR900/RR911/RAND_RR911.pdf (accessed on 18 November 2016).
- Livermore, D.M.; Hope, R.; Reynolds, R.; Blackburn, R.; Johnson, A.P.; Woodford, N. Declining cephalosporin and fluoroquinolone non-susceptibility among bloodstream Enterobacteriaceae from the UK: Links to prescribing change? J. Antimicrob. Chemother. 2013, 68, 2667–2674. [Google Scholar] [CrossRef] [PubMed]
- Giani, T.; Pini, B.; Arena, F.; Conte, V.; Bracco, S.; Migliavacca, R. Epidemic diffusion of KPC carbapenemase-producing Klebsiella pneumoniae in Italy: Results of the first countrywide survey, 15 May to 30 June 2011. Euro. Surveill. 2013, 30, 2–10. [Google Scholar]
- ECDC. Surveillance Atlas of Infectious Disease. Available online: http://atlas.ecdc.europa.eu/public/index.aspx?Instance=GeneralAtlas (accessed on 18 November 2016).
- Findlay, J.; Hopkins, K.L.; Doumith, M.; Meunier, D.; Wiuff, C.; Hill, R.; Pike, R.; Loy, R.; Mustafa, S.; Livermore, D.M.; et al. KPC enzymes in the UK: An analysis of the first 160 cases outside the North-West region. J. Antimicrob. Chemother. 2016, 71, 1199–1206. [Google Scholar] [CrossRef] [PubMed]
- Leavitt, A.; Chmelnitsky, I.; Carmeli, Y.; Navon-Venezia, S. Complete nucleotide sequence of KPC-3-encoding plasmid pKpQIL in the epidemic Klebsiella pneumoniae sequence type 258. Antimicrob. Agents Chemother. 2010, 54, 4493–4496. [Google Scholar] [CrossRef] [PubMed]
- Bonura, C.; Giuffrè, M.; Aleo, A.; Fasciana, T.; Di Bernardo, F.; Stampone, T.; Giammanco, A. An update of the evolving epidemic of blaKPC carrying Klebsiella pneumoniae in Sicily, Italy, 2014: Emergence of multiple non-ST258 clones. PLoS ONE 2015, 10, e0132936. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control/European Medicines Agency. The Bacterial Challenge: Time to React. European Centre for Disease Prevention and Control: Stockholm, 2009. Available online: http://ecdc.europa.eu/en/publications/Publications/0909_TER_The_Bacterial_Challenge_Time_to_React.pdf (accessed on 18 November 2016).
- Shallcross, L.J.; Davies, S.C. The World Health Assembly resolution on antimicrobial resistance. J. Antimicrob. Chemother. 2014, 69, 2883–2885. [Google Scholar] [CrossRef] [PubMed]
- WHO. High-Level Meeting on Antimicrobial Resistance. Available online: http://www.un.org/pga/71/2016/09/21/press-release-hl-meeting-on-antimicrobial-resistance/ (accessed on 18 November 2016).
- Oakley, J.E.; O’Hagan, A. SHELF: The Sheffield Elicitation Framework (version 2.0), School of Mathematics and Statistics, University of Sheffield, UK, 2010. Available online: http://tonyohagan.co.uk/shelf (accessed on 18 November 2016).
- Morris, D.E.; Oakley, J.E.; Crowe, J.A. A web-based tool for eliciting probability distributions from experts. Environ. Model. Softw. 2014, 52, 1–4. [Google Scholar] [CrossRef]
- British Society for Antimicrobial Chemotherapy Resistance Surveillance Project. Available online: http://www.bsacsurv.org/reports/bacteraemia (accessed on 18 November 2016).
- Hospital Episode Statistics: Admitted Patient Care, England—2012-13. Available online: http://content.digital.nhs.uk/catalogue/PUB12566/hosp-epis-stat-admi-summ-rep-2012–13-rep.pdf (accessed on 18 November 2016).
- Crowley, L.; Pitcher, D.; Wilson, J.; Guy, R.; Fluck, R. UK Renal Registry 16th Annual Report: Chapter 15 Epidemiology of Reported Infections amongst Patients Receiving Dialysis for Established Renal Failure in England from May 2011 to April 2012: A Joint Report from Public Health England and the UK Renal Registry. UK Renal Registry: Bristol, 2013. Available online: http://www.renalreg.com/Reports/2013.html (accessed on 18 November 2016).
- Public Health England. Surgical Site Infection Reports. Available online: http://webarchive.nationalarchives.gov.uk/20140722091854/http://www.hpa.org.uk/Publications/InfectiousDiseases/SurgicalSiteInfectionReports/ (accessed on 18 November 2016).
- Reuter, S.; Kern, W.V.; Sigge, A.; Dohner, H.; Marre, R.; Kerm, P.; von Baum, H. Impact of fluoroquinolone prophylaxis on reduced infection-related mortality among patients with neutropenia and hematologic malignancies. Clin. Infect. Dis. 2005, 40, 1087–1093. [Google Scholar] [CrossRef] [PubMed]
- Maraha, B.; Bonten, H.; van Hooff, H.; Fiolet, H.; Buiting, A.G.; Stobberingh, E.E. Infectious complications and antibiotic use in renal transplant recipients during a 1-year follow-up. Clin. Microbiol. Infect. 2001, 7, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Almallah, Y.Z.; Rennie, C.D.; Stone, J.; Lancashire, M.J. Urinary tract infection and patient satisfaction after flexible cystoscopy and urodynamic evaluation. Urology 2000, 56, 37–39. [Google Scholar] [CrossRef]
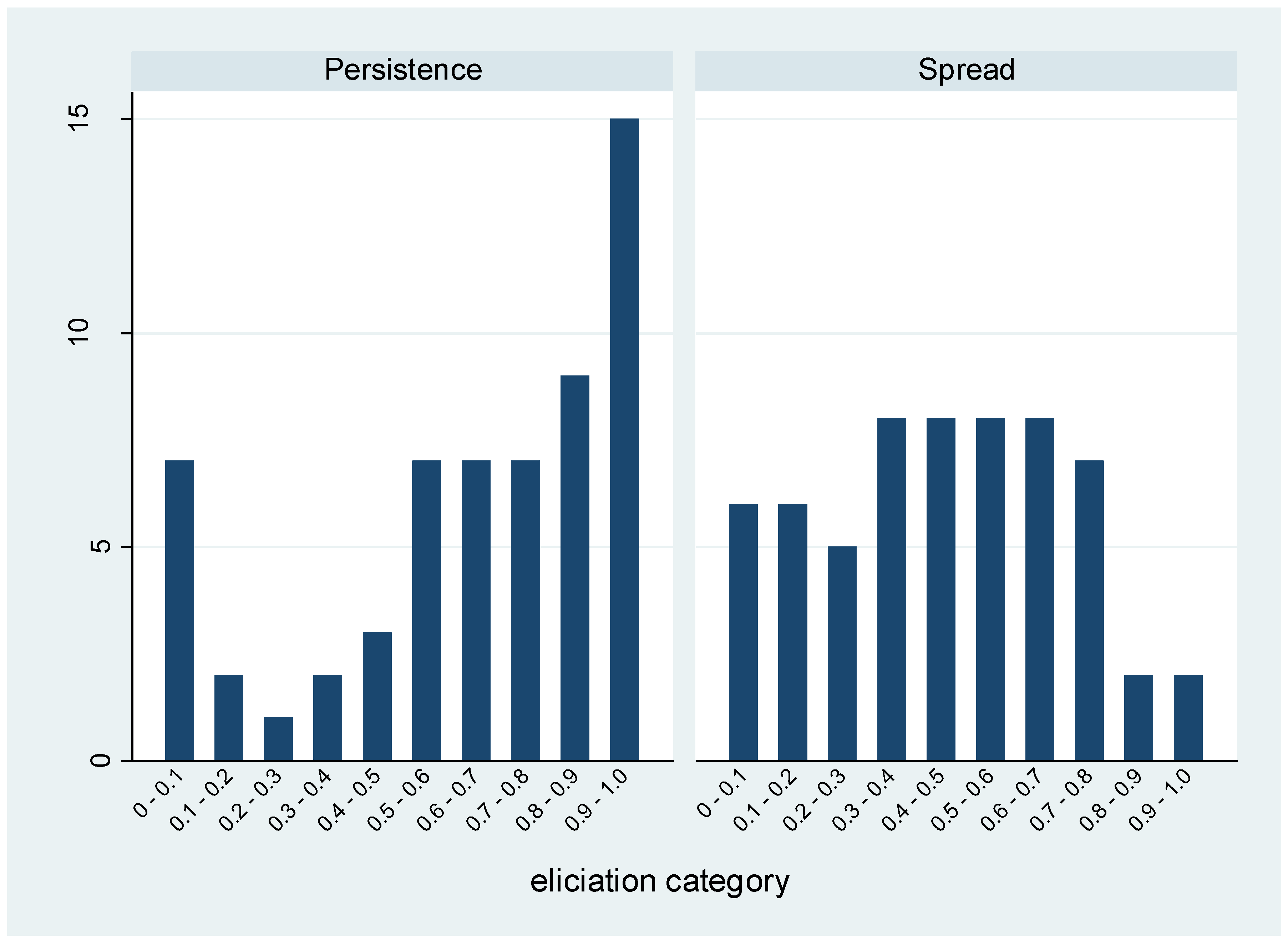
| Parameter | β Distribution | Percentiles | |||||
|---|---|---|---|---|---|---|---|
| α | β | Mean | Variance | 50 | 2.5 | 97.5 | |
| Persistence | 2.96 | 0.99 | 0.75 | 0.04 | 0.79 | 0.29 | 0.99 |
| Spread | 1.46 | 1.72 | 0.46 | 0.06 | 0.45 | 0.05 | 0.91 |
| Year | Median | 95% CrI | Median | 95% CrI |
|---|---|---|---|---|
| 1 | 1200 | 70–7400 | 1200 | 70–7400 |
| 5 | 6800 | 400–58,600 | 19,600 | 1100–158,000 |
| 10 | 14,300 | 800–114,000 | 77,800 | 4400–614,000 |
| 20 | 22,800 | 1500–160,000 | 283,700 | 17,000–1,989,000 |
| Year of Scenario | Annual | Cumulative | ||
|---|---|---|---|---|
| Median | 95% CrI | Median | 95% CrI | |
| 1 | 300 | 0–3100 | 300 | 0–3100 |
| 5 | 1900 | 0–23,000 | 5500 | 0–63,000 |
| 10 | 4100 | 0–47,000 | 22,000 | 0–248,000 |
| 20 | 6400 | 0–64,000 | 79,000 | 0–821,000 |
| Year of Scenario | Annual | Cumulative | ||
|---|---|---|---|---|
| Median | 95% CrI | Median | 95% CrI | |
| 1 | 10,000 | 500–119,000 | 10,000 | 500–119,100 |
| 5 | 60,000 | 2600–875,000 | 170,000 | 8000–2,400,000 |
| 10 | 124,000 | 5500–1,730,000 | 676,000 | 30,000–9,500,000 |
| 20 | 195,000 | 10,000–2,400,000 | 2,440,000 | 120,000–31,900,000 |
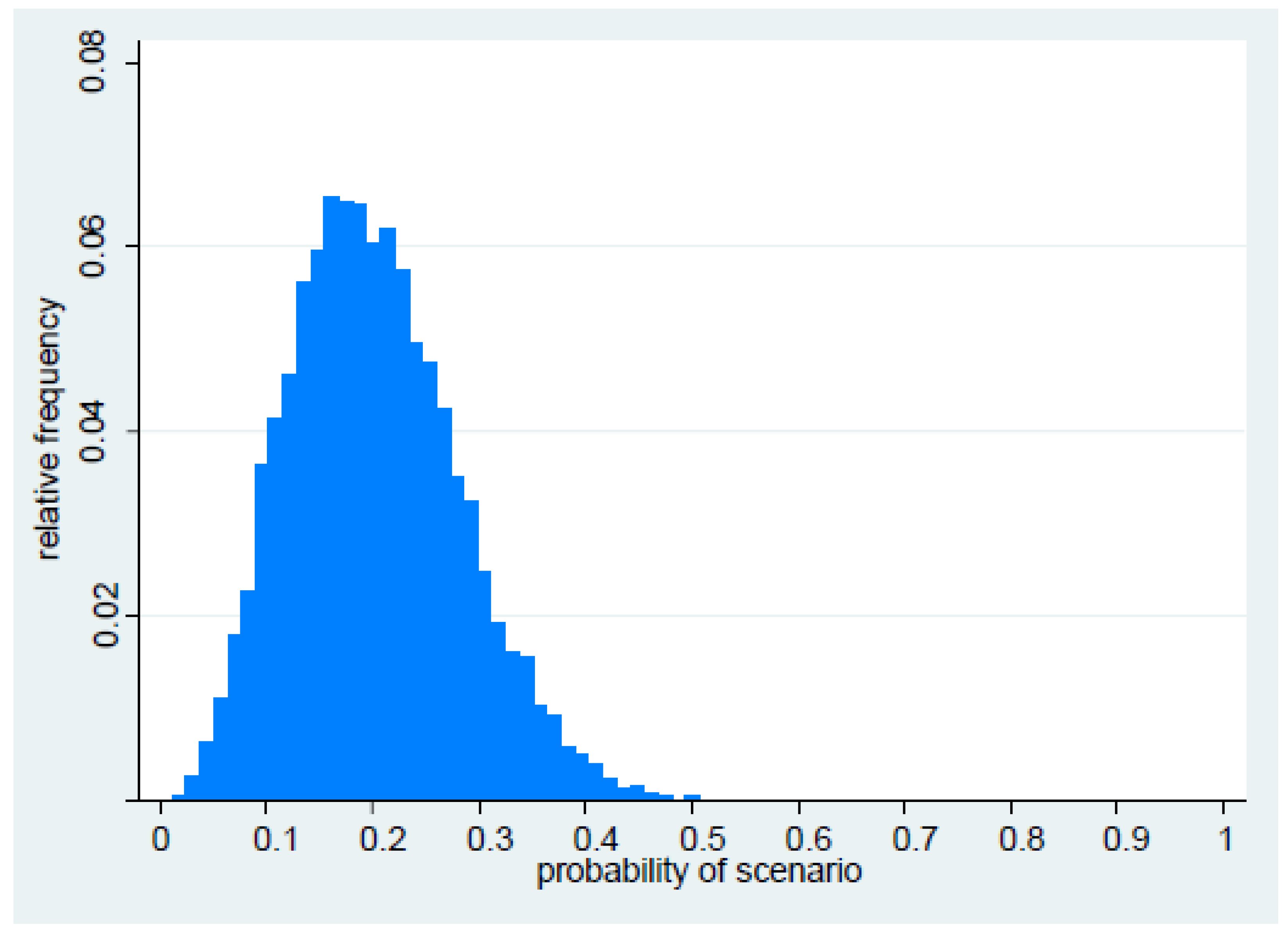
| Parameter 1: What is the probability that PDR (resulting in loss of susceptibility to all remaining drug classes) in Gram-negative organisms will emerge in or enter the UK within the next five years (i.e., by 2019)? |
| Parameter 2: In the UK, what proportion of drug class-bug resistance patterns become established, such that they persist over time? |
| Parameter 3: In the UK, what proportion of established drug class-bug resistance patterns go on to become widespread? |
| Parameter 4: What is the overall probability that PDR will emerge in or enter the UK within the next five years, and become established and widespread? |
| Parameter 5: During the scenario, what peak proportion of Gram-negative isolates will demonstrate PDR? |
| Parameter 6: How many years will elapse from the emergence of PDR, until the peak proportion is reached? |
| Parameter 7: What cumulative number of PDR Gram-negative bacteraemia will occur during the first five years of the scenario (i.e., 2016–2020)? |
| Parameter 8: What is the odds ratio for 30-day mortality amongst patients with PDR Gram-negative bacteraemia compared to similar patients with no infection? |
| Parameter 9: By how many days is length of stay (LoS) greater amongst patients with PDR Gram-negative bacteraemia compared to similar patients with no infection? |
| Parameter 10: Amongst various potential trajectories for the epidemic curve of PDR Gram-negative bacteraemia (defined in terms of peak prevalence, time to peak prevalence, and the presence or absence of a decline once the peak prevalence is reached), which is considered by the Expert Panel to be the most plausible? |
| Parameter 11: In addition, panel members were asked to describe the trajectory by which the baseline number of Gram-negative bacteraemias (i.e., non-PDR Gram-negative bacteraemia) may be expected to change over time, to 2035. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carter, D.; Charlett, A.; Conti, S.; Robotham, J.V.; Johnson, A.P.; Livermore, D.M.; Fowler, T.; Sharland, M.; Hopkins, S.; Woodford, N.; et al. A Risk Assessment of Antibiotic Pan-Drug-Resistance in the UK: Bayesian Analysis of an Expert Elicitation Study. Antibiotics 2017, 6, 9. https://doi.org/10.3390/antibiotics6010009
Carter D, Charlett A, Conti S, Robotham JV, Johnson AP, Livermore DM, Fowler T, Sharland M, Hopkins S, Woodford N, et al. A Risk Assessment of Antibiotic Pan-Drug-Resistance in the UK: Bayesian Analysis of an Expert Elicitation Study. Antibiotics. 2017; 6(1):9. https://doi.org/10.3390/antibiotics6010009
Chicago/Turabian StyleCarter, Daniel, André Charlett, Stefano Conti, Julie V. Robotham, Alan P. Johnson, David M. Livermore, Tom Fowler, Mike Sharland, Susan Hopkins, Neil Woodford, and et al. 2017. "A Risk Assessment of Antibiotic Pan-Drug-Resistance in the UK: Bayesian Analysis of an Expert Elicitation Study" Antibiotics 6, no. 1: 9. https://doi.org/10.3390/antibiotics6010009
APA StyleCarter, D., Charlett, A., Conti, S., Robotham, J. V., Johnson, A. P., Livermore, D. M., Fowler, T., Sharland, M., Hopkins, S., Woodford, N., Burgess, P., & Dobra, S. (2017). A Risk Assessment of Antibiotic Pan-Drug-Resistance in the UK: Bayesian Analysis of an Expert Elicitation Study. Antibiotics, 6(1), 9. https://doi.org/10.3390/antibiotics6010009






