Moxifloxacin Increases Heart Rate in Humans
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Basudde, C.D. Clinical signs and biochemical changes in calves caused by injection of ivermectin. Vet. Q. 1989, 11, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Colbert, W.E.; Turk, J.A.; Williams, P.D.; Buening, M.K. Cardiovascular and autonomic pharmacology of the macrolide antibiotic ly281389 in anesthetized beagles and in isolated smooth and cardiac muscles. Antimicrob. Agents Chemother. 1991, 35, 1365–1369. [Google Scholar] [CrossRef] [PubMed]
- De Lima, J.J.; Xue, H.; Coburn, L.; Andoh, T.F.; McCarron, D.A.; Bennett, W.M.; Roullet, J.B. Effects of fk506 in rat and human resistance arteries. Kidney Int. 1999, 55, 1518–1527. [Google Scholar] [CrossRef] [PubMed]
- Main, B.W.; Means, J.R.; Rinkema, L.E.; Smith, W.C.; Sarazan, R.D. Cardiovascular effects of the macrolide antibiotic tilmicosin, administered alone and in combination with propranolol or dobutamine, in conscious unrestrained dogs. J. Vet. Pharmacol. Ther. 1996, 19, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Ozkanlar, Y.; Nishijima, Y.; da Cunha, D.; Hamlin, R.L. Acute effects of tacrolimus (fk506) on left ventricular mechanics. Pharmacol. Res. 2005, 52, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Tamargo, J.; de Miguel, B.; Tejerina, M.T. A comparison of josamycin with macrolides and related antibiotics on isolated rat atria. Eur. J. Pharmacol. 1982, 80, 285–293. [Google Scholar] [CrossRef]
- Kim, E.J.; Shin, W.H.; Kim, K.S.; Han, S.S. Safety pharmacology of dw-224a, a novel fluoroquinolone antibiotic agent. Drug Chem. Toxicol. 2004, 27, 295–307. [Google Scholar] [CrossRef] [PubMed]
- Basyigit, I.; Kahraman, G.; Ilgazli, A.; Yildiz, F.; Boyaci, H. The effects of levofloxacin on ECG parameters and late potentials. Am. J. Ther. 2005, 12, 407–410. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Friedman, H.S.; Sinha, A.K. The effects of erythromycin on the electrocardiogram. Chest 1999, 115, 983–986. [Google Scholar] [CrossRef] [PubMed]
- Keating, G.M.; Scott, L.J. Moxifloxacin: A review of its use in the management of bacterial infections. Drugs 2004, 64, 2347–2377. [Google Scholar] [CrossRef] [PubMed]
- Bloomfield, D.M.; Kost, J.T.; Ghosh, K.; Hreniuk, D.; Hickey, L.A.; Guitierrez, M.J.; Gottesdiener, K.; Wagner, J.A. The effect of moxifloxacin on QTc and implications for the design of thorough QT studies. Clin. Pharmacol. Ther. 2008, 84, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.K.; Zhang, J.; Ng, M.J.; Dang, Q. Statistical characteristics of moxifloxacin-induced QTc effect. J. Biopharm. Stat. 2010, 20, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration. Guidance for Industry: E14 Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential for Non-Antiarhythmic Drugs. Avaliable online: http://www.Fda.Gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm073153.Pdf (accessed on 29 January 2015).
- Mason, J.W.; Florian, J.A., Jr.; Garnett, C.E.; Moon, T.E.; Selness, D.S.; Spaulding, R.R. Pharmacokinetics and pharmacodynamics of three moxifloxacin dosage forms: Implications for blinding in active-controlled cardiac repolarization studies. J. Clin. Pharmacol. 2010, 50, 1249–1259. [Google Scholar] [CrossRef] [PubMed]
- Fridericia, L.S. The duration of systole in the electrocardiogram of normal subjects and of patients with heart disease. Acta Med. Scand. 1920, 53, 469–486. [Google Scholar] [CrossRef]
- A Thorough QT Study to Define the ECG Effects of Cethromycin Using a Clinal and a Suprtherpeutic Dose Compared to Placebo and Moxifloxacin in Healthy Subjects. Avaliable online: https://idsa.confex.com/idsa/2008/webprogram/Paper25062.html (accessed on 22 August 2016).
- Noel, G.J.; Goodman, D.B.; Chien, S.; Solanki, B.; Padmanabhan, M.; Natarajan, J. Measuring the effects of supratherapeutic doses of levofloxacin on healthy volunteers using four methods of qt correction and periodic and continuous ecg recordings. J. Clin. Pharmacol. 2004, 44, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Electrocardiographic Effects of WCK 2349. Avaliable online: https://www.escmid.org/escmid_publications/escmid_elibrary/?q=mason&tx_solr%5Bfilter%5D%5B0%5D=author%253AJay%2BMason (accessed on 22 August 2016).
- Solithromycin, a 4th Generation Macrolide and the 1st Fluoroketolide, Does Not Prolong the QTC Interval: Results of a Definitive QT Study. Avaliable online: https://www.escmid.org/escmid_publications/escmid_elibrary/?q=darpo&id=2173&L=0&tx_solr%5Bfilter%5D%5B0%5D=main_filter_eccmid%253Atrue&tx_solr%5Bfilter%5D%5B1%5D=pub_date%253A201501010000–201512312359&x=17&y=20 (accessed on 25 August 2016).
- Emea. Ketek Scientific Discussion—Product Information. (page 20). Avaliable online: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Scientific_Discussion/human/000354/WC500041893.pdf (accessed on 11 November 2016).
- Cheng, Y.J.; Nie, X.Y.; Chen, X.M.; Lin, X.X.; Tang, K.; Zeng, W.T.; Mei, W.Y.; Liu, L.J.; Long, M.; Yao, F.J.; et al. The role of macrolide antibiotics in increasing cardiovascular risk. J. Am. Coll Cardiol. 2015, 66, 2173–2184. [Google Scholar] [CrossRef] [PubMed]
- Ray, W.A.; Murray, K.T.; Meredith, S.; Narasimhulu, S.S.; Hall, K.; Stein, C.M. Oral erythromycin and the risk of sudden death from cardiac causes. N. Engl. J. Med. 2004, 351, 1089–1096. [Google Scholar] [CrossRef] [PubMed]
- Haverkamp, W.; Kruesmann, F.; Fritsch, A.; van Veenhuyzen, D.; Arvis, P. Update on the cardiac safety of moxifloxacin. Curr. Drug Saf. 2012, 7, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Wisialowski, T.; Crimin, K.; Engtrakul, J.; O′Donnell, J.; Fermini, B.; Fossa, A.A. Differentiation of arrhythmia risk of the antibacterials moxifloxacin, erythromycin, and telithromycin based on analysis of monophasic action potential duration alternans and cardiac instability. J. Pharmacol. Exp. Ther. 2006, 318, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Shimokawa, A.; Kunitake, T.; Takasaki, M.; Kannan, H. Differential effects of anesthetics on sympathetic nerve activity and arterial baroreceptor reflex in chronically instrumented rats. J. Auton. Nerv. Syst. 1998, 72, 46–54. [Google Scholar] [CrossRef]
- Herrmann, S.; Layh, B.; Ludwig, A. Novel insights into the distribution of cardiac HCN channels: An expression study in the mouse heart. J. Mol. Cell. Cardiol. 2011, 51, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Verrier, R.L.; Bonatti, R.; Silva, A.F.; Batatinha, J.A.; Nearing, B.D.; Liu, G.; Rajamani, S.; Zeng, D.; Belardinelli, L. If inhibition in the atrioventricular node by ivabradine causes rate-dependent slowing of conduction and reduces ventricular rate during atrial fibrillation. Heart Rhythm 2014, 11, 2288–2296. [Google Scholar] [CrossRef] [PubMed]
- Dogan, A.; Tunc, E.; Varol, E.; Ozaydin, M.; Ozturk, M. Comparison of the four formulas of adjusting QT interval for the heart rate in the middle-aged healthy turkish men. Ann. Noninvasive Electrocardiol. 2005, 10, 134–141. [Google Scholar] [CrossRef] [PubMed]
- E14 Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential for Non-Antiarrhythmic Drugs—Questions and Answers (r1). Avaliable online: http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm073153.pdf (accessed on 29 January 2015).
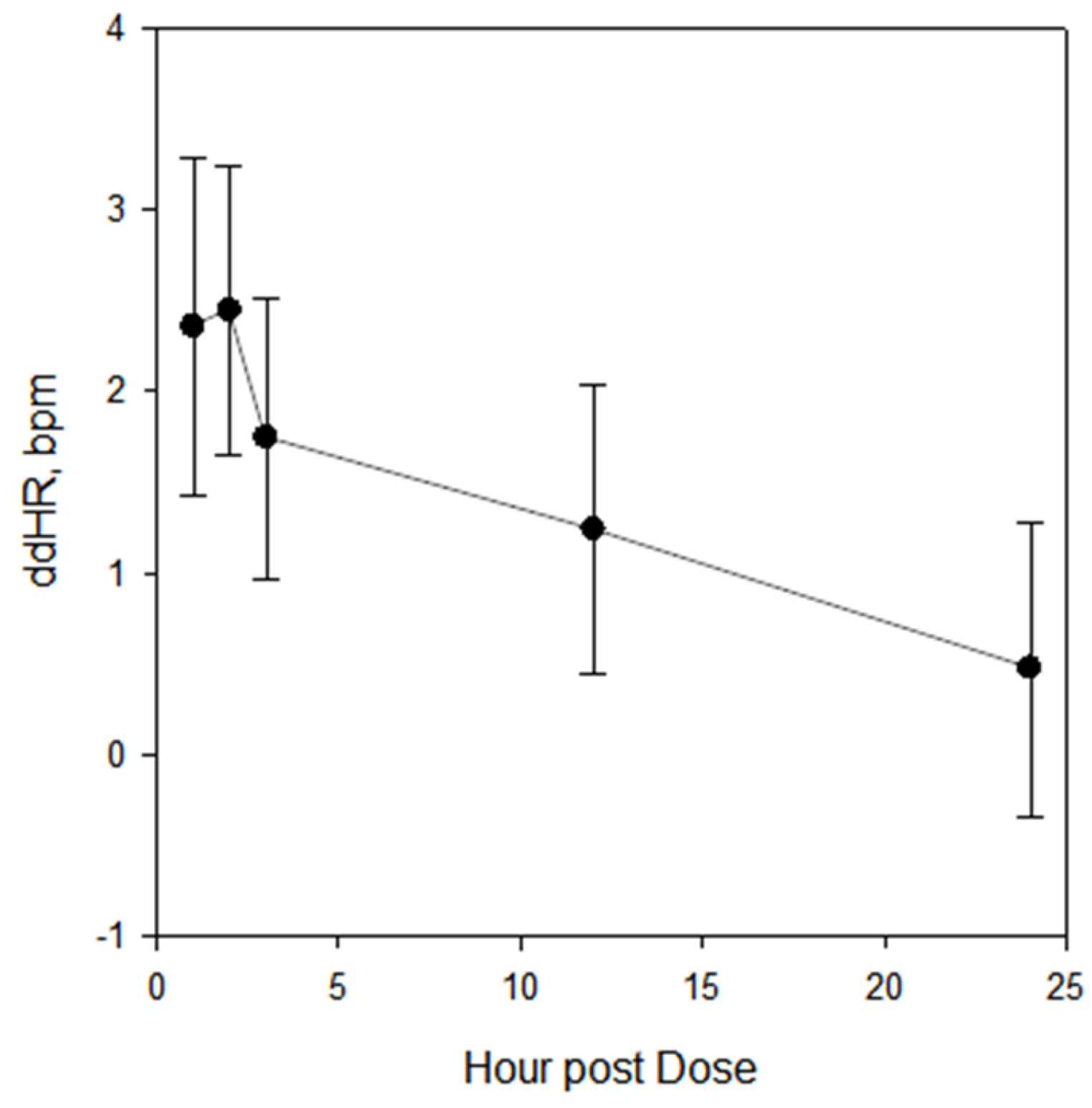
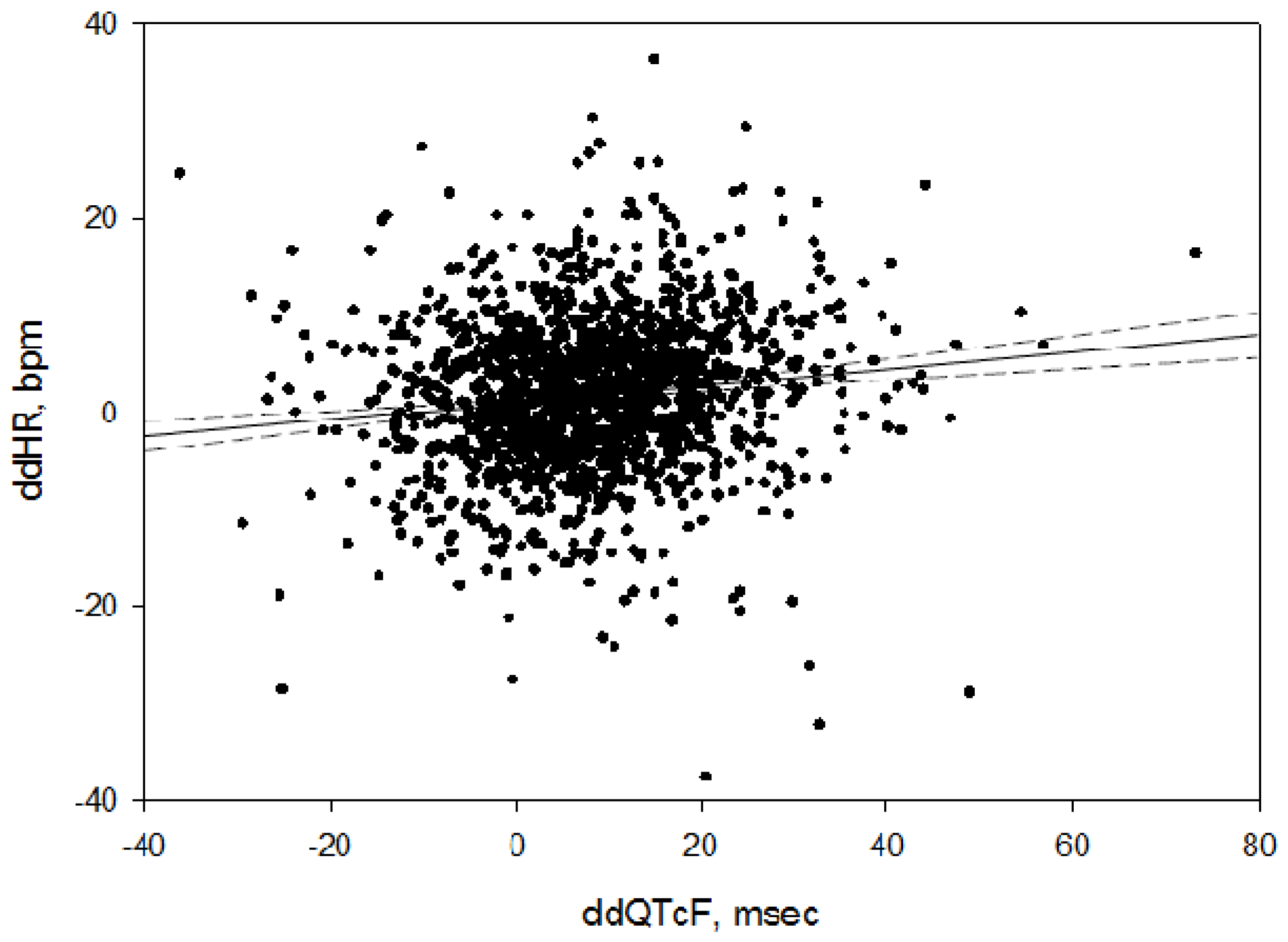
| Study | N Subjects | Mean Age (Years) | Sex (% M) | Mean Baseline Pre-Dose HR | Treatments |
|---|---|---|---|---|---|
| 1 | 50 | 31 ± 8.7 | 60 | 61.8 ± 9.2 | Placebo, moxifloxacin, study drug dose 1, study drug dose 2 |
| 2 | 49 | 31 ± 9.0 | 57 | 57.7 ± 9.1 | Placebo, moxifloxacin, study drug dose 1, study drug dose 2 |
| 3 | 53 | 30 ± 8.0 | 42 | 63.6 ± 9.8 | Placebo, moxifloxacin, study drug |
| 4 | 48 | 36 ± 9.1 | 50 | 59.8 ± 8.6 | Placebo, moxifloxacin, study drug dose 1, study drug dose 2 |
| 6 | 47 | 36 ± 9.7 | 67 | 60.4 ± 9.4 | Placebo, moxifloxacin, study drug |
| 5 | 41 | 32 ± 8.2 | 63 | 64.5 ± 9.6 | Placebo, moxifloxacin, study drug |
| 7 | 47 | 32 ± 10.0 | 64 | 60.0 ± 8.4 | Placebo, moxifloxacin, study drug dose 1, study drug dose 2 |
| Study | Time Point, Hour | Unadjusted (bpm) | Mixed Model (bpm) | ||
|---|---|---|---|---|---|
| ddHR | 95% CI | ddHR | 95% CI | ||
| All | 1 | 2.4 | 1.5, 3.2 | 2.4 | 1.6, 3.2 |
| 2 | 2.4 | 1.6, 3.3 | 2.5 | 1.7, 3.3 | |
| 3 | 1.7 | 0.9, 2.6 | 1.8 | 1.0, 2.6 | |
| 12 | 1.2 | 0.4, 2.1 | 1.3 | 0.4, 2.1 | |
| 24 | 0.5 | −0.4, 1.3 | 0.5 | −0.3, 1.3 | |
| 1 | 1 | 2.1 | 0.3, 4.0 | 2.2 | 0.4, 4.1 |
| 2 | 0.8 | −1.1, 2.6 | 0.9 | −1.0, 2.8 | |
| 3 | 1.0 | −0.8, 2.9 | 1.1 | −0.8, 3.0 | |
| 12 | −0.0 | −1.9, 1.9 | 0.1 | −1.8, 2.0 | |
| 24 | −0.7 | −2.5, 1.2 | −0.6 | −2.5, 1.3 | |
| 2 | 1 | 2.5 | 0.1, 4.9 | 2.4 | −0.0, 4.8 |
| 2 | 2.4 | −0.0, 4.8 | 2.3 | −0.1, 4.7 | |
| 3 | 1.7 | −0.7, 4.1 | 1.6 | −0.8, 4.0 | |
| 12 | 1.5 | −0.9, 3.9 | 1.4 | −1.0, 3.8 | |
| 24 | 0.5 | −1.9, 2.9 | 0.4 | −2.0, 2.8 | |
| 3 | 1 | 1.0 | −1.5, 3.5 | 1.0 | −1.6, 3.5 |
| 2 | 3.2 | 0.7, 5.7 | 3.2 | 0.6, 5.7 | |
| 3 | 2.5 | 0.1, 5.0 | 2.5 | −0.0, 5.1 | |
| 12 | 0.6 | −1.9, 3.1 | 0.6 | −2.0, 3.1 | |
| 24 | 1.3 | −1.2, 3.8 | 1.3 | −1.3, 3.8 | |
| 4 | 1 | 4.3 | 2.6, 6.0 | 4.3 | 2.7, 6.0 |
| 2 | 3.6 | 1.9, 5.3 | 3.6 | 2.0, 5.3 | |
| 3 | 2.9 | 1.2, 4.6 | 2.9 | 1.2, 4.5 | |
| 12 | 3.5 | 1.8, 5.2 | 3.5 | 1.8, 5.1 | |
| 24 | 1.7 | 0.0, 3.4 | 1.7 | 0.1, 3.4 | |
| 5 | 1 | 2.1 | −0.1, 4.3 | 2.0 | −0.3, 4.3 |
| 2 | 3.0 | 0.8, 5.2 | 2.9 | 0.6, 5.3 | |
| 3 | 1.4 | −0.8, 3.6 | 1.3 | −1.0, 3.6 | |
| 12 | 1.1 | −1.1, 3.3 | 1.0 | −1.3, 3.3 | |
| 24 | −1.0 | −3.2, 1.2 | −1.3 | −3.7, 1.0 | |
| 6 | 1 | 2.4 | 0.3, 4.5 | 2.7 | 0.5, 4.9 |
| 2 | 1.5 | −0.7, 3.6 | 1.8 | −0.4, 3.9 | |
| 3 | 0.3 | −1.8, 2.4 | 0.6 | −1.5, 2.8 | |
| 12 | 0.7 | −1.4, 2.9 | 1.0 | −1.1, 3.2 | |
| 24 | 0.0 | −2.1, 2.1 | 0.3 | −1.9, 2.5 | |
| 7 | 1 | 2.3 | −0.1, 4.6 | 2.5 | 0.0, 5.0 |
| 2 | 2.5 | 0.2, 4.8 | 2.8 | 0.3, 5.2 | |
| 3 | 2.1 | −0.2, 4.5 | 2.4 | −0.1, 4.9 | |
| 12 | 1.3 | −1.0, 3.6 | 1.6 | −0.9, 4.0 | |
| 24 | 1.3 | −1.0, 3.6 | 1.6 | −0.9, 4.1 | |
| Drug | Mean Maximum HR Increase | Dose | Antibiotic Class | Comment |
|---|---|---|---|---|
| Cethromycin [16] | 4.4 bpm 11.3 bpm | 300 mg po 900 mg po | Macrolide (ketolide) | Not approved |
| Erythromycin [9] | 4 bpm | 500 mg iv | Macrolide | Approved |
| Levofloxacin [17] | 8 bpm | 1500 mg po | Fluoroquinolone | Approved |
| Levonadifloxacin [18] | 15 bpm | 2600 mg po | Fluoroquinolone | In development |
| Moxifloxacin (this study) | 2.4 bpm | 400 mg po | Fluoroquinolone | Approved |
| Solithromycin [19] | 15 bpm | 800 mg iv | Macrolide (ketolide) | In development |
| Telithromycin [20] | 13 bpm | 3200 mg | Macrolide (ketolide) | Approved |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mason, J.W.; Moon, T.E. Moxifloxacin Increases Heart Rate in Humans. Antibiotics 2017, 6, 5. https://doi.org/10.3390/antibiotics6010005
Mason JW, Moon TE. Moxifloxacin Increases Heart Rate in Humans. Antibiotics. 2017; 6(1):5. https://doi.org/10.3390/antibiotics6010005
Chicago/Turabian StyleMason, Jay W., and Thomas E. Moon. 2017. "Moxifloxacin Increases Heart Rate in Humans" Antibiotics 6, no. 1: 5. https://doi.org/10.3390/antibiotics6010005
APA StyleMason, J. W., & Moon, T. E. (2017). Moxifloxacin Increases Heart Rate in Humans. Antibiotics, 6(1), 5. https://doi.org/10.3390/antibiotics6010005






