Pharmacokinetic/Toxicity Properties of the New Anti-Staphylococcal Lead Compound SK-03-92
Abstract
:1. Introduction
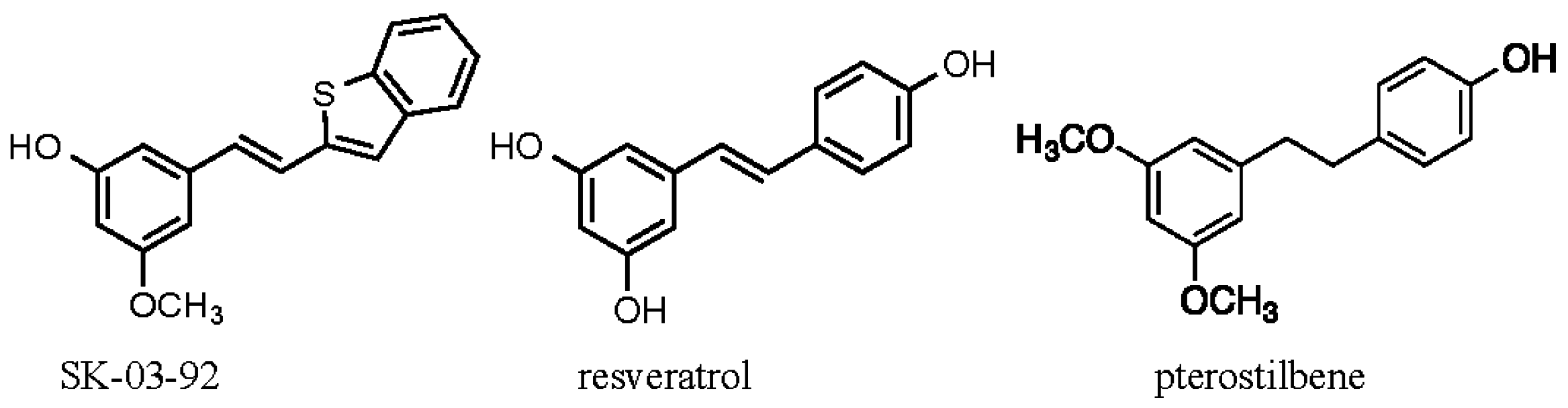
2. Results and Discussion
2.1. Physical Properties of SK-03-92
2.2. Determining the Solubility of SK-03-92 in Other Solutions
2.3. Safety/Cytotoxicity Testing of SK-03-92
2.4. Single Dose PK Analysis of SK-03-92
| Route | Half-Life (min) | Tmax c (min) | Cmax c (μg/mL) | Cl_F_obs c (mL/min/g) | Vz_F_obs c (mL/g) | AUCINF_obs c (min·μg/mL) |
|---|---|---|---|---|---|---|
| ip a | 22.46 ± 17.81 | 30.00 ± 0.00 | 1.64 ± 0.59 | 1.46 ± 0.26 | 47.71 ± 33.25 | 70.15 ± 12.76 |
| oral b | 30.40 ± 17.81 | 21.43 ± 11.80 | 0.37 ± 0.35 | 21.00 ± 6.71 | 810.67 ± 173.87 | 5.14 ± 1.87 |
2.5. Relative Bioavailability of SK-03-92
2.6. Multi-Dose Effects and Protein Analyses of SK-03-92
2.7. Protein Binding by SK-03-92
3. Experimental Section
3.1. Synthesis of SK-03-92
3.2. Physical Property Assessment
3.3. Mice
3.4. Cytotoxicity Assays
3.5. Initial Safety Testing in Mice
3.6. HPLC Assay Development
3.7. Single Dose PK Assay
3.8. Bioavailability Assay
3.9. Multi-Dose Analysis
3.10. Protein Binding Assay
4. Conclusions
Acknowledgments
Author Contributions
Conflict of Interest
References
- Suaya, J.A.; Mera, R.M.; Cassidy, A.; O’Hara, P.; Amrine-Madsen, H.; Burstin, S.; Miller, L.G. Incidence and cost of hospitalizations associated with Staphylococcus aureus skin and soft tissue infections in the United States from 2001 to 2009. BMC Infect. Dis. 2014, 14. [Google Scholar] [CrossRef] [PubMed]
- Kallen, A.J.; Mu, Y.; Bulens, S.; Reingold, A.; Petit, S.; Gershman, K.; Ray, S.M.; Harrison, L.H.; Lynfield, R.; Dumyati, G.; et al. Active Bacterial Core surveillance (ABCs) MRSA Investigators of the Emerging Infections Program. Health care-associated invasive MRSA infections, 2005–2008. JAMA 2010, 304, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Stryjewski, M.E.; Corey, G.R. Methicillin-resistant Staphylococcus aureus: An evolving pathogen. Clin. Infect. Dis. 2014, 58 (Suppl. S1), S10–S19. [Google Scholar] [CrossRef] [PubMed]
- Infectious Diseases Society of America. The 10 × '20 initiative: Pursuing a global commitment to develop 10 new antibacterial drugs by 2020. Clin. Infect. Dis. 2010, 50, 1081–1083. [Google Scholar]
- Kabir, M.S.; Engelbrecht, K.; Polanowski, R.; Krueger, S.M.; Ignasiak, R.; Rott, M.; Schwan, W.R.; Stemper, M.E.; Reed, K.D.; Sherman, D.; et al. New classes of Gram-positive selective antibacterials: Inhibitors of MRSA and surrogates of the causative agents of anthrax and tuberculosis. Bioorg. Med. Chem. Lett. 2010, 18, 5745–5749. [Google Scholar] [CrossRef] [PubMed]
- Schwan, W.R.; Kabir, M.S.; Kallaus, M.; Krueger, S.; Monte, A.; Cook, J.M. Synthesis and minimum inhibitory concentrations of SK-03-92 against Staphylococcus aureus and other gram-positive bacteria. J. Infect. Chemother. 2012, 18, 124–126. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Lin, H.-S.; Ho, P.C.; Ng, K.-Y. The impact of aqueous solubility and dose on the pharmacokinetic profile of resveratrol. Pharm. Res. 2008, 25, 2593–2600. [Google Scholar] [CrossRef] [PubMed]
- Strickley, D.G. Solubilizing excipients in oral and injectable formulations. Pharm. Res. 2004, 21, 201–230. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- OECD. Acute Oral Toxicity—Acute Toxic Class Method. In OECD Guidelines for the Testing of Chemicals; Organisation for Economic Cooperation and Development: Paris, France, 2001. [Google Scholar]
- Ruiz, M.J.; Fernandez, M.; Pico, Y.; Manes, J.; Asensi, M.; Carda, C.; Asensio, G.; Estrela, J.M. Dietary administration of high doses of pterostilbene and quercetin to mice is not toxic. J. Agric. Food Chem. 2009, 57, 3180–3186. [Google Scholar] [CrossRef] [PubMed]
- Johnson, W.D.; Morrissey, R.L.; Usborne, A.L.; Kapetanovic, I.; Crowell, J.A.; Muzzio, M.; McCormick, D.L. Subchronic oral toxicity and cardiovascular safety pharmacology studies of resveratrol, a naturally occurring polyphenol with cancer preventive activity. Food Chem. Toxicol. 2011, 49, 3319–3327. [Google Scholar] [CrossRef] [PubMed]
- Walle, T.; Hsieh, F.; DeLegge, M.H.; Oatis, J.E., Jr.; Walle, U.K. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab. Dispos. 2004, 32, 1377–1382. [Google Scholar] [CrossRef] [PubMed]
- Martinez, S.E.; Sayre, C.K.; Davies, N.M. Pharmacometrics of 3-methoxypterostilbene: A component of traditional Chinese medicinal plants. Evid. Based Complement. Alternat. Med. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Kapetanovic, I.M.; Muzzio, M.; Huang, Z.; Thompson, T.N.; McCormick, D.L. Pharmacokinetics, oral bioavailability, and metabolic profile of resveratrol and its dimethylether analog, pterostilbene, in rats. Cancer Chemother. Pharmacol. 2011, 68, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Yeo, S.C.M.; Ho, P.; Lin, H.-S. Pharmacokinetics of pterostilbene in Sprague-Dawley rats: The impacts of aqueous solubility, fasting, dose escalation, and dosing route on bioavailability. Mol. Nutr. Food Res. 2013, 57, 1015–1025. [Google Scholar] [CrossRef] [PubMed]
- Asensi, M.; Medina, I.; Ortega, A.; Carretero, J.; Bano, M.C.; Obrador, E.; Estrela, J.M. Inhibition of cancer growth by reservatrol is related to its low bioavailability. Free Radic. Biol. Med. 2002, 33, 387–398. [Google Scholar] [CrossRef]
- Lin, H.-S.; Spatafora, C.; Tringali, C.; Ho, P.C. Determination of trans-2,4,3′,4′,5′-pentamethoxystilbene in rat plasma and its application to a pharmacokinetic study. J. Pharm. Biomed. Anal. 2012, 57, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.-S.; Ho, P.C. Preclinical pharmacokinetic evaluation of resveratrol trimethyl ether in Sprague-Dawley rats: The impacts of aqueous solubility. Dose escalation, food and repeated dosing on oral bioavailability. J. Pharm. Sci. 2011, 100, 4491–4500. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.S.; Sviripa, V.M.; Watt, D.S.; Liu, C.; Xiang, T.X.; Anderson, B.D.; Ong, P.S.; Ho, P.C. Quantification of trans-2,6-difluoro-4′-N,N-dimethylaminostilbene in rat plasma: Application to a pharmacokinetic study. J. Pharm. Biomed. Anal. 2013, 72, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Bethune, S.J.; Schultheiss, N.; Henck, J.-O. Improving the poor aqueous solubility of nutraceutical compound pterostilbene through cocrystal formation. Cryst. Growth Des. 2011, 11, 2817–2823. [Google Scholar] [CrossRef]
- Shin, H.C.; Cho, H.; Lai, T.C.; Kozak, K.R.; Kolesar, J.M.; Kwon, G.S. Pharmacokinetic study of 3-in-1 poly(ethylene glycol)-block-poly(d,l-lactic acid) micelles carrying paclitaxel, 17-allylamino-17-demethoxygeldanamycin, and rapamycin. J. Control. Release 2012, 163, 93–99. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schwan, W.R.; Kolesar, J.M.; Kabir, M.S.; Elder, E.J.; Williams, J.B.; Minerath, R.; Cook, J.M.; Witzigmann, C.M.; Monte, A.; Flaherty, T. Pharmacokinetic/Toxicity Properties of the New Anti-Staphylococcal Lead Compound SK-03-92. Antibiotics 2015, 4, 617-626. https://doi.org/10.3390/antibiotics4040617
Schwan WR, Kolesar JM, Kabir MS, Elder EJ, Williams JB, Minerath R, Cook JM, Witzigmann CM, Monte A, Flaherty T. Pharmacokinetic/Toxicity Properties of the New Anti-Staphylococcal Lead Compound SK-03-92. Antibiotics. 2015; 4(4):617-626. https://doi.org/10.3390/antibiotics4040617
Chicago/Turabian StyleSchwan, William R., Jill M. Kolesar, M. Shahjahan Kabir, Edmund J. Elder, Jeffrey B. Williams, Rachel Minerath, James M. Cook, Christopher M. Witzigmann, Aaron Monte, and Tricia Flaherty. 2015. "Pharmacokinetic/Toxicity Properties of the New Anti-Staphylococcal Lead Compound SK-03-92" Antibiotics 4, no. 4: 617-626. https://doi.org/10.3390/antibiotics4040617
APA StyleSchwan, W. R., Kolesar, J. M., Kabir, M. S., Elder, E. J., Williams, J. B., Minerath, R., Cook, J. M., Witzigmann, C. M., Monte, A., & Flaherty, T. (2015). Pharmacokinetic/Toxicity Properties of the New Anti-Staphylococcal Lead Compound SK-03-92. Antibiotics, 4(4), 617-626. https://doi.org/10.3390/antibiotics4040617








