Antibiotic Use in Horses: Analysis of 57 German Veterinary Practices (2018–2023)
Abstract
1. Introduction
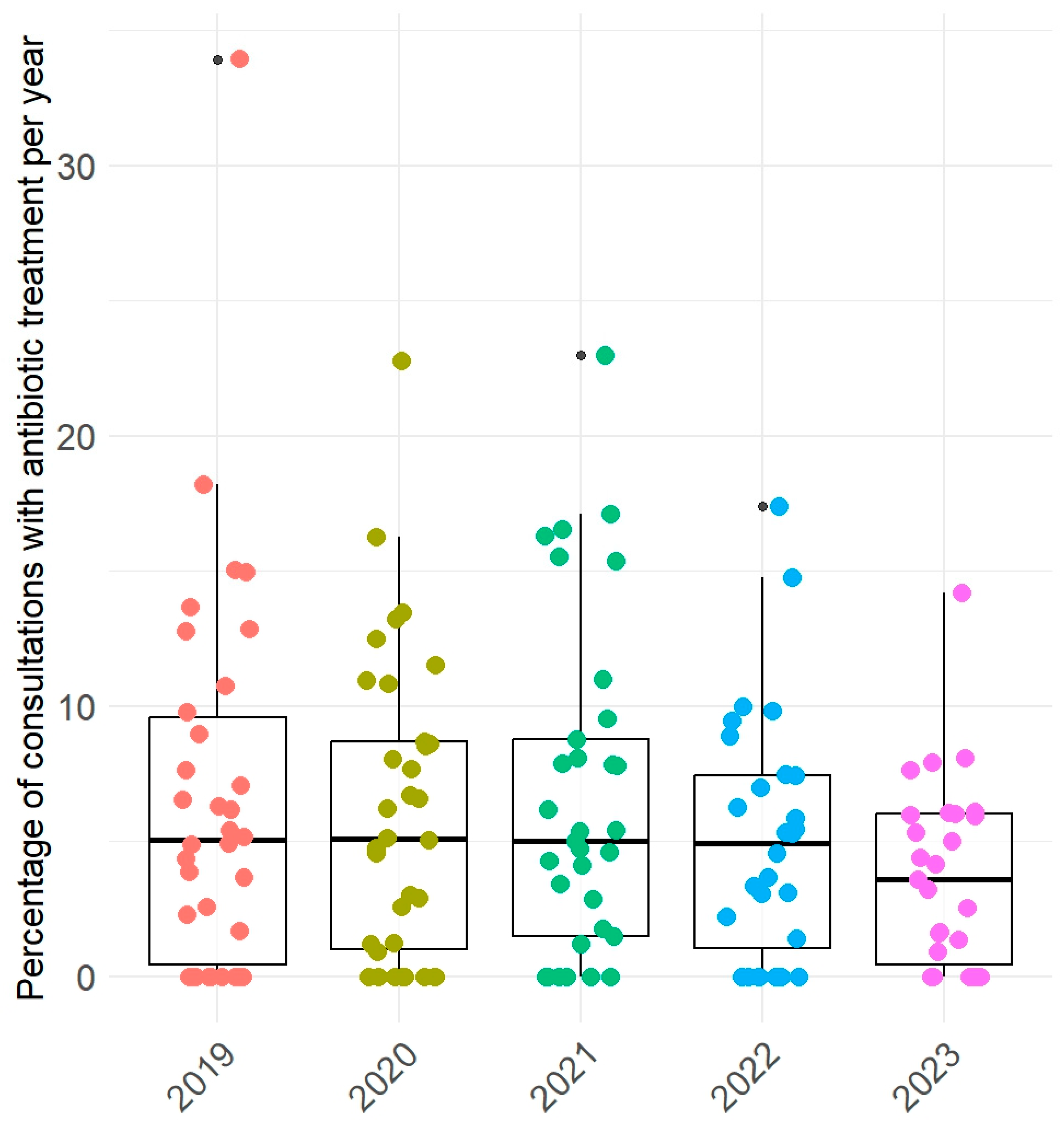
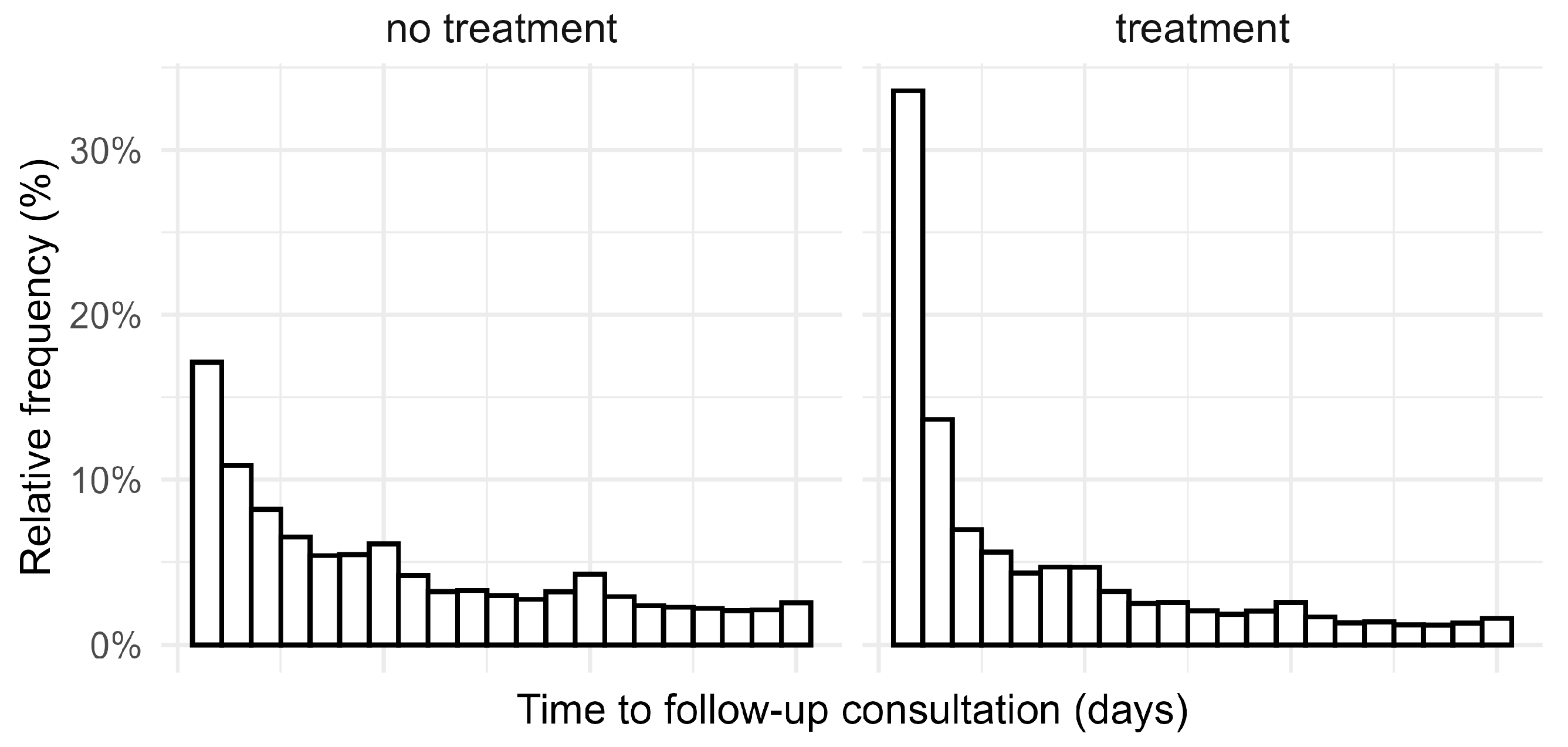
2. Results
2.1. Use of Antibiotics by Substance Class
2.2. Use of Antibiotics by Indication
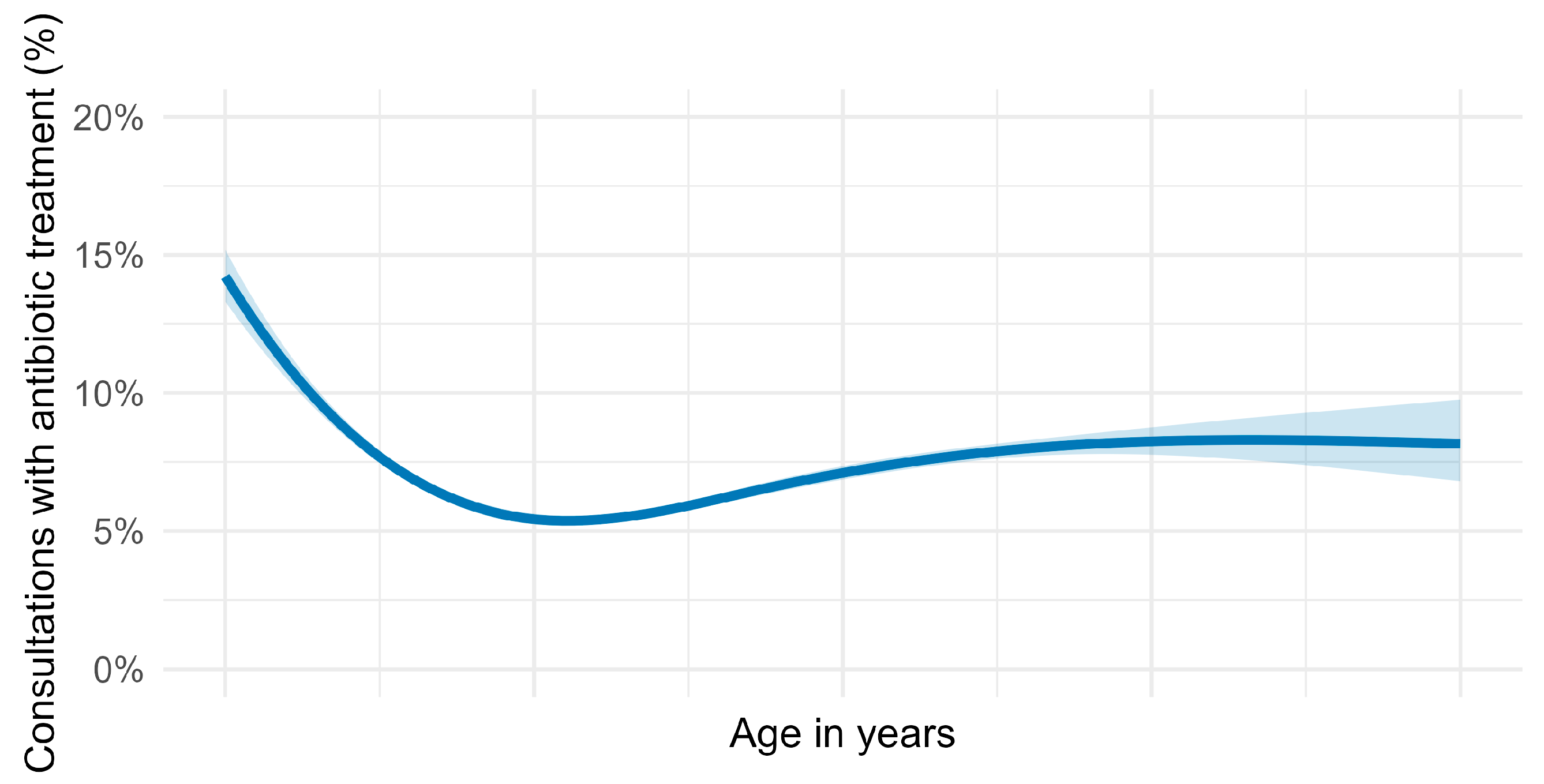
2.3. Use of Antibiotics by Horse’s Age
3. Discussion
4. Materials and Methods
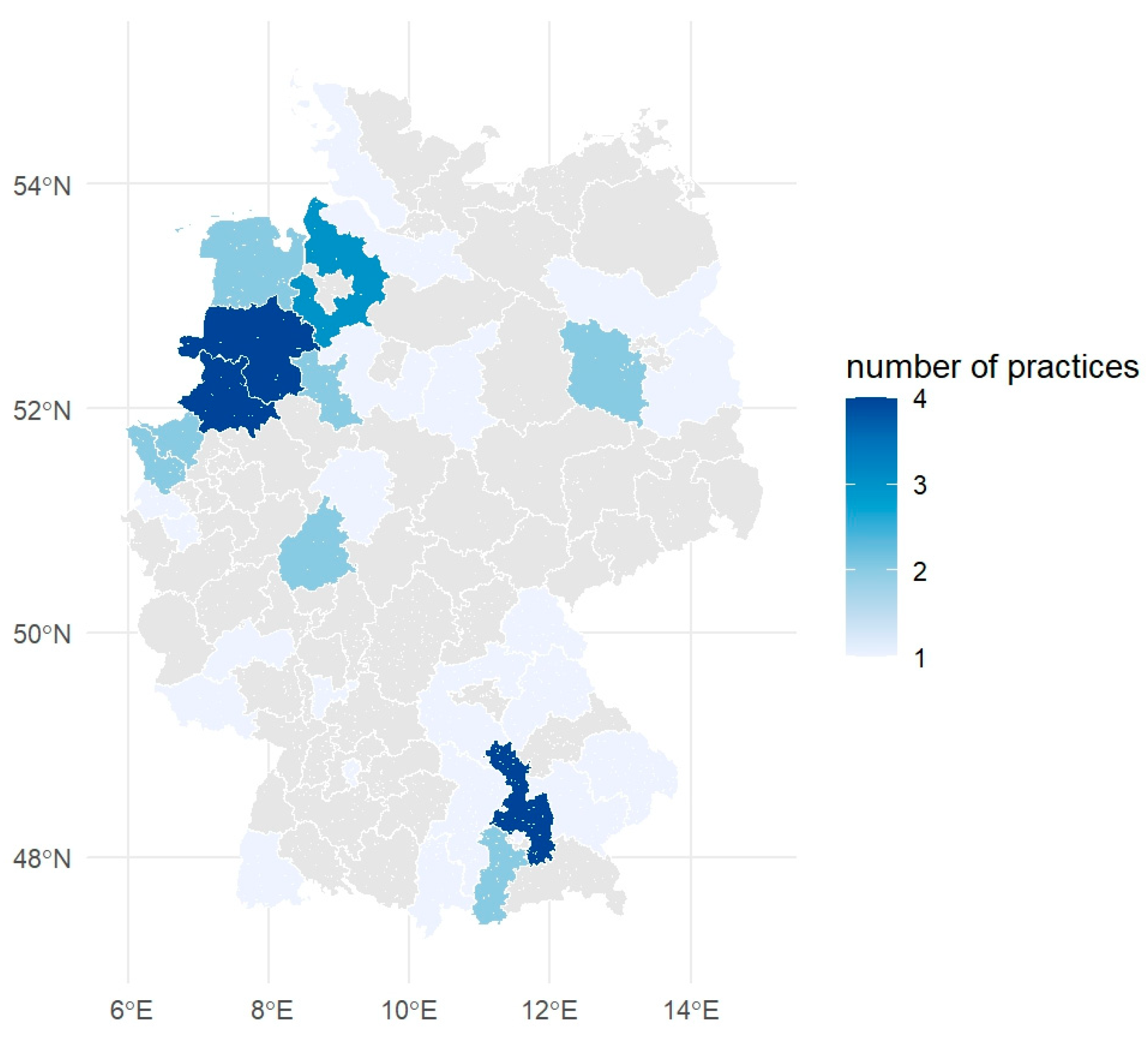
4.1. Data Collection and Processing
4.2. Classification of Diagnoses and Treatments
4.3. Classification of Antibiotics and Source of Pharmaceutical Data
- Quantity per package.
- Active substance(s) and concentrations.
- Route of administration.
4.4. Inclusion Criteria and Study Period
4.5. Statistical Analyses
4.6. Mapping
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
Abbreviations
| 95% CI | 95% Confidence Interval |
| HPCIA | Highest-Priority Critically Important Antimicrobials |
| WHO | World Health Organization |
Appendix A
References
- Barbosa, T.M.; Levy, S.B. The impact of antibiotic use on resistance development and persistence. Drug Resist. Update 2000, 3, 303–311. [Google Scholar] [CrossRef]
- Argudín, M.A.; Deplano, A.; Meghraoui, A.; Dodémont, M.; Heinrichs, A.; Denis, O.; Nonhoff, C.; Roisin, S. Bacteria from Animals as a Pool of Antimicrobial Resistance Genes. Antibiotics 2017, 6, 12. [Google Scholar] [CrossRef] [PubMed]
- van den Bogaard, A.E.; Stobberingh, E.E. Antibiotic usage in animals: Impact on bacterial resistance and public health. Drugs 1999, 58, 589–607. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO List of Medically Important Antimicrobials: A Risk Management Tool for Mitigating Antimicrobial Resistance Due to Non-Human Use; World Health Organization: Geneva, Switzerland, 2024; ISBN 978-92-4-008461-. [Google Scholar]
- European Medicines Agency. Categorisation of Antibiotics in the European Union: Answer to the Request from the European Commission for Updating the Scientific Advice on the Impact on Public Health and Animal Health of the use of Antibiotics in Animals; European Medicines Agency: Amsterdam, The Netherlands, 2019; Available online: https://www.ema.europa.eu/en/documents/report/categorisation-antibiotics-european-union-answer-request-european-commission-updating-scientific-advice-impact-public-health-and-animal-health-use-antibiotics-animals_en.pdf (accessed on 7 February 2025).
- Schnepf, A.; Bienert-Zeit, A.; Ertugrul, H.; Wagels, R.; Werner, N.; Hartmann, M.; Feige, K.; Kreienbrock, L. Antimicrobial Usage in Horses: The Use of Electronic Data, Data Curation, and First Results. Front. Vet. Sci. 2020, 7, 343. [Google Scholar] [CrossRef]
- Sinclair, C.; Schofield, I.; Mair, T. Antibiotic use in first opinion equine practice in the United Kingdom: Serial point prevalence surveys in 17 practices. Equine Vet. Educ. 2025, 37, 68–75. [Google Scholar] [CrossRef]
- Mair, T.S.; Parkin, T.D. Audit of antimicrobial use in eleven equine practices over a five-year period (2014–2018). Equine Vet. Educ. 2022, 34, 404–408. [Google Scholar] [CrossRef]
- Prouillac, C. Use of Antimicrobials in a French Veterinary Teaching Hospital: A Retrospective Study. Antibiotics 2021, 10, 1369. [Google Scholar] [CrossRef]
- Kabir, A.; Lamichhane, B.; Habib, T.; Adams, A.; El-Sheikh Ali, H.; Slovis, N.M.; Troedsson, M.H.; Helmy, Y.A. Antimicrobial resistance in equines: A growing threat to horse health and beyond—A comprehensive review. Antibiotics 2024, 18, 713. [Google Scholar] [CrossRef]
- Lönker, N.S.; Fechner, K.; El Wahed, A.A. Horses as a crucial part of One Health. Vet. Sci. 2020, 7, 28. [Google Scholar] [CrossRef]
- European Parliament and Council of the European Union. Regulation (EU) 2019/6 of the European Parliament and of the Council of 11 December 2018 on Veterinary Medicinal Products and Repealing Directive 2001/82/EC. Off. J. Eur. Union 2019, 4, 43–167. [Google Scholar]
- Bacci, S.; Meucci, V.; Sgorbini, M.; de Marchi, L.; Pirone, A.; Pretti, C.; Tognetti, R.; Intorre, L. Pattern of prescriptions and prudent use of antimicrobial in horse practice at a Veterinary Teaching Hospital. Res. Vet. Sci. 2024, 168, 105140. [Google Scholar] [CrossRef]
- Allen, S.E.; Verheyen, K.L.P.; O’Neill, D.G.; Brodbelt, D.C. Use of antimicrobials licensed for systemic administration in UK equine practice. Equine Vet. J. 2023, 55, 808–819. [Google Scholar] [CrossRef] [PubMed]
- Federal Ministry of Food, Nutrition and Homeland. Viehbestände nach der Erhebung zum 1. März 2020: Fachserie 3, Reihe 2.1.3 [Livestock Numbers After the Survey on 1 March 2020]. Available online: https://www.bmel-statistik.de/landwirtschaft/tierhaltung/viehbestand (accessed on 15 August 2025).
- Dallap Schaer, B.L.; Linton, J.K.; Aceto, H. Antimicrobial use in horses undergoing colic surgery. J. Vet. Intern. Med. 2012, 26, 1449–1456. [Google Scholar] [CrossRef] [PubMed]
- Kauter, A.; Epping, L.; Ghazisaeedi, F.; Lübke-Becker, A.; Wolf, S.A.; Kannapin, D.; Stoeckle, S.D.; Semmler, T.; Günther, S.; Gehlen, H.; et al. Frequency, Local Dynamics, and Genomic Characteristics of ESBL-Producing Escherichia coli Isolated from Specimens of Hospitalized Horses. Front. Microbiol. 2021, 12, 671676. [Google Scholar] [CrossRef] [PubMed]
- Teschner, D.; Barton, A.K.; Klaus, C.; Gehlen, H. Antibiotikaeinsatz bei operierten Kolikpferden in Deutschland [Antibiotic use in colic horses that underwent surgery in Germany]. Pferdeheilkunde 2015, 31, 235–240. [Google Scholar] [CrossRef]
- Perkins, G.A.; Wagner, B. The development of equine immunity: Current knowledge on immunology in the young horse. Equine Vet. J. 2015, 47, 267–274. [Google Scholar] [CrossRef]
- Federal Ministry of Food and Agriculture (BMEL). German Regulation on Equine Passports (Equidenpass-Verordnung—EQPassV). Federal Law Gazette (Bundesgesetzblatt) I 2000, 166; Last Amended by Article 5 of the Regulation of 14 December 2020 (BGBl. I, 2987). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:32015R0262 (accessed on 15 August 2025).
- Schneider, S.T.; Meemken, D.; Gehlen, H.; Merle, R.; Langkabel, N. A comparative survey of veterinarians, equine owners, and equine keepers regarding the knowledge and implementation of legal requirements in Germany for the use and documentation of veterinary medicine in equines intended for slaughter. PLoS ONE 2023, 18, e0283371. [Google Scholar] [CrossRef]
- Gustafsson, K.; Sykes, B.W.; Verwilghen, D.; Palmers, K.; Sullivan, S.; van Galen, G. Trimethoprim-sulfonamide: A valid antimicrobial treatment in foals? J. Am. Vet. Med. Assoc. 2024, 262, 825–833. [Google Scholar] [CrossRef]
- Hardefeldt, L.Y.; Bailey, K.E.; Slater, J. Overview of the use of antimicrobial drugs for the treatment of bacterial infections in horses. Equine Vet. Educ. 2021, 33, 602–611. [Google Scholar] [CrossRef]
- Federal Ministry of Food and Agriculture (BMEL). Verordnung über Tierärztliche Hausapotheken: TÄHAV [23rd Ordinance on Veterinary Dispensaries]; Bundesanzeiger Verlag: Bonn, Germany, 2024. [Google Scholar]
- Hughes, L.A.; Pinchbeck, G.; Callaby, R.; Dawson, S.; Clegg, P.; Williams, N. Antimicrobial prescribing practice in UK equine veterinary practice. Equine Vet. J. 2013, 45, 141–147. [Google Scholar] [CrossRef]
- Welsh, C.E.; Parkin, T.D.H.; Marshall, J.F. Use of large-scale veterinary data for the investigation of antimicrobial prescribing practices in equine medicine. Equine Vet. J. 2017, 49, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Rule, E.K.; Boyle, A.G.; Redding, L.E. Antimicrobial prescribing patterns in equine ambulatory practice. Prev. Vet. Med. 2021, 193, 105411. [Google Scholar] [CrossRef]
- Tallon, R.E.; Whitt, B.; Bladon, B.M. Antibiotic usage in 14 equine practices over a 10-year period (2012–2021). Equine Vet. J. 2024, 56, 544–551. [Google Scholar] [CrossRef]
- Altermatt, N.; Dolf, G.; Ramseyer, A.; Burger, D.; Gerber, V. Auftreten gesundheitlicher Probleme beim Schweizer Warmblutpferd mittleren Alters [Occurrence of health problems in middle-aged Swiss warmblood horses]. Schweiz Arch Tierheilkd 2021, 163, 339–350. [Google Scholar] [CrossRef]
- Merle, R.; Feuer, L.; Frenzer, K.; Plenio, J.-L.; Bethe, A.; Sarnino, N.; Lübke-Becker, A.; Bäumer, W. Use of Antibiotics in Companion Animals from 133 German Practices from 2018 to 2023. Antibiotics 2025, 14, 58. [Google Scholar] [CrossRef]
- Farrell, S.; McKernan, C.; Benson, T.; Elliott, C.; Dean, M. Understanding farmers’ and veterinarians’ behavior in relation to antimicrobial use and resistance in dairy cattle: A systematic review. J. Dairy Sci. 2021, 104, 4584–4603. [Google Scholar] [CrossRef]
- Gohrbandt, S. Erarbeitung eines Diagnoseschlüssels in der Veterinärmedizin. Ph.D. Dissertation, Freie Universität Berlin, Berlin, Germany, 2019. [Google Scholar]
- Allenspach, K.; Burgener; Dahlem, D.; Gerber, B.; Glanemann; Glaus, T.; Griebsch, C.; Hazuchova, K.; Hildebrandt, N.; Kandel-Tschiederer, B.; et al. Differenzialdiagnosen Innere Medizin bei Hund und Katze: Vom Leitsymptom zur Diagnose [Differential Diagnoses of Internal Medicine in Dogs and Cats: From the Main Symptom to the Diagnosis], 3rd ed.; Georg Thieme Verlag: Stuttgart, Germany, 2020; ISBN 9783132423398. [Google Scholar]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikit-learn: Machine Learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Veterinärmedizinischer Informationsdienst für Arzneimittelanwendung, Toxikologie und Arzneimittelrecht. VETIDATA; University of Leipzig. Available online: www.vetidata.de (accessed on 25 February 2025).
- Landschneider, C. ROTE LISTE® 2011. Pharm. unserer Zeit 2011, 40, 286. [Google Scholar] [CrossRef]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. [Google Scholar]
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- R Studio Team. RStudio: Integrated Development for R; RStudio: Boston, MA, USA, 2022. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Müller, K.E. _Here: A Simpler Way to Find Your Files_; CRAN: Wien, Austria, 2020. [Google Scholar]
- Wood, S. Generalized Additive Models: An Introduction with R, 2nd ed; Chapman and Hall; CRC: Boca Raton, FL, USA, 2017. [Google Scholar]
- Schwarzer, G.; Carpenter, A.J.; Rücker, G. meta: An R package for meta-analysis. R News 2015, 15, 40–45. [Google Scholar]
- Pebesma, E.J.; Bivand, R. Spatial Data Science: With Applications in R, 1st ed.; CRC Press: Boca Raton, FL, USA; London, UK; New York, NY, USA, 2023; ISBN 9780429459016. [Google Scholar]



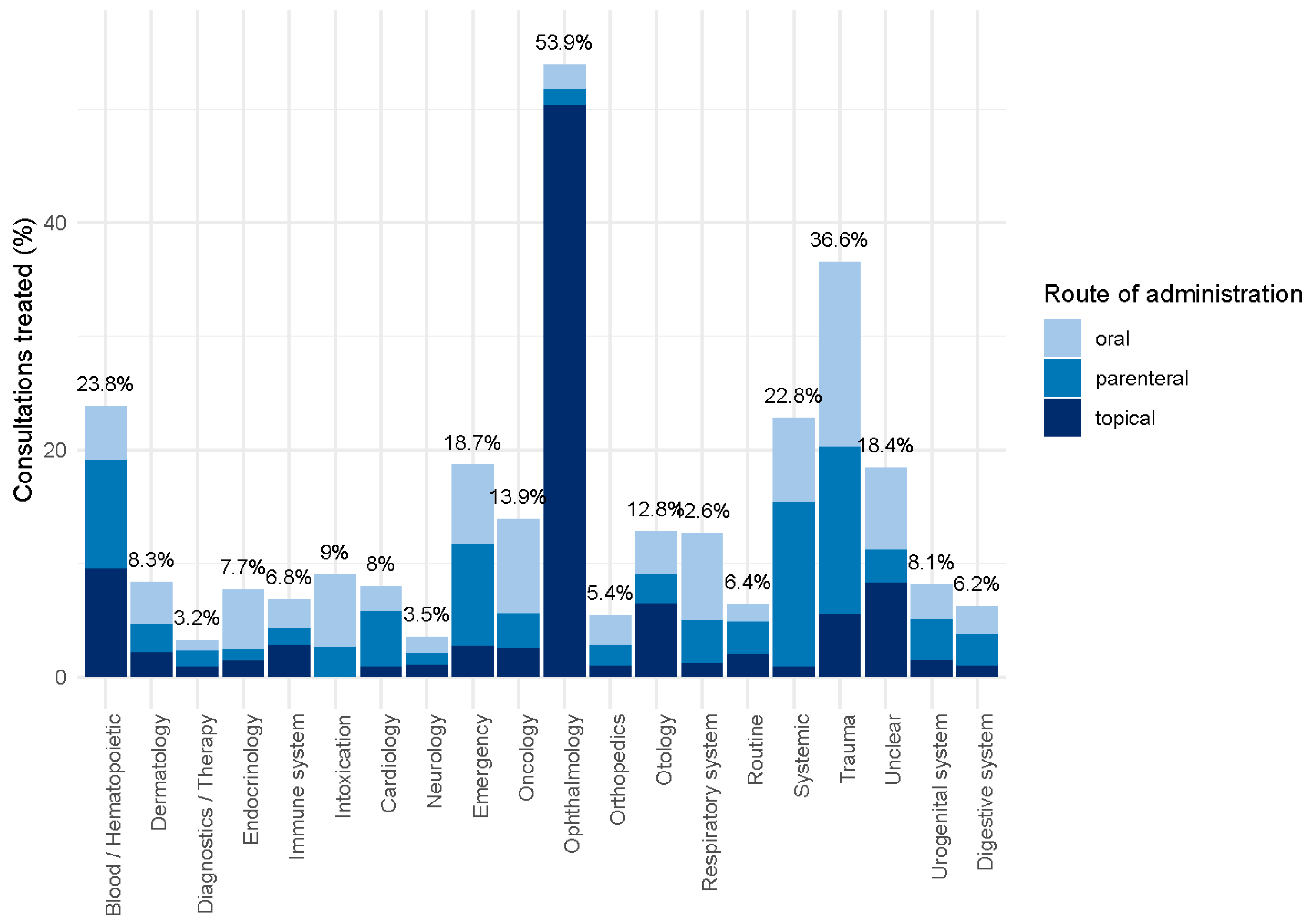
| Indication | No. of Consultations | % of All Consultations with 95% CI | % Treated (Total) with 95% CI | Oral (%) | Parenteral (%) | Topical (%) |
|---|---|---|---|---|---|---|
| Blood/Hematopoietic organs | 20 | 0% (0–0%) | 23.8% (14.2–37.2%) | 20.0 | 40.0 | 40.0 |
| Dermatology | 13,761 | 18.5% (18.2–18.8%) | 8.3% (7.9–8.7%) | 44.5 | 29.8 | 25.8 |
| Diagnostics/Therapy 1 | 17,302 | 23.3% (23.0–23.6%) | 3.2% (2.9–3.4%) | 29.7 | 43.5 | 26.8 |
| Endocrinology 2 | 570 | 0.8% (0.7–0.8%) | 7.7% (5.6–10.4%) | 68.2 | 13.6 | 18.2 |
| Immune system | 396 | 0.5% (0.5–0.6%) | 6.8% (4.6–9.9%) | 37.0 | 22.2 | 40.7 |
| Intoxication | 78 | 0.1% (0.1–0.1%) | 9.0% (4.1–17.5%) | 71.4 | 28.6 | 0.0 |
| Cardiology | 1354 | 1.8% (1.7–1.9%) | 8.0% (6.6–9.6%) | 27.5 | 61.5 | 11.0 |
| Neurology | 2205 | 3% (2.8–3.1%) | 3.5% (2.8–4.3%) | 40.8 | 30.3 | 28.9 |
| Emergency | 548 | 0.7% (0.7–0.8%) | 18.7% (15.6–22.3%) | 37.5 | 48.1 | 14.4 |
| Oncology | 469 | 0.6% (0.6–0.7%) | 13.9% (10.9–17.4%) | 59.7 | 22.4 | 17.9 |
| Ophthalmology | 2192 | 2.9% (2.8–3.1%) | 53.9% (51.9–55.9%) | 4.0 | 2.6 | 93.4 |
| Orthopedics | 8948 | 12% (11.8–12.3%) | 5.4% (4.9–5.9%) | 48.7 | 33.8 | 17.5 |
| Otology | 152 | 0.2% (0.2–0.2%) | 12.8% (8.4–18.9%) | 30.0 | 20.0 | 50.0 |
| Respiratory system | 7708 | 10.4% (10.2–10.6%) | 12.6% (11.8–13.4%) | 60.3 | 30.6 | 9.1 |
| Routine exam/prevention 3 | 6667 | 9% (8.8–9.2%) | 6.4% (5.8–7.0%) | 24.6 | 44.3 | 31.1 |
| Systemic disease 4 | 567 | 0.8% (0.7–0.8%) | 22.8% (19.5–26.5%) | 32.8 | 63.4 | 3.8 |
| Trauma | 3079 | 4.1% (4–4.3%) | 36.6% (35.0–38.3%) | 44.6 | 40.4 | 15.0 |
| Unclear/unspecified 5 | 408 | 0.5% (0.5–0.6%) | 18.4% (14.8–22.6%) | 39.5 | 15.8 | 44.7 |
| Urogenital system | 4564 | 6.1% (6–6.3%) | 8.1% (7.3–8.8%) | 37.6 | 44.5 | 17.9 |
| Digestive system | 3338 | 4.5% (4.3–4.6%) | 6.2% (5.5–6.9%) | 40.2 | 44.5 | 15.3 |
| Total | 74,326 | 100.0% | 9.7% (9.5–10.0%) | 36.9 | 31.3 | 31.8 |
| Indication | Aminoglycosides | Beta-Lactams | Sulfonamides | Amphenicols | Cephalosporins (1st/2nd Gen) | Cephalosporins (3rd/4th Gen) | Fluoroquinolones | Polymyxins | Steroid Antibiotics | Tetracyclines | Macrolides | Nitroimidazoles |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Blood/Hematopoietic organs | 3 (60.0%) | 1 (20.0%) | 1 (20.0%) | - | - | - | - | - | - | - | - | - |
| Dermatology | 222 (19.2%) | 250 (21.6%) | 517 (44.7%) | 3 (0.3%) | 3 (0.3%) | 36 (3.1%) | 59 (5.1%) | 1 (0.1%) | 2 (0.2%) | 63 (5.4%) | - | - |
| Diagnostics/Therapy | 123 (22.1%) | 176 (31.7%) | 167 (30.0%) | 1 (0.2%) | - | 14 (2.5%) | 20 (3.6%) | 2 (0.4%) | - | 51 (9.2%) | 2 (0.4%) | - |
| Endocrinology | 7 (15.9%) | 4 (9.1%) | 24 (54.5%) | - | - | 1 (2.3%) | 7 (15.9%) | - | - | 1 (2.3%) | - | - |
| Immune system | 9 (33.3%) | 5 (18.5%) | 10 (37.0%) | - | - | - | 1 (3.7%) | - | - | 2 (7.4%) | - | - |
| Intoxication | - | 1 (14.3%) | 5 (71.4%) | - | - | - | 1 (14.3%) | - | - | - | - | - |
| Cardiology | 10 (9.2%) | 48 (44.0%) | 35 (32.1%) | - | 1 (0.9%) | 5 (4.6%) | 6 (5.5%) | - | - | 4 (3.7%) | - | - |
| Neurology | 16 (21.1%) | 15 (19.7%) | 32 (42.1%) | - | - | 1 (1.3%) | 5 (6.6%) | - | - | 7 (9.2%) | - | - |
| Emergency | 11 (10.6%) | 32 (30.8%) | 43 (41.3%) | - | - | 4 (3.8%) | 8 (7.7%) | - | - | 6 (5.8%) | - | - |
| Oncology | 11 (16.4%) | 10 (14.9%) | 41 (61.2%) | - | - | - | 1 (1.5%) | - | - | 4 (6.0%) | - | - |
| Ophthalmology | 662 (53.6%) | 30 (2.4%) | 51 (4.1%) | 39 (3.2%) | - | 2 (0.2%) | 112 (9.1%) | - | 2 (0.2%) | 337 (27.3%) | - | - |
| Orthopedics | 59 (12.2%) | 124 (25.6%) | 236 (48.7%) | 1 (0.2%) | - | 6 (1.2%) | 16 (3.3%) | - | - | 43 (8.9%) | - | - |
| Otology | 7 (35.0%) | 3 (15.0%) | 5 (25.0%) | - | - | - | 3 (15.0%) | 2 (10.0%) | - | - | - | - |
| Respiratory system | 54 (5.5%) | 185 (18.9%) | 618 (63.2%) | 4 (0.4%) | 1 (0.1%) | 18 (1.8%) | 57 (5.8%) | - | - | 40 (4.1%) | 1 (0.1%) | - |
| Routine exam/prevention | 86 (20.7%) | 174 (41.9%) | 96 (23.1%) | - | 2 (0.5%) | - | 16 (3.9%) | 1 (0.2%) | - | 36 (8.7%) | 4 (1.0%) | - |
| Systemic disease | 6 (4.6%) | 66 (50.4%) | 50 (38.2%) | - | - | - | 7 (5.3%) | - | - | 2 (1.5%) | - | - |
| Trauma | 119 (9.9%) | 398 (33.1%) | 561 (46.7%) | 1 (0.1%) | 1 (0.1%) | 23 (1.9%) | 36 (3.0%) | - | - | 62 (5.2%) | - | - |
| Unclear/unspecified | 13 (17.1%) | 9 (11.8%) | 26 (34.2%) | 2 (2.6%) | - | - | 13 (17.1%) | - | - | 13 (17.1%) | - | - |
| Urogenital system | 47 (12.5%) | 117 (31.2%) | 146 (38.9%) | - | 1 (0.3%) | 14 (3.7%) | 21 (5.6%) | - | - | 29 (7.7%) | - | - |
| Digestive system | 29 (13.9%) | 60 (28.7%) | 91 (43.5%) | - | - | 2 (1.0%) | 14 (6.7%) | - | - | 12 (5.7%) | - | 1 (0.5%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merle, R.; Feuer, L.; Frenzer, K.; Plenio, J.-L.; Bethe, A.; Sarnino, N.; Lübke-Becker, A.; Bäumer, W. Antibiotic Use in Horses: Analysis of 57 German Veterinary Practices (2018–2023). Antibiotics 2025, 14, 953. https://doi.org/10.3390/antibiotics14090953
Merle R, Feuer L, Frenzer K, Plenio J-L, Bethe A, Sarnino N, Lübke-Becker A, Bäumer W. Antibiotic Use in Horses: Analysis of 57 German Veterinary Practices (2018–2023). Antibiotics. 2025; 14(9):953. https://doi.org/10.3390/antibiotics14090953
Chicago/Turabian StyleMerle, Roswitha, Leonie Feuer, Katharina Frenzer, Jan-Lukas Plenio, Astrid Bethe, Nunzio Sarnino, Antina Lübke-Becker, and Wolfgang Bäumer. 2025. "Antibiotic Use in Horses: Analysis of 57 German Veterinary Practices (2018–2023)" Antibiotics 14, no. 9: 953. https://doi.org/10.3390/antibiotics14090953
APA StyleMerle, R., Feuer, L., Frenzer, K., Plenio, J.-L., Bethe, A., Sarnino, N., Lübke-Becker, A., & Bäumer, W. (2025). Antibiotic Use in Horses: Analysis of 57 German Veterinary Practices (2018–2023). Antibiotics, 14(9), 953. https://doi.org/10.3390/antibiotics14090953






