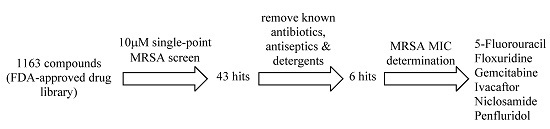
An FDA-Drug Library Screen for Compounds with Bioactivities against Meticillin-Resistant Staphylococcus aureus (MRSA)
Abstract
:1. Introduction
2. Results and Discussion
| Drugs Found in This Screen | Original Indication | Administration Route | Keratinocyte GI50 (μM) | MRSA MICs (μM) | |||
|---|---|---|---|---|---|---|---|
| USA 100 | USA 300 | USA 400 | VISA | ||||
| Ivacaftor | cystic fibrosis | OR | 5.9 ± 0.5 | 6.25 | 6.25 | 6.25 | 6.25 |
| 5-fluorouracil | cancer | IV, TO | 33.3 ± 2.6 | 6.25 | 3.13 | 3.13 | 6.25 |
| Penfluridol | psychosis | OR | 2.1 ± 0.6 | 3.13 | 3.13 | 3.13 | 3.13 |
| Niclosamide | anthelmintic | OR | <0.2 | 0.78 | 0.39 | 0.78 | 0.39 |
| Gemcitabine | cancer | IV | 1.9 ± 0.2 | 0.10 | 0.05 | 0.10 | 0.05 |
| Floxuridine | cancer | IV | >50 | 0.025 | 0.00313 | 0.0125 | 0.00625 |
| FDA-approved MRSA drugs | |||||||
| Clindamycin | MRSA infection | IV, OR, TO | >50 | >100 | 0.20 | 0.20 | >100 |
| Doxycycline | MRSA infection | IV, OR | 36.9 ± 3.7 | 0.39 | 0.78 | 1.56 | 12.5 |
| Linezolid | MRSA infection | IV, OR | >100 | 6.25 | 6.25 | 6.25 | 3.13 |
| Mupirocin | MRSA infection | TO | >50 | 0.78 | 0.39 | 0.39 | >100 |
| Tigecycline | MRSA infection | IV | 20.6 ± 4.2 | 0.78 | 0.78 | 0.78 | 1.56 |
| Vancomycin | MRSA infection | IV | >100 | 0.78 | 0.78 | 0.78 | 6.25 |
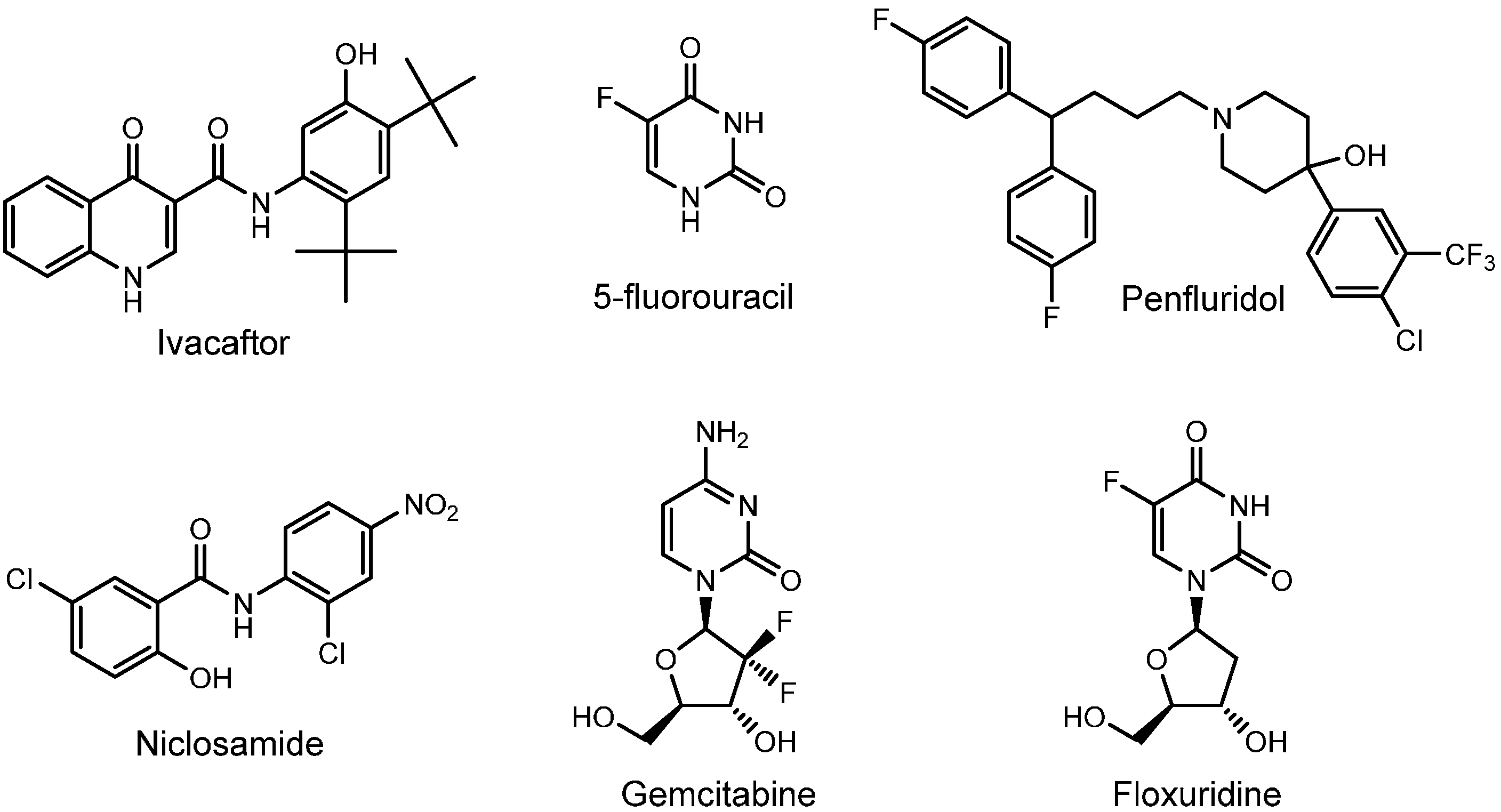
2.1. Ivacaftor
2.2. 5-Flurouracil
2.3. Penfluridol
2.4. Niclosamide
2.5. Gemcitabine
2.6. Floxuridine

3. Experimental Section
3.1. Compound Library, Test Compounds and Bacteria
3.2. Single-Point Bacteria Growth Inhibition Assay
3.3. Minimum Inhibitory Concentration (MIC) Assay
3.4. Human Epidermal Keratinocyte Viability (GI50) Assay
3.5. Bactericidal/Static Determination Assay
3.6. Time-Kill Assay
4. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interests
References
- Boucher, H.W.; Talbot, G.H.; Bradley, J.S.; Edwards, J.E.; Gilbert, D.; Rice, L.B.; Scheld, M.; Spellberg, B.; Bartlett, J. Bad bugs, no drugs: No ESKAPE! An update from the Infectious Diseases Society of America. Clin. Infect. Dis. 2009, 48, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Silver, L.L. Challenges of antibacterial discovery. Clin. Microbiol. Rev. 2011, 24, 71–109. [Google Scholar] [CrossRef] [PubMed]
- Bush, K.; Courvalin, P.; Dantas, G.; Davies, J.; Eisenstein, B.; Huovinen, P.; Jacoby, G.A.; Kishony, R.; Kreiswirth, B.N.; Kutter, E.; et al. Tackling antibiotic resistance. Nat. Rev. Microbiol. 2011, 9, 894–896. [Google Scholar] [CrossRef] [PubMed]
- Kinch, M.S.; Patridge, E.; Plummer, M.; Hoyer, D. An analysis of FDA-approved drugs for infectious disease: Antibacterial agents. Drug Discov. Today 2014, 19, 1283–1287. [Google Scholar] [CrossRef]
- Gould, I.M. MRSA bacteraemia. Int. J. Antimicrob. Agents 2007, 30, S66–S70. [Google Scholar] [CrossRef] [PubMed]
- Gordon, R.J.; Lowy, F.D. Pathogenesis of methicillin-resistant Staphylococcus aureus infection. Clin. Infect. Dis. 2008, 46, S350–S359. [Google Scholar] [CrossRef] [PubMed]
- Wertheim, H.F.L.; Melles, D.C.; Vos, M.C.; van Leeuwen, W.; van Belkum, A.; Verbrugh, H.A.; Nouwen, J.L. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect. Dis. 2005, 5, 751–762. [Google Scholar] [CrossRef]
- David, M.Z.; Daum, R.S. Community-associated methicillin-resistant Staphylococcus aureus: Epidemiology and clinical consequences of an emerging epidemic. Clin. Microbiol. Rev. 2010, 23, 616–687. [Google Scholar] [CrossRef] [PubMed]
- DeLeo, F.R.; Otto, M.; Kreiswirth, B.N.; Chambers, H.F. Community-associated meticillin-resistant Staphylococcus aureus. Lancet 2010, 375, 1557–1568. [Google Scholar] [CrossRef]
- Moran, G.J.; Krishnadasan, A.; Gorwitz, R.J.; Gorwitz, M.D.; Gregory, E.; Fosheim, L.K.; McDougal, M.S.; Roberta, B.; Talan, D.A. Methicillin-resistant S. aureus infections among patients in the emergency department. N. Engl. J. Med. 2006, 355, 666–674. [Google Scholar] [CrossRef] [PubMed]
- Dryden, M.S. Complicated skin and soft tissue infection. J. Antimicrob. Chemother. 2010, 65, iii35–iii44. [Google Scholar] [CrossRef] [PubMed]
- Gould, I.M. Costs of hospital-acquired methicillin-resistant Staphylococcus aureus (MRSA) and its control. Int. J. Antimicrob. Agents 2006, 28, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, A.D. Epidemiology and virulence of community-associated MRSA. Clin. Microbiol. News 2009, 31, 153–160. [Google Scholar] [CrossRef]
- Lee, B.Y.; Singh, A.; David, M.Z.; Bartsch, S.M.; Slayton, R.B.; Huang, S.S.; Zimmer, S.M.; Potter, M.A.; Macal, C.M.; Lauderdale, D.S.; et al. The economic burden of community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA). Clin. Microbiol. Infect. 2013, 19, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Frieden, T. Antibiotic Resistance Threats in the United States, 2013. Executive Summary; Centers for Disease Control and Prevention: Atlanta, GA, USA. Available online: http://www.cdc.gov/drugresistance/threat-report-2013/ (accessed on 13 June 2015).
- Brenner, R.; Ellis-Grosse, E.J.; Echols, R. Moving goalposts—regulatory oversight of antibacterial drugs. Nat. Biotechnol. 2006, 24, 1515–1520. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, P. Antibacterial discovery and development—The failure of success? Nat. Biotechnol. 2006, 24, 1497–1503. [Google Scholar] [CrossRef] [PubMed]
- Cooper, M.A.; Shlaes, D. Fix the antibiotics pipeline. Nature 2011, 472. [Google Scholar] [CrossRef] [PubMed]
- Nambiar, S.; Laessig, K.; Toerner, J.; Farley, J.; Cox, E. Antibacterial drug development: Challenges, recent developments, and future considerations. Clin. Pharmacol. Ther. 2014, 96, 147–149. [Google Scholar] [CrossRef]
- Ashburn, T.T.; Thor, K.B. Drug repositioning: Identifying and developing new uses for existing drugs. Nat. Rev. Drug Discov. 2004, 3, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Oprea, T.I.; Mestres, J. Drug repurposing: Far beyond new targets for old drugs. AAPS J. 2012, 14, 759–763. [Google Scholar] [CrossRef] [PubMed]
- Sleigh, S.H.; Barton, C.L. Repurposing strategies for therapeutics. Pharm. Med. 2010, 24, 151–159. [Google Scholar] [CrossRef]
- Barratt, M.J.; Frail, D. Drug Repositioning: Bringing New Life to Shelved Assets and Existing Drugs; Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Limbago, B.; Fosheim, G.E.; Schoonover, V.; Crane, C.E.; Nadle, J.; Petit, S.; Heltzel, D.; Ray, S.M.; Harrison, L.H.; Lynfield, R.; et al. Characterization of methicillin-resistant Staphylococcus aureus isolates collected in 2005 and 2006 from patients with invasive disease: A population-based analysis. J. Clin. Microbiol. 2009, 47, 1344–1351. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Bayer, A.; Cosgrove, S.E.; Robert, S.D.; Scott, K.F.; Rachel, J.G.; Sheldon, L.K.; Adolf, W.K.; Donald, P.L.; Barbara, E.M.; et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin. Infect. Dis. 2011, 52. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, B.W.; Davis, J.; McElvaney, G.; Robert, S.D.; Scott, K.F.; Rachel, J.G.; Sheldon, L.K.; Adolf, W.K.; Donald, P.L.; Barbara, E.M.; et al. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N. Engl. J. Med. 2011, 163, 1663–1672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reznikov, L.R.; Alaiwa, M.H.A.; Dohrn, C.L.; Gansemer, N.D.; Diekema, D.J.; Stoltz, D.A.; Welsh, M.J. Antibacterial properties of the CFTR potentiator ivacaftor. J. Cyst. Fibros. 2014, 13, 515–519. [Google Scholar] [CrossRef] [PubMed]
- DrugBank: Ivacaftor (DB08820). Available online: http://www.drugbank.ca/drugs/DB08820 (accessed on 13 June 2015).
- Heidelberger, C.; Chaudhuri, N.K.; Danneberg, P.; Mooren, D.; Griesbach, L. Fluorinated pyrimidines, a new class of tumour-inhibitory compounds. Nature 1957, 179, 663–666. [Google Scholar] [CrossRef] [PubMed]
- Grem, J.L. 5-Fluorouracil: Forty-plus and still ticking. A review of its preclinical and clinical development. Investig. New Drugs 2000, 18, 299–313. [Google Scholar] [CrossRef]
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-Fluorouracil: Mechanisms of action and clinical strategies. Nat. Rev. Cancer 2003, 3, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Janssen, P.A.; Niemegeers, C.J.; Schellekens, K.H.; Lenaerts, F.M.; Verbruggen, F.J.; van Nueten, J.M.; Schaper, W.K.A. The pharmacology of penfluridol (R 16341) a new potent and orally long-acting neuroleptic drug. Eur. J. Pharmacol. 1970, 11, 139–154. [Google Scholar] [CrossRef]
- Weinbach, E.C.; Garbus, J. Mechanism of action of reagents that uncouple oxidative phosphorylation. Nature 1969, 221, 1016–1018. [Google Scholar] [CrossRef] [PubMed]
- Rajamuthiah, R.; Fuchs, B.B.; Conery, A.L.; Kim, W.; Jayamani, E.; Kwon, B.; Ausubel, F.M.; Mylonakis, E. Repurposing salicylanilide anthelmintic drugs to combat drug resistant Staphylococcus aureus. PLoS ONE 2015, 10, e0124595. [Google Scholar] [CrossRef] [PubMed]
- Appelbaum, P.C. Reduced glycopeptide susceptibility in methicillin-resistant Staphylococcus aureus (MRSA). Int. J. Antimicrob. Agents 2007, 30, 398–408. [Google Scholar] [CrossRef] [PubMed]
- Pearson, R.D.; Hewlett, E.L. Niclosamide therapy for tapeworm infections. Ann. Intern. Med. 1985, 102, 550–551. [Google Scholar] [CrossRef] [PubMed]
- Rimland, D.; Roberson, B. Gastrointestinal carriage of methicillin-resistant Staphylococcus aureus. J. Clin. Microbiol. 1986, 24, 137–138. [Google Scholar] [PubMed]
- Boyce, J.M.; Havill, N.L.; Otter, J.A.; Adams, N.M.T. Widespread environmental contamination associated with patients with diarrhea and methicillin-resistant Staphylococcus aureus colonization of the gastrointestinal tract. Infect. Control Hosp. Epidemiol. 2007, 28, 1142–1147. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Agency. Niclosamide. Available online: http://www.epa.gov/pesticides/reregistration/REDs/factsheets/2455fact.pdf (accessed on 13 June 2015).
- MacDonald, M.; Lamerdin, J.; Owens, S.; Keon, B.; Bilter, G.; Shang, Z.; Huang, Z.; Yu, H.; Dias, J.; Minami, T.; et al. Identifying off-target effects and hidden phenotypes of drugs in human cells. Nat. Chem. Biol. 2006, 2, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, N.M.; Fernandes, P.A.; Ramos, M.J. Understanding ribonucleotide reductase inactivation by gemcitabine. Chem. Eur. J. 2007, 13, 8507–8515. [Google Scholar] [CrossRef] [PubMed]
- DrugBank: Gemcitabine (DB00441). Available online: http://www.drugbank.ca/drugs/DB00441 (accessed on 13 June 2015).
- Berger, F.G.; Berger, S.H. Thymidylate synthase as a chemotherapeutic drug target. Where are we after fifty years? Cancer Biol. Ther. 2006, 5, 1238–1241. [Google Scholar] [CrossRef] [PubMed]
- Power, D.G.; Kemeny, N.E. The role of floxuridine in metastatic liver disease. Mol. Cancer Ther. 2009, 8, 1015–1025. [Google Scholar] [CrossRef] [PubMed]
- DrugBank: Floxuridine (DB00322). Available online: http://www.drugbank.ca/drugs/DB00322 (accessed on 13 June 2015).
- Pankey, G.A.; Sabath, L.D. Clinical relevance of bacteriostatic versus bactericidal mechanisms of action in the treatment of Gram-positive bacterial infections. Clin. Infect. Dis. 2004, 38, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, B.T.; Brown, T.; Parasrampuria, R.; Brazeau, D.A.; Forrest, A.; Kelchlin, P.A.; Holden, P.N.; Peloquin, C.A.; Hanna, D.; Bulitta, J.B. Front-loaded Linezolid regimens result in increased killing and suppression of the accessory gene regulator system of Staphylococcus aureus. Antimicrob. Agents Chemother. 2012, 56, 3712–3719. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically, Approved Standard—Ninth edition, Document M07-A9; CLSI: Wayne, PA, USA, 2012; Volume 32, No. 2. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lau, Q.Y.; Tan, Y.Y.F.; Goh, V.C.Y.; Lee, D.J.Q.; Ng, F.M.; Ong, E.H.Q.; Hill, J.; Chia, C.S.B. An FDA-Drug Library Screen for Compounds with Bioactivities against Meticillin-Resistant Staphylococcus aureus (MRSA). Antibiotics 2015, 4, 424-434. https://doi.org/10.3390/antibiotics4040424
Lau QY, Tan YYF, Goh VCY, Lee DJQ, Ng FM, Ong EHQ, Hill J, Chia CSB. An FDA-Drug Library Screen for Compounds with Bioactivities against Meticillin-Resistant Staphylococcus aureus (MRSA). Antibiotics. 2015; 4(4):424-434. https://doi.org/10.3390/antibiotics4040424
Chicago/Turabian StyleLau, Qiu Ying, Yoke Yan Fion Tan, Vanessa Chai Yin Goh, David Jing Qin Lee, Fui Mee Ng, Esther H. Q. Ong, Jeffrey Hill, and Cheng San Brian Chia. 2015. "An FDA-Drug Library Screen for Compounds with Bioactivities against Meticillin-Resistant Staphylococcus aureus (MRSA)" Antibiotics 4, no. 4: 424-434. https://doi.org/10.3390/antibiotics4040424
APA StyleLau, Q. Y., Tan, Y. Y. F., Goh, V. C. Y., Lee, D. J. Q., Ng, F. M., Ong, E. H. Q., Hill, J., & Chia, C. S. B. (2015). An FDA-Drug Library Screen for Compounds with Bioactivities against Meticillin-Resistant Staphylococcus aureus (MRSA). Antibiotics, 4(4), 424-434. https://doi.org/10.3390/antibiotics4040424