Unlocking Antimicrobial Peptides from Marine Invertebrates: A Comprehensive Review of Antimicrobial Discovery
Abstract
1. Introduction
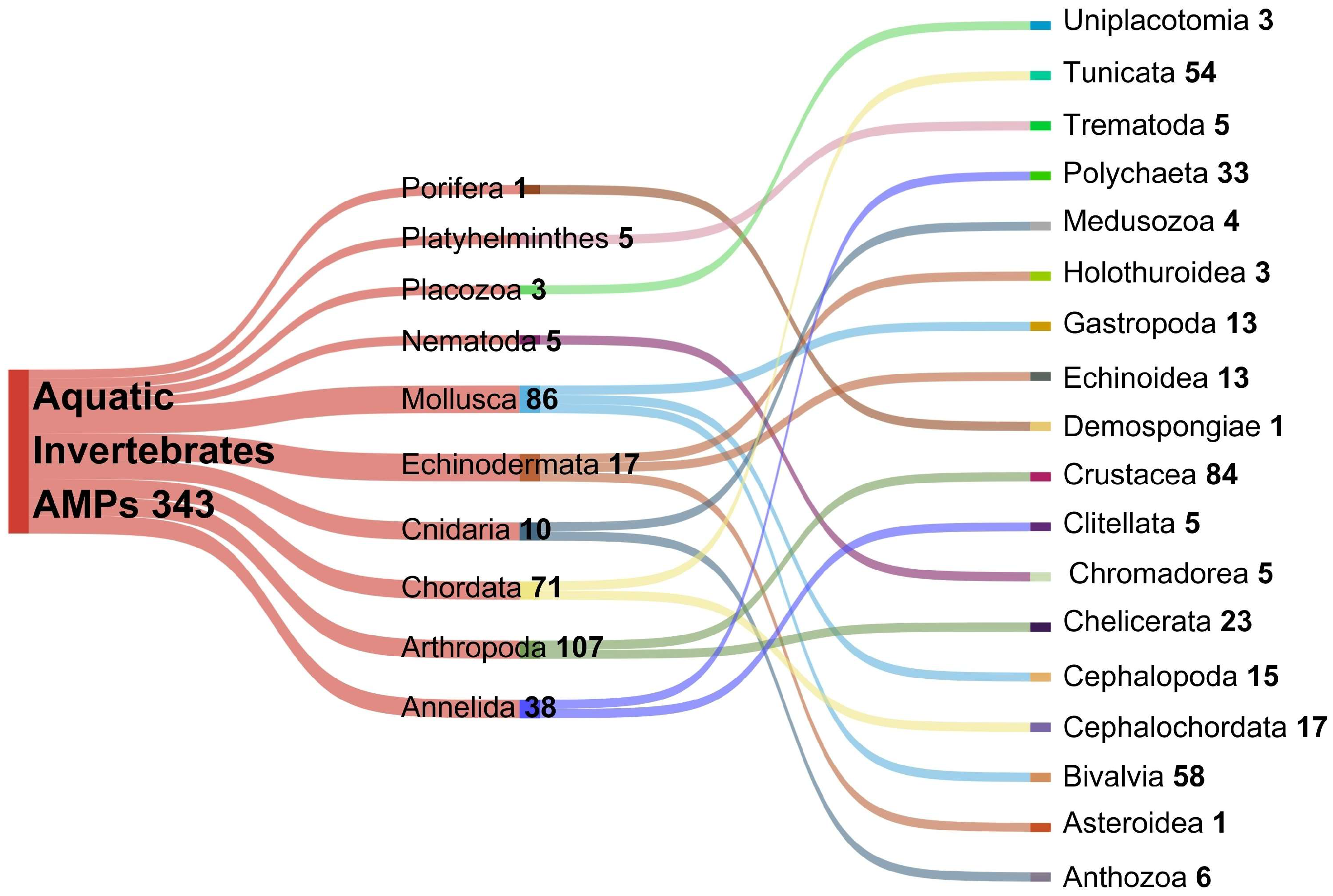
2. Marine Invertebrate-Based Antimicrobial Peptides
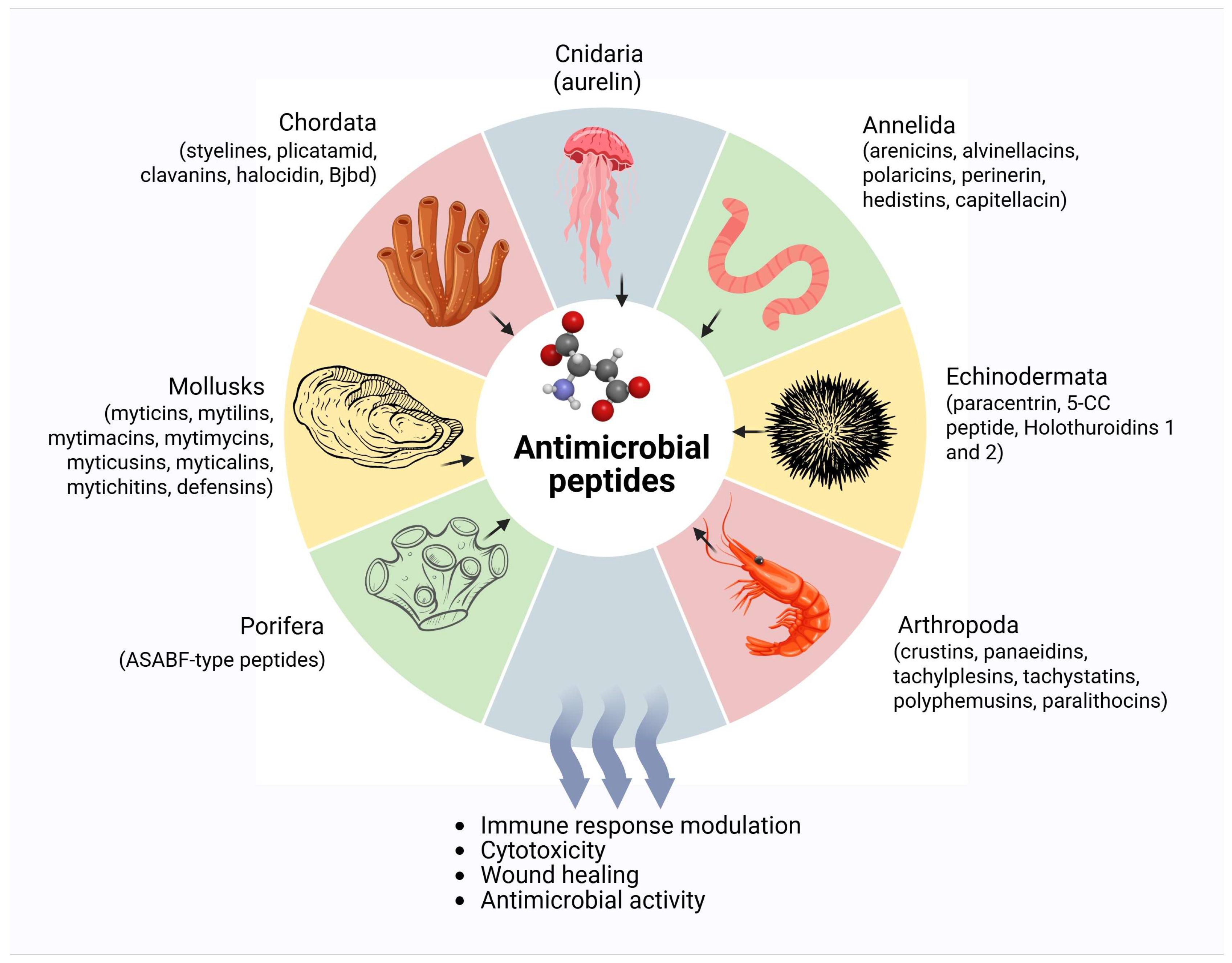
3. Marine Phyla Producing AMPs
3.1. Annelida
3.2. Arthopoda
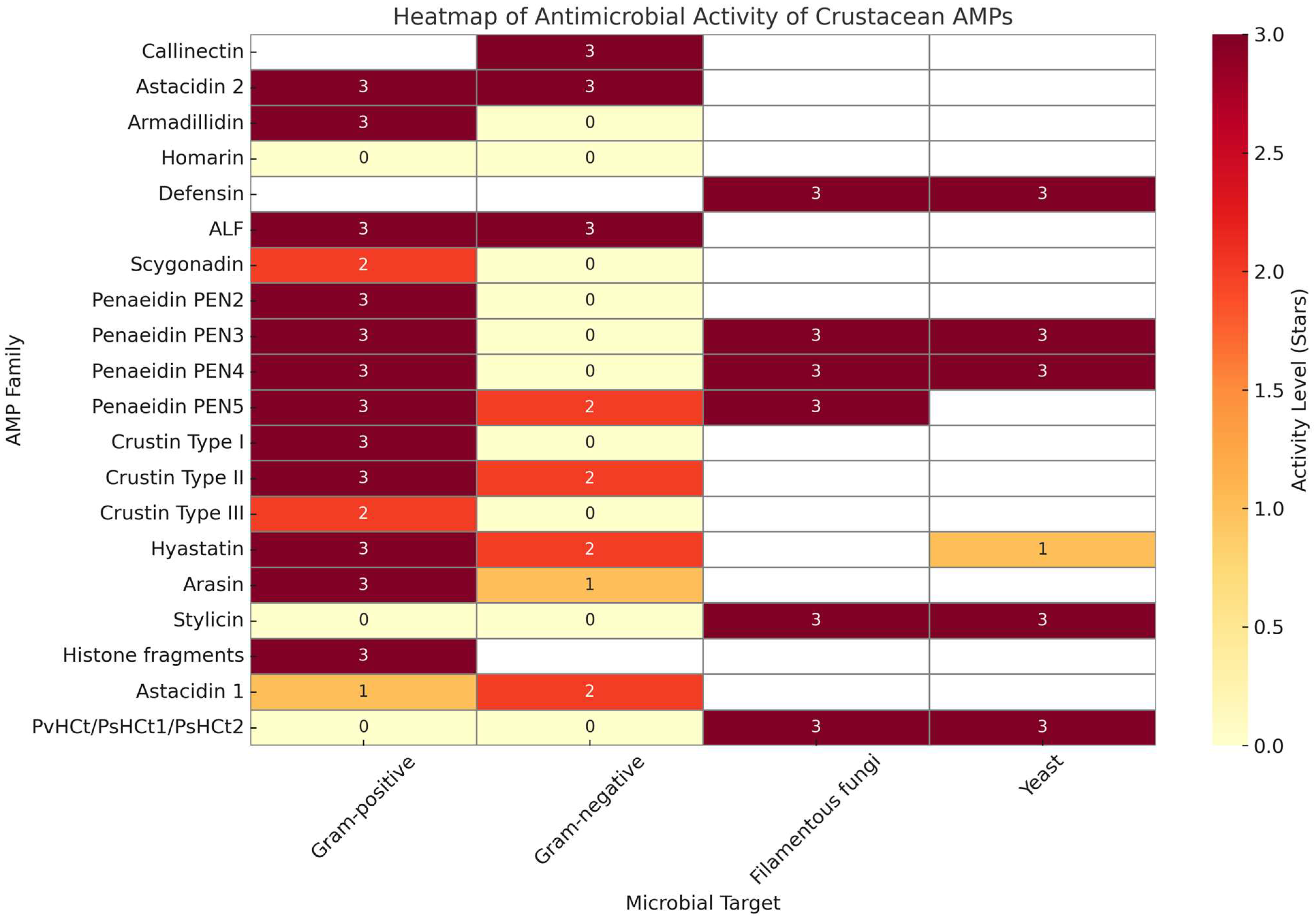
Crustacea
| Group | Peptide Name | Source Organism | Structure/Class | Key Features | Antimicrobial Activity | Reference(s) |
|---|---|---|---|---|---|---|
| Chelicerata | Tachyplesin | T. tridentatus, C. rotundicauda, T. gigas | Short cationic peptide, 2 disulfide bonds | Cyclized forms, enhanced stability | Bacteria, fungi, cancer cells, biofilms | [52,56,58,70,71,72,73,74] |
| Polyphemusin | Limulus polyphemus | Tachyplesin-like structure | Amphipathic variants, high activity | Broad-spectrum | [52,58,71,75] | |
| Tachycitin | T. tridentatus | αβ-motif, chitin-binding, 10 cysteines | Synergistic with big defensin | Broad-spectrum | [76,77] | |
| Tachystatin | T. tridentatus, T. gigas | β-sheet, chitin-binding, 3 disulfide bonds | Isoform C: amphiphilic and hemolytic; A2: stable, non-toxic | Broad-spectrum | [78,79,80,81,82,83,84] | |
| Tatritin | T. tridentatus | α-helix + β-sheet, 6 disulfide bonds | Chitin-binding, stable structure | Broad-spectrum | [82,85,86] | |
| Crustacea | Arasin | C. sapidus, various crabs, crayfish, prawns | Pro/Arg-rich N-terminal, Cys-rich C-terminal | Chitin-binding; immune regulation | Broad-spectrum | [30,65,87,88,89,90,91,92,93,94,95,96,97,98,99] |
| Crustin | C. maenas, penaeid shrimp | WAP-domain-based peptide | 7 structural types (I–VII); diverse bioactivities | Gram ± bacteria, fungi, viruses | [65,66] | |
| Proline-rich AMP | C. maenas, S. paramamosain | 6.5 kDa, proline-rich | Non-lytic, blocks protein synthesis | Broad-spectrum | [32,36,100,101,102,103] | |
| Glycine-rich AMP | S. paramamosain | Gly-rich motifs, Cys-terminal | Thermally stable | Broad-spectrum | [89,104,105,106] | |
| Hyastatin | Hyas araneus | Multi-domain, chitin-binding, 6 Cys | Highly diverse; 14 variants in P. trituberculatus | Bacteria, chitin-binding | [107,108,109,110,111] |
3.3. Chordata
3.4. Cnidaria
3.5. Echinodermata
3.6. Mollusca
3.7. Nematoda
3.8. Placozoa
3.9. Platyhelminthes
3.10. Marine Porifera-Derived Antimicrobial Peptides
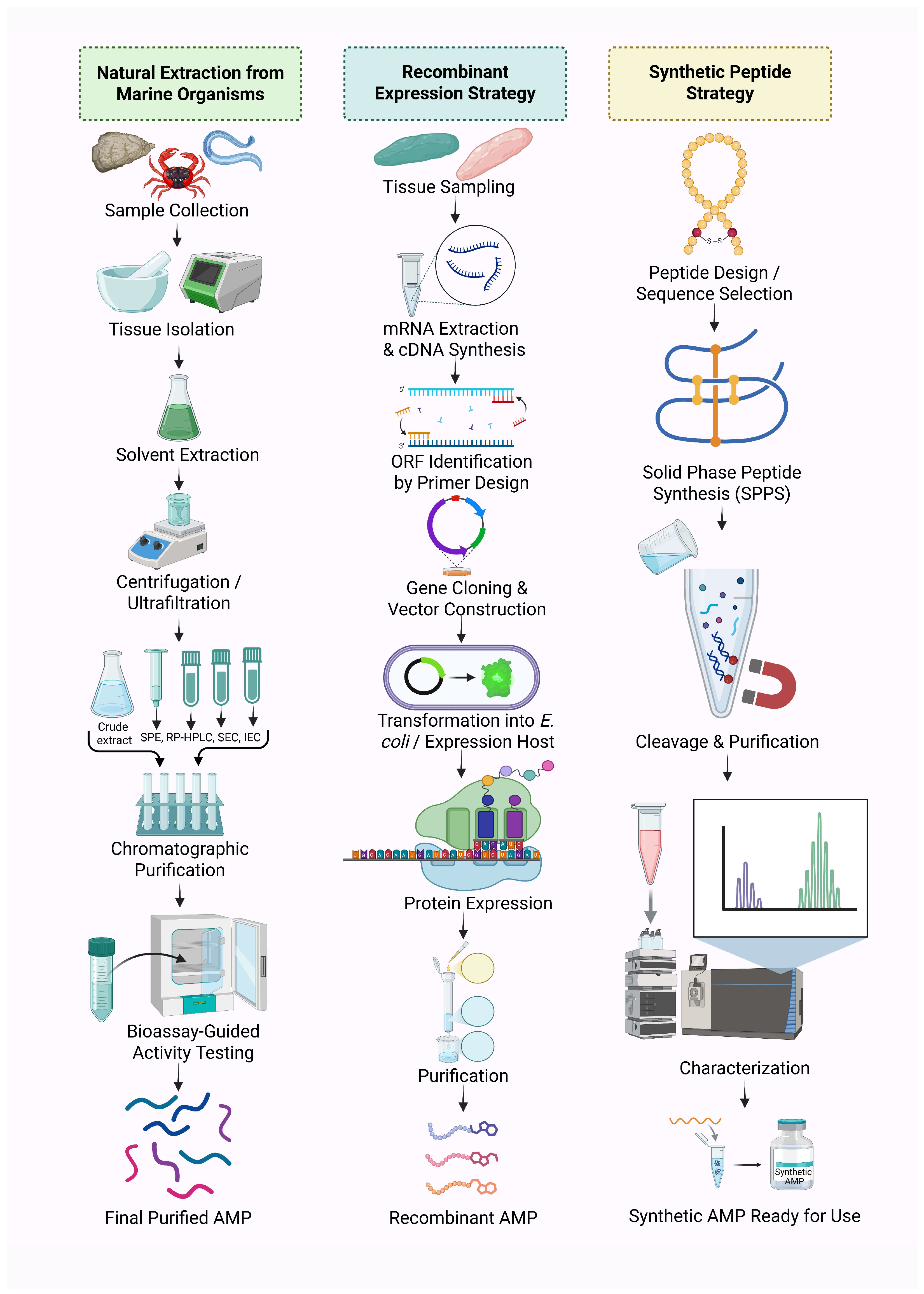
4. Isolation and Production of Marine-Derived AMPs
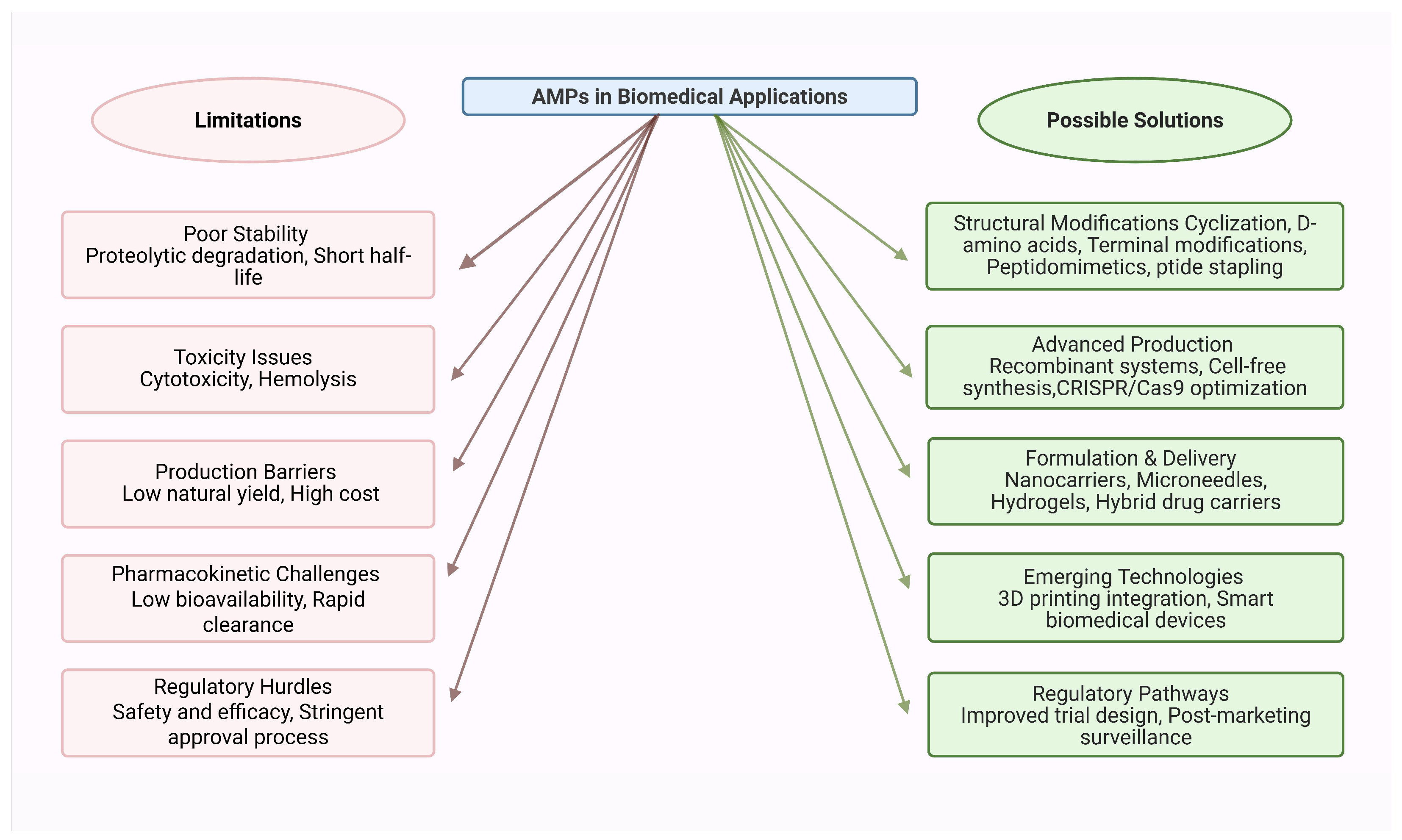
5. Potential Biotechnological and Therapeutic Applications
5.1. Use in Human and Veterinary Medicine
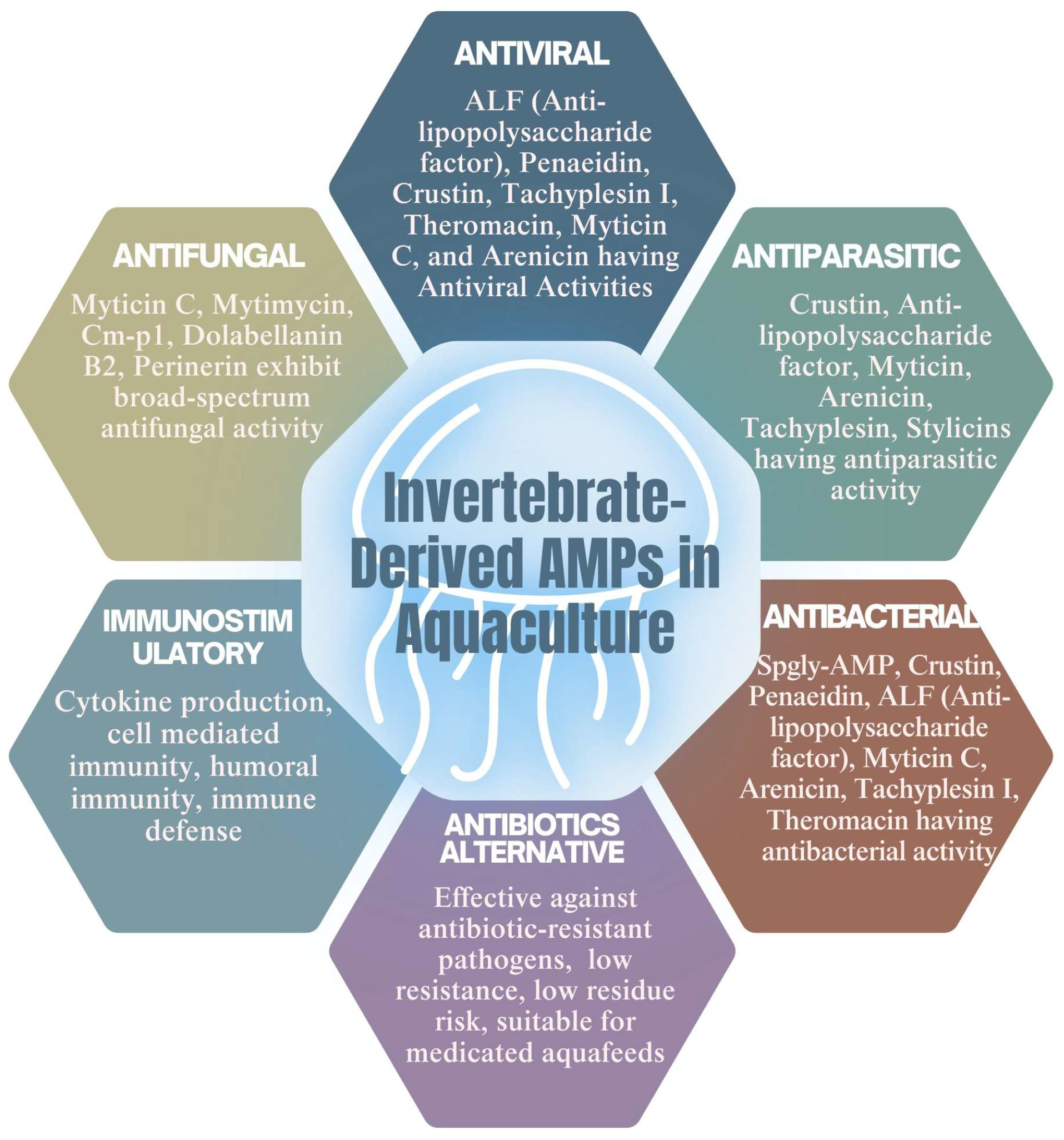
5.2. Role in Aquaculture: Disease Control and Health Management
5.2.1. Antifungal Activities of AMPs
5.2.2. The Antibacterial Activity of AMPs
5.3. Representative Case Studies of Marine Invertebrate AMPs
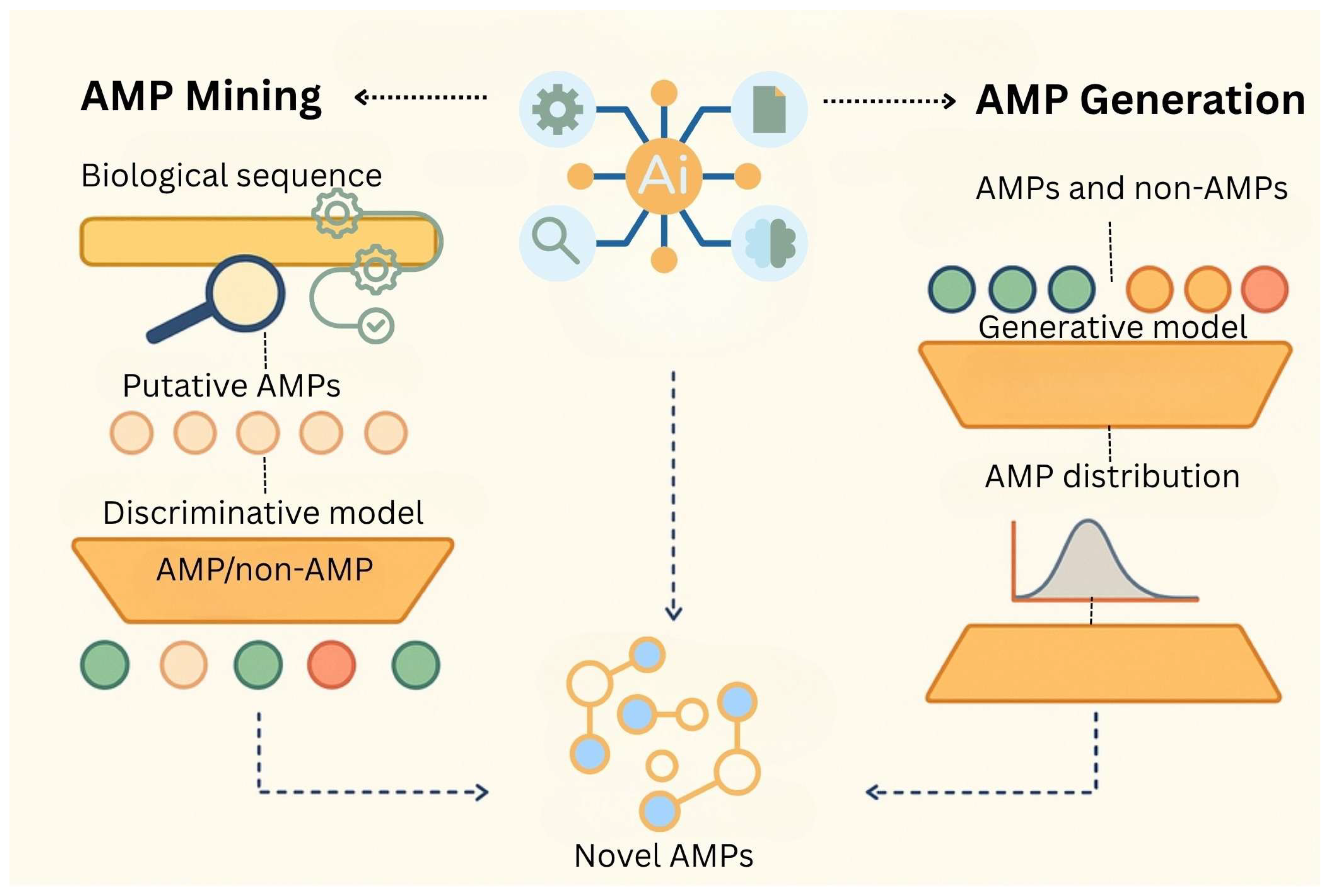
6. Artificial Intelligence in Antimicrobial Peptide Discovery, Design and Production
7. Clinical Translation and Market Status of Marine Invertebrate AMPs
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Medeiros, W.; Kralova, S.; Oliveira, V.; Ziemert, N.; Sehnal, L. Antarctic Bacterial Natural Products: From Genomic Insights to Drug Discovery. Nat. Prod. Rep. 2025, 42, 774–787. [Google Scholar] [CrossRef]
- Agrawal, S.; Adholeya, A.; Deshmukh, S.K. The Pharmacological Potential of Non-Ribosomal Peptides from Marine Sponge and Tunicates. Front. Pharmacol. 2016, 7, 333. [Google Scholar] [CrossRef]
- Spiridonov, V.; Ćurić, M.; Novkovski, N. Biosphere: Ecosystem Diversity and Environmental Change; Springer: London, UK, 2025; pp. 51–81. [Google Scholar] [CrossRef]
- Guryanova, S.V.; Balandin, S.V.; Belogurova-Ovchinnikova, O.Y.; Ovchinnikova, T.V. Marine Invertebrate Antimicrobial Peptides and Their Potential as Novel Peptide Antibiotics. Mar. Drugs 2023, 21, 503. [Google Scholar] [CrossRef]
- Masso-Silva, J.; Diamond, G. Antimicrobial Peptides from Fish. Pharmaceuticals 2014, 7, 265–310. [Google Scholar] [CrossRef]
- Agrawal, S.; Chavan, P.; Badiger, A. Marine Fungi of the Genera Aspergillus and Penicillium: A Promising Reservoir of Chemical Diversity for Developing Anti-Viral Drug Candidates. Microbe 2024, 3, 100081. [Google Scholar] [CrossRef]
- Ghosh, S.; Sarkar, T.; Pati, S.; Kari, Z.A.; Edinur, H.A.; Chakraborty, R. Novel Bioactive Compounds From Marine Sources as a Tool for Functional Food Development. Front. Mar. Sci. 2022, 9, 832957. [Google Scholar] [CrossRef]
- Magalhães, R.; Mil-Homens, D.; Cruz, S.; Oliveira, M. Marine Antimicrobial Peptides: Emerging Strategies Against Multidrug-Resistant and Biofilm-Forming Bacteria. Antibiotics 2025, 14, 808. [Google Scholar] [CrossRef]
- Mayer, A.; Rodríguez, A.; Taglialatela-Scafati, O.; Fusetani, N. Marine Pharmacology in 2009–2011: Marine Compounds with Antibacterial, Antidiabetic, Antifungal, Anti-Inflammatory, Antiprotozoal, Antituberculosis, and Antiviral Activities; Affecting the Immune and Nervous Systems, and Other Miscellaneous Mechanisms of Action. Mar. Drugs 2013, 11, 2510–2573. [Google Scholar] [CrossRef]
- Macedo, M.W.F.S.; da Cunha, N.B.; Carneiro, J.A.; da Costa, R.A.; de Alencar, S.A.; Cardoso, M.H.; Franco, O.L.; Dias, S.C. Marine Organisms as a Rich Source of Biologically Active Peptides. Front. Mar. Sci. 2021, 8, 1–23. [Google Scholar] [CrossRef]
- Sørensen, O.E.; Cole, N.B.A.M. Antimicrobial Peptides in Innate Immune Responses. Trends Innate Immun. 2008, 15, 61–77. [Google Scholar]
- Rodrigues, T.; Guardiola, F.A.; Almeida, D.; Antunes, A. Aquatic Invertebrate Antimicrobial Peptides in the Fight Against Aquaculture Pathogens. Microorganisms 2025, 13, 156. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.K.; Lee, H.H.; Seo, C.H.; Park, Y. Antimicrobial and Immunomodulatory Properties and Applications of Marine-Derived Proteins and Peptides. Mar. Drugs 2019, 17, 350. [Google Scholar] [CrossRef]
- Lee, S.; Silverman, N.; Gao, F.-B. Emerging Roles of Antimicrobial Peptides in Innate Immunity, Neuronal Function, and Neurodegeneration. Trends Neurosci. 2024, 47, 949–961. [Google Scholar] [CrossRef]
- Ganz, T. The Role of Antimicrobial Peptides in Innate Immunity. Integr. Comp. Biol. 2003, 43, 300–304. [Google Scholar] [CrossRef]
- Kang, H.; Seo, C.; Park, Y. Marine Peptides and Their Anti-Infective Activities. Mar. Drugs 2015, 13, 618–654. [Google Scholar] [CrossRef]
- Kawsar, M.A.; Alam, M.T.; Pandit, D.; Rahman, M.M.; Mia, M.; Talukdar, A.; Sumon, T.A. Status of Disease Prevalence, Drugs and Antibiotics Usage in Pond-Based Aquaculture at Narsingdi District, Bangladesh: A Major Public Health Concern and Strategic Appraisal for Mitigation. Heliyon 2022, 8, e09060. [Google Scholar] [CrossRef]
- Marino, A.; Maniaci, A.; Lentini, M.; Ronsivalle, S.; Nunnari, G.; Cocuzza, S.; Parisi, F.M.; Cacopardo, B.; Lavalle, S.; La Via, L. The Global Burden of Multidrug-Resistant Bacteria. Epidemiologia 2025, 6, 21. [Google Scholar] [CrossRef]
- Fischbach, M.A.; Walsh, C.T. Antibiotics for Emerging Pathogens. Science 2009, 325, 1089–1093. [Google Scholar] [CrossRef]
- Zhu, M.; Liu, Y.; Huang, B.; Huang, W. Research Trends on Antimicrobial Peptides in Aquaculture: A Thematic and Bibliometric Analysis. Aquac. Res. 2025, 2025, 4375783. [Google Scholar] [CrossRef]
- Guzmán, F.; Wong, G.; Román, T.; Cárdenas, C.; Alvárez, C.; Schmitt, P.; Albericio, F.; Rojas, V. Identification of Antimicrobial Peptides from the Microalgae Tetraselmis suecica (Kylin) Butcher and Bactericidal Activity Improvement. Mar. Drugs 2019, 17, 453. [Google Scholar] [CrossRef]
- Algammal, A.M.; Mabrok, M.; Sivaramasamy, E.; Youssef, F.M.; Atwa, M.H.; El-kholy, A.W.; Hetta, H.F.; Hozzein, W.N. Emerging MDR-Pseudomonas aeruginosa in Fish Commonly Harbor OprL and ToxA Virulence Genes and BlaTEM, BlaCTX-M, and TetA Antibiotic-Resistance Genes. Sci. Rep. 2020, 10, 15961. [Google Scholar] [CrossRef]
- Oliveira Júnior, N.G.; Souza, C.M.; Buccini, D.F.; Cardoso, M.H.; Franco, O.L. Antimicrobial Peptides: Structure, Functions and Translational Applications. Nat. Rev. Microbiol. 2025, 1–14. [Google Scholar] [CrossRef]
- Villa, F.A.; Gerwick, L. Marine Natural Product Drug Discovery: Leads for Treatment of Inflammation, Cancer, Infections, and Neurological Disorders. Immunopharmacol. Immunotoxicol. 2010, 32, 228–237. [Google Scholar] [CrossRef]
- Blunt, J.W.; Copp, B.R.; Munro, M.H.G.; Northcote, P.T.; Prinsep, M.R. Marine Natural Products. Nat. Prod. Rep. 2011, 28, 196–268. [Google Scholar] [CrossRef]
- Wang, G.; Li, X.; Wang, Z. APD3: The Antimicrobial Peptide Database as a Tool for Research and Education. Nucleic Acids Res. 2015, 44, D1087–D1093. [Google Scholar] [CrossRef]
- Veltri, D.; Kamath, U.; Shehu, A. Deep Learning Improves Antimicrobial Peptide Recognition. Bioinformatics 2018, 34, 2740–2747. [Google Scholar] [CrossRef]
- Zhang, L.; Gallo, R.L. Antimicrobial Peptides. Curr. Biol. 2016, 26, R14–R19. [Google Scholar] [CrossRef]
- Patra, A.; Das, J.; Agrawal, N.R.; Kushwaha, G.S.; Ghosh, M.; Son, Y.-O. Marine Antimicrobial Peptides-Based Strategies for Tackling Bacterial Biofilm and Biofouling Challenges. Molecules 2022, 27, 7546. [Google Scholar] [CrossRef]
- Rajanbabu, V.; Chen, J.Y.; Wu, J.L. Antimicrobial Peptides from Marine Organisms; Springer: Berlin, Germany, 2015; pp. 747–758. [Google Scholar] [CrossRef]
- Otero-Gonzáiez, A.J.; Magalhães, B.S.; Garcia-Villarino, M.; López-Abarrategui, C.; Sousa, D.A.; Dias, S.C.; Franco, O.L. Antimicrobial Peptides from Marine Invertebrates as a New Frontier for Microbial Infection Control. FASEB J. 2010, 24, 1320–1334. [Google Scholar] [CrossRef]
- Schmid-Hempel, P. Variation in Immune Defence as a Question of Evolutionary Ecology. Proc. R. Soc. Lond. B Biol. Sci. 2003, 270, 357–366. [Google Scholar] [CrossRef]
- Lanz-Mendoza, H.; Contreras-Garduño, J. Innate Immune Memory in Invertebrates: Concept and Potential Mechanisms. Dev. Comp. Immunol. 2022, 127, 104285. [Google Scholar] [CrossRef] [PubMed]
- Tincu, J.A.; Taylor, S.W. Antimicrobial Peptides from Marine Invertebrates. Antimicrob. Agents Chemother. 2004, 48, 3645–3654. [Google Scholar] [CrossRef] [PubMed]
- Shinn, A.P.; Pratoomyot, J.; Bron, J.E.; Paladini, G. Economic Impacts of Aquatic Parasites on Global Finfish Production. Glob. Aquac. Advocate 2015, 2015, 58–61. [Google Scholar]
- Schmitt, P.; Rosa, R.D.; Destoumieux-Garzón, D. An Intimate Link between Antimicrobial Peptide Sequence Diversity and Binding to Essential Components of Bacterial Membranes. Biochim. Biophys. Acta (BBA) Biomembr. 2016, 1858, 958–970. [Google Scholar] [CrossRef]
- Falanga, A.; Lombardi, L.; Franci, G.; Vitiello, M.; Iovene, M.; Morelli, G.; Galdiero, M.; Galdiero, S. Marine Antimicrobial Peptides: Nature Provides Templates for the Design of Novel Compounds against Pathogenic Bacteria. Int. J. Mol. Sci. 2016, 17, 785. [Google Scholar] [CrossRef]
- Bertrand, B.; Munoz-Garay, C. Marine Antimicrobial Peptides: A Promising Source of New Generation Antibiotics and Other Bio-Active Molecules. Int. J. Pept. Res. Ther. 2018, 25, 1441–1450. [Google Scholar] [CrossRef]
- Tasiemski, A.; Schikorski, D.; Le Marrec-Croq, F.; Pontoire-Van Camp, C.; Boidin-Wichlacz, C.; Sautière, P.-E. Hedistin: A Novel Antimicrobial Peptide Containing Bromotryptophan Constitutively Expressed in the NK Cells-like of the Marine Annelid, Nereis Diversicolor. Dev. Comp. Immunol. 2007, 31, 749–762. [Google Scholar] [CrossRef]
- Bir, J.; Islam, S.S.; Sabbir, W.; Islam, R.; Huq, K.A. Ecology and Reproductive Biology of Mud Crab Scylla Spp: A Study of Commercial Mud Crab in Bangladesh. Int. J. Acad. Res. Dev. 2020, 5, 1–7. [Google Scholar]
- Salzet, M.; Tasiemski, A.; Cooper, E. Innate Immunity in Lophotrochozoans: The Annelids. Curr. Pharm. Des. 2006, 12, 3043–3050. [Google Scholar] [CrossRef]
- Bruno, R.; Maresca, M.; Canaan, S.; Cavalier, J.-F.; Mabrouk, K.; Boidin-Wichlacz, C.; Olleik, H.; Zeppilli, D.; Brodin, P.; Massol, F.; et al. Worms’ Antimicrobial Peptides. Mar. Drugs 2019, 17, 512. [Google Scholar] [CrossRef]
- Schikorski, D.; Cuvillier-Hot, V.; Leippe, M.; Boidin-Wichlacz, C.; Slomianny, C.; Macagno, E.; Salzet, M.; Tasiemski, A. Microbial Challenge Promotes the Regenerative Process of the Injured Central Nervous System of the Medicinal Leech by Inducing the Synthesis of Antimicrobial Peptides in Neurons and Microglia. J. Immunol. 2008, 181, 1083–1095. [Google Scholar] [CrossRef]
- Cho, J.H.; Park, C.B.; Yoon, Y.G.; Kim, S.C. Lumbricin I, a Novel Proline-Rich Antimicrobial Peptide from the Earthworm: Purification, CDNA Cloning and Molecular Characterization. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 1998, 1408, 67–76. [Google Scholar] [CrossRef]
- Bodó, K.; Boros, Á.; Rumpler, É.; Molnár, L.; Böröcz, K.; Németh, P.; Engelmann, P. Identification of Novel Lumbricin Homologues in Eisenia Andrei Earthworms. Dev. Comp. Immunol. 2019, 90, 41–46. [Google Scholar] [CrossRef]
- Li, W.; Li, S.; Zhong, J.; Zhu, Z.; Liu, J.; Wang, W. A Novel Antimicrobial Peptide from Skin Secretions of the Earthworm, Pheretima Guillelmi (Michaelsen). Peptides 2011, 32, 1146–1150. [Google Scholar] [CrossRef] [PubMed]
- Tasiemski, A.; Vandenbulcke, F.; Mitta, G.; Lemoine, J.; Lefebvre, C.; Sautière, P.-E.; Salzet, M. Molecular Characterization of Two Novel Antibacterial Peptides Inducible upon Bacterial Challenge in an Annelid, the Leech Theromyzon Tessulatum. J. Biol. Chem. 2004, 279, 30973–30982. [Google Scholar] [CrossRef] [PubMed]
- Ovchinnikova, T.V.; Aleshina, G.M.; Balandin, S.V.; Krasnosdembskaya, A.D.; Markelov, M.L.; Frolova, E.I.; Leonova, Y.F.; Tagaev, A.A.; Krasnodembsky, E.G.; Kokryakov, V.N. Purification and Primary Structure of Two Isoforms of Arenicin, a Novel Antimicrobial Peptide from Marine Polychaeta Arenicola marina. FEBS Lett. 2004, 577, 209–214. [Google Scholar] [CrossRef]
- Ovchinnikova, T.V.; Shenkarev, Z.O.; Nadezhdin, K.D.; Balandin, S.V.; Zhmak, M.N.; Kudelina, I.A.; Finkina, E.I.; Kokryakov, V.N.; Arseniev, A.S. Recombinant Expression, Synthesis, Purification, and Solution Structure of Arenicin. Biochem. Biophys. Res. Commun. 2007, 360, 156–162. [Google Scholar] [CrossRef]
- Orlov, D.S.; Shamova, O.V.; Eliseev, I.E.; Zharkova, M.S.; Chakchir, O.B.; Antcheva, N.; Zachariev, S.; Panteleev, P.V.; Kokryakov, V.N.; Ovchinnikova, T.V.; et al. Redesigning Arenicin-1, an Antimicrobial Peptide from the Marine Polychaeta Arenicola Marina, by Strand Rearrangement or Branching, Substitution of Specific Residues, and Backbone Linearization or Cyclization. Mar. Drugs 2019, 17, 376. [Google Scholar] [CrossRef]
- Safronova, V.N.; Bolosov, I.A.; Kruglikov, R.N.; Korobova, O.V.; Pereskokova, E.S.; Borzilov, A.I.; Panteleev, P.V.; Ovchinnikova, T. V Novel β-Hairpin Peptide from Marine Polychaeta with a High Efficacy against Gram-Negative Pathogens. Mar. Drugs 2022, 20, 517. [Google Scholar] [CrossRef]
- Panteleev, P.; Tsarev, A.; Bolosov, I.; Paramonov, A.S.; Marggraf, M.B.; Sychev, S.V.; Shenkarev, Z.O.; Ovchinnikova, T.V. Novel Antimicrobial Peptides from the Arctic Polychaeta Nicomache Minor Provide New Molecular Insight into Biological Role of the BRICHOS Domain. Mar. Drugs 2018, 16, 401. [Google Scholar] [CrossRef]
- Tasiemski, A.; Jung, S.; Boidin-Wichlacz, C.; Jollivet, D.; Cuvillier-Hot, V.; Pradillon, F.; Vetriani, C.; Hecht, O.; Sönnichsen, F.D.; Gelhaus, C.; et al. Characterization and Function of the First Antibiotic Isolated from a Vent Organism: The Extremophile Metazoan Alvinella Pompejana. PLoS ONE 2014, 9, e95737. [Google Scholar] [CrossRef]
- Shepperson, O.A.; Hanna, C.C.; Brimble, M.A.; Harris, P.W.R.; Cameron, A.J. Total Synthesis of Novel Antimicrobial β-Hairpin Capitellacin Via Rapid Flow-Based SPPS Assembly and Regioselective On-Resin Disulfide Cyclisation. Int. J. Pept. Res. Ther. 2021, 28, 32. [Google Scholar] [CrossRef]
- Bruno, R.; Boidin-Wichlacz, C.; Melnyk, O.; Zeppilli, D.; Landon, C.; Thomas, F.; Cambon, M.-A.; Lafond, M.; Mabrouk, K.; Massol, F.; et al. The Diversification of the Antimicrobial Peptides from Marine Worms Is Driven by Environmental Conditions. Sci. Total Environ. 2023, 879, 162875. [Google Scholar] [CrossRef]
- Panteleev, P.V.; Safronova, V.N.; Duan, S.; Komlev, A.S.; Bolosov, I.A.; Kruglikov, R.N.; Kombarova, T.I.; Korobova, O.V.; Pereskokova, E.S.; Borzilov, A.I.; et al. Novel BRICHOS-Related Antimicrobial Peptides from the Marine Worm Heteromastus Filiformis: Transcriptome Mining, Synthesis, Biological Activities, and Therapeutic Potential. Mar. Drugs 2023, 21, 639. [Google Scholar] [CrossRef]
- Safronova, V.N.; Panteleev, P.V.; Kruglikov, R.N.; Bolosov, I.A.; Finkina, E.I.; Ovchinnikova, T.V. Novel BRICHOS-Related Defensin-like Antimicrobial Peptide from the Marine Polychaeta Arenicola Marina. Russ. J. Bioorg. Chem. 2024, 50, 629–643. [Google Scholar] [CrossRef]
- Pan, W.; Liu, X.; Ge, F.; Han, J.; Zheng, T. Perinerin, A Novel Antimicrobial Peptide Purified from the Clamworm Perinereis Aibuhitensis Grube and Its Partial Characterization. J. Biochem. 2004, 135, 297–304. [Google Scholar] [CrossRef]
- Schierwater, B.; DeSalle, R. Invertebrate Zoology: A Tree of Life Approach; CRC Press: Boca Raton, FL, USA, 2021; ISBN 9781482235821. [Google Scholar]
- Dijkstra, K.-D.B.; Monaghan, M.T.; Pauls, S.U. Freshwater Biodiversity and Aquatic Insect Diversification. Annu. Rev. Entomol. 2014, 59, 143–163. [Google Scholar] [CrossRef]
- Wei, Y.; Lin, D.; Xu, Z.; Gao, X.; Zeng, C.; Ye, H. A Possible Role of Crustacean Cardioactive Peptide in Regulating Immune Response in Hepatopancreas of Mud Crab. Front. Immunol. 2020, 11, 711. [Google Scholar] [CrossRef]
- Tassanakajon, A.; Amparyup, P.; Somboonwiwat, K.; Supungul, P. Cationic Antimicrobial Peptides in Penaeid Shrimp. Mar. Biotechnol. 2011, 13, 639–657. [Google Scholar] [CrossRef]
- Somboonwiwat, K.; Marcos, M.; Tassanakajon, A.; Klinbunga, S.; Aumelas, A.; Romestand, B.; Gueguen, Y.; Boze, H.; Moulin, G.; Bachere, E. Recombinant expression and anti-microbial activity of anti-lipopolysaccharide factor (ALF) from the black tiger shrimp. Dev. Comp. Immunol. 2005, 29, 841–851. [Google Scholar] [CrossRef]
- Löfgren, S.E.; Smânia, A.; Smânia, E.D.F.A.; Bachère, E.; Barracco, M.A. Comparative Activity and Stability under Salinity Conditions of Different Antimicrobial Peptides Isolated from Aquatic Animals. Aquac. Res. 2009, 40, 1805–1812. [Google Scholar] [CrossRef]
- Relf, J.M.; Chisholm, J.R.S.; Kemp, G.D.; Smith, V.J. Purification and Characterization of a Cysteine-rich 11.5-kDa Antibacterial Protein from the Granular Haemocytes of the Shore Crab, Carcinus maenas. Eur. J. Biochem. 1999, 264, 350–357. [Google Scholar] [CrossRef]
- Smith, V.J.; Fernandes, J.M.O.; Kemp, G.D.; Hauton, C. Crustins: Enigmatic WAP Domain-Containing Antibacterial Proteins from Crustaceans. Dev. Comp. Immunol. 2008, 32, 758–772. [Google Scholar] [CrossRef]
- McTaggart, S.J.; Conlon, C.; Colbourne, J.K.; Blaxter, M.L.; Little, T.J. The Components of the Daphnia Pulex Immune System as Revealed by Complete Genome Sequencing. BMC Genom. 2009, 10, 175. [Google Scholar] [CrossRef] [PubMed]
- Destoumieux, D.; Bulet, P.; Strub, J.; van Dorsselaer, A.; Bachère, E. Recombinant Expression and Range of Activity of Penaeidins, Antimicrobial Peptides from Penaeid Shrimp. Eur. J. Biochem. 1999, 266, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Smith, V.J.; Desbois, A.P.; Dyrynda, E.A. Conventional and Unconventional Antimicrobials from Fish, Marine Invertebrates and Micro-Algae. Mar. Drugs 2010, 8, 1213–1262. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Furunaka, H.; Miyata, T.; Tokunaga, F.; Muta, T.; Iwanaga, S.; Niwa, M.; Takao, T.; Shimonishi, Y. Tachyplesin, a Class of Antimicrobial Peptide from the Hemocytes of the Horseshoe Crab (Tachypleus tridentatus). Isolation and Chemical Structure. J. Biol. Chem. 1988, 263, 16709–16713. [Google Scholar] [CrossRef]
- Paredes-Gamero, E.J.; Martins, M.N.C.; Cappabianco, F.A.M.; Ide, J.S.; Miranda, A. Characterization of Dual Effects Induced by Antimicrobial Peptides: Regulated Cell Death or Membrane Disruption. Biochim. Biophys. Acta (BBA) Gen. Subj. 2012, 1820, 1062–1072. [Google Scholar] [CrossRef]
- Vernen, F.; Harvey, P.J.; Dias, S.A.; Veiga, A.S.; Huang, Y.-H.; Craik, D.J.; Lawrence, N.; Troeira Henriques, S. Characterization of Tachyplesin Peptides and Their Cyclized Analogues to Improve Antimicrobial and Anticancer Properties. Int. J. Mol. Sci. 2019, 20, 4184. [Google Scholar] [CrossRef]
- Pallavicini, A.; del Mar Costa, M.; Gestal, C.; Dreos, R.; Figueras, A.; Venier, P.; Novoa, B. High Sequence Variability of Myticin Transcripts in Hemocytes of Immune-Stimulated Mussels Suggests Ancient Host–Pathogen Interactions. Dev. Comp. Immunol. 2008, 32, 213–226. [Google Scholar] [CrossRef]
- Panteleev, P.V.; Tsarev, A.V.; Safronova, V.N.; Reznikova, O.V.; Bolosov, I.A.; Sychev, S.V.; Shenkarev, Z.O.; Ovchinnikova, T. V Structure Elucidation and Functional Studies of a Novel β-Hairpin Antimicrobial Peptide from the Marine Polychaeta Capitella Teleta. Mar. Drugs 2020, 18, 620. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Scott, M.G.; Yan, H.; Mayer, L.D.; Hancock, R.E.W. Interaction of Polyphemusin I and Structural Analogs with Bacterial Membranes, Lipopolysaccharide, and Lipid Monolayers. Biochemistry 2000, 39, 14504–14514. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, S.-I.; Nagayama, R.; Hirata, M.; Shigenaga, T.; Agarwala, K.L.; Saito, T.; Cho, J.; Nakajima, H.; Takagi, T.; Iwanaga, S. Tachycitin, a Small Granular Component in Horseshoe Crab Hemocytes, Is an Antimicrobial Protein with Chitin-Binding Activity. J. Biochem. 1996, 120, 1253–1260. [Google Scholar] [CrossRef] [PubMed]
- Suetake, T.; Tsuda, S.; Kawabata, S.; Miura, K.; Iwanaga, S.; Hikichi, K.; Nitta, K.; Kawano, K. Chitin-Binding Proteins in Invertebrates and Plants Comprise a Common Chitin-Binding Structural Motif. J. Biol. Chem. 2000, 275, 17929–17932. [Google Scholar] [CrossRef]
- Pereira, A.; Valdés-Muñoz, E.; Marican, A.; Cabrera-Barjas, G.; Vijayakumar, S.; Valdés, O.; Rafael, D.; Andrade, F.; Abaca, P.; Bustos, D.; et al. Rational Design of Hydrogels for Cationic Antimicrobial Peptide Delivery: A Molecular Modeling Approach. Pharmaceutics 2023, 15, 474. [Google Scholar] [CrossRef]
- Plaza, A.; Bifulco, G.; Keffer, J.L.; Lloyd, J.R.; Baker, H.L.; Bewley, C.A. Celebesides A−C and Theopapuamides B−D, Depsipeptides from an Indonesian Sponge That Inhibit HIV-1 Entry. J. Org. Chem. 2008, 74, 504–512. [Google Scholar] [CrossRef]
- Portet, A.; Toulza, E.; Lokmer, A.; Huot, C.; Duval, D.; Galinier, R.; Gourbal, B. Experimental Infection of the Biomphalaria glabrata Vector Snail by Schistosoma mansoni Parasites Drives Snail Microbiota Dysbiosis. Microorganisms 2021, 9, 1084. [Google Scholar] [CrossRef]
- Qin, C.L.; Huang, W.; Zhou, S.Q.; Wang, X.C.; Liu, H.H.; Fan, M.H.; Wang, R.X.; Gao, P.; Liao, Z. Characterization of a Novel Antimicrobial Peptide with Chiting-Biding Domain from Mytilus Coruscus. Fish Shellfish Immunol. 2014, 41, 362–370. [Google Scholar] [CrossRef]
- Quinn, G.A.P.; Heymans, R.; Rondaj, F.; Shaw, C.; de Jong-Brink, M. Schistosoma mansoni dermaseptin-like peptide: Structural and functional characterization. J. Parasitol. 2005, 91, 1340–1351. [Google Scholar] [CrossRef]
- Fujitani, N.; Kawabata, S.; Osaki, T.; Kumaki, Y.; Demura, M.; Nitta, K.; Kawano, K. Structure of the Antimicrobial Peptide Tachystatin, A.J. Biol. Chem. 2002, 277, 23651–23657. [Google Scholar] [CrossRef]
- Fujitani, N.; Kouno, T.; Nakahara, T.; Takaya, K.; Osaki, T.; Kawabata, S.; Mizuguchi, M.; Aizawa, T.; Demura, M.; Nishimura, S.; et al. The Solution Structure of Horseshoe Crab Antimicrobial Peptide Tachystatin B with an Inhibitory Cystine-knot Motif. J. Pept. Sci. 2007, 13, 269–279. [Google Scholar] [CrossRef]
- Wang, G.; Cui, Y.; Liu, H.; Tian, Y.; Li, S.; Fan, Y.; Sun, S.; Wu, D.; Peng, C. Antibacterial Peptides-Loaded Bioactive Materials for the Treatment of Bone Infection. Colloids Surf. B Biointerfaces 2023, 225, 113255. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-F.; Xie, X.-Y.; Huang, Y.; Li, Y.-K.; Liu, H.; Chen, X.-L.; Wang, H.-L. Identification of a Novel Antimicrobial Peptide From the Ancient Marine Arthropod Chinese Horseshoe Crab, Tachypleus tridentatus. Front. Immunol. 2022, 13, 794779. [Google Scholar] [CrossRef] [PubMed]
- Stensvåg, K.; Haug, T.; Sperstad, S.V.; Rekdal, Ø.; Indrevoll, B.; Styrvold, O.B. Arasin 1, a Proline–Arginine-Rich Antimicrobial Peptide Isolated from the Spider Crab, Hyas araneus. Dev. Comp. Immunol. 2008, 32, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Noga, E.J.; Stone, K.L.; Wood, A.; Gordon, W.L.; Robinette, D. Primary Structure and Cellular Localization of Callinectin, an Antimicrobial Peptide from the Blue Crab. Dev. Comp. Immunol. 2011, 35, 409–415. [Google Scholar] [CrossRef]
- Imjongjirak, C.; Amparyup, P.; Tassanakajon, A. Molecular Cloning, Genomic Organization and Antibacterial Activity of a Second Isoform of Antilipopolysaccharide Factor (ALF) from the Mud Crab, Scylla paramamosain. Fish Shellfish Immunol. 2011, 30, 58–66. [Google Scholar] [CrossRef]
- Anju, A.; Smitha, C.K.; Preetha, K.; Boobal, R.; Rosamma, P. Molecular Characterization, Recombinant Expression and Bioactivity Profile of an Antimicrobial Peptide, Ss-Arasin from the Indian Mud Crab, Scylla serrata. Fish Shellfish Immunol. 2019, 88, 352–358. [Google Scholar] [CrossRef]
- Chai, L.-Q.; Li, W.-W.; Wang, X.-W. Identification and Characterization of Two Arasin-like Peptides in Red Swamp Crayfish Procambarus clarkii. Fish Shellfish Immunol. 2017, 70, 673–681. [Google Scholar] [CrossRef]
- Zhang, Q.; Zou, D.; Zeng, X.; Li, L.; Wang, A.; Liu, F. Effect of the Direct Use of Biomass in Agricultural Soil on Heavy Metals Activation or Immobilization? Environ. Pollut. 2021, 272, 115989. [Google Scholar] [CrossRef]
- Paulsen, V.S.; Blencke, H.-M.; Benincasa, M.; Haug, T.; Eksteen, J.J.; Styrvold, O.B.; Scocchi, M.; Stensvåg, K. Structure-Activity Relationships of the Antimicrobial Peptide Arasin 1—And Mode of Action Studies of the N-Terminal, Proline-Rich Region. PLoS ONE 2013, 8, e53326. [Google Scholar] [CrossRef]
- Rey-Campos, M.; Novoa, B.; Pallavicini, A.; Gerdol, M.; Figueras, A. Comparative Genomics Reveals 13 Different Isoforms of Mytimycins (A–M) in Mytilus galloprovincialis. Int. J. Mol. Sci. 2021, 22, 3235. [Google Scholar] [CrossRef]
- Rey-Campos, M.; Novoa, B.; Pallavicini, A.; Gerdol, M.; Figueras, A. Comparative Genomics Reveals a Significant Sequence Variability of Myticin Genes in Mytilus galloprovincialis. Biomolecules 2020, 10, 943. [Google Scholar] [CrossRef]
- Rey-Campos, M.; Moreira, R.; Romero, A.; Medina-Gali, R.M.; Novoa, B.; Gasset, M.; Figueras, A. Transcriptomic Analysis Reveals the Wound Healing Activity of Mussel Myticin C. Biomolecules 2020, 10, 133. [Google Scholar] [CrossRef]
- Ravichandr, S.; Kumaravel, K.; Rameshkuma, G.; AjithKumar, T.T. Antimicrobial Peptides from the Marine Fishes. Res. J. Immunol. 2010, 3, 146–156. [Google Scholar] [CrossRef]
- Raju, S.V.; Sarkar, P.; Kumar, P.; Arockiaraj, J. Piscidin, Fish Antimicrobial Peptide: Structure, Classification, Properties, Mechanism, Gene Regulation and Therapeutical Importance. Int. J. Pept. Res. Ther. 2020, 27, 91–107. [Google Scholar] [CrossRef]
- Rajapaksha, D.C.; Jayathilaka, E.H.T.T.; Edirisinghe, S.L.; Nikapitiya, C.; Lee, J.; Whang, I.; De Zoysa, M. Octopromycin: Antibacterial and Antibiofilm Functions of a Novel Peptide Derived from Octopus Minor against Multidrug-Resistant Acinetobacter baumannii. Fish Shellfish Immunol. 2021, 117, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Scocchi, M.; Tossi, A.; Gennaro, R. Proline-Rich Antimicrobial Peptides: Converging to a Non-Lytic Mechanism of Action. Cell. Mol. Life Sci. 2011, 68, 2317–2330. [Google Scholar] [CrossRef] [PubMed]
- Imjongjirak, C.; Amphaiphan, P.; Charoensapsri, W.; Amparyup, P. Characterization and Antimicrobial Evaluation of Sp PR-AMP1, a Proline-Rich Antimicrobial Peptide from the Mud Crab Scylla paramamosain. Dev. Comp. Immunol. 2017, 74, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, P.; de Lorgeril, J.; Gueguen, Y.; Destoumieux-Garzón, D.; Bachère, E. Expression, Tissue Localization and Synergy of Antimicrobial Peptides and Proteins in the Immune Response of the Oyster Crassostrea gigas. Dev. Comp. Immunol. 2012, 37, 363–370. [Google Scholar] [CrossRef]
- Li, W.; Tailhades, J.; O’Brien-Simpson, N.M.; Separovic, F.; Otvos, L.; Hossain, M.A.; Wade, J.D. Proline-Rich Antimicrobial Peptides: Potential Therapeutics against Antibiotic-Resistant Bacteria. Amino Acids 2014, 46, 2287–2294. [Google Scholar] [CrossRef]
- Xie, Y.; Wan, H.; Zeng, X.; Zhang, Z.; Wang, Y. Characterization and Antimicrobial Evaluation of a New Spgly-AMP, Glycine-Rich Antimicrobial Peptide from the Mud Crab Scylla paramamosain. Fish Shellfish Immunol. 2020, 106, 384–392. [Google Scholar] [CrossRef]
- Semreen, M.H.; El-Gamal, M.I.; Abdin, S.; Alkhazraji, H.; Kamal, L.; Hammad, S.; El-Awady, F.; Waleed, D.; Kourbaj, L. Recent Updates of Marine Antimicrobial Peptides. Saudi Pharm. J. 2018, 26, 396–409. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.-K.; Go, H.-J.; Kim, C.-H.; Nam, B.-H.; Park, N.G. Antimicrobial Peptide, HdMolluscidin, Purified from the Gill of the Abalone, Haliotis discus. Fish Shellfish Immunol. 2016, 52, 289–297. [Google Scholar] [CrossRef]
- Sperstad, S.V.; Haug, T.; Vasskog, T.; Stensvåg, K. Hyastatin, a Glycine-Rich Multi-Domain Antimicrobial Peptide Isolated from the Spider Crab (Hyas araneus) Hemocytes. Mol. Immunol. 2009, 46, 2604–2612. [Google Scholar] [CrossRef] [PubMed]
- Shan, Z.; Zhu, K.; Peng, H.; Chen, B.; Liu, J.; Chen, F.; Ma, X.; Wang, S.; Qiao, K.; Wang, K. The New Antimicrobial Peptide SpHyastatin from the Mud Crab Scylla paramamosain with Multiple Antimicrobial Mechanisms and High Effect on Bacterial Infection. Front. Microbiol. 2016, 7, 1140. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.-K.; Lee, M.J.; Nam, B.-H.; Park, N.G. CgMolluscidin, a Novel Dibasic Residue Repeat Rich Antimicrobial Peptide, Purified from the Gill of the Pacific Oyster, Crassostrea gigas. Fish Shellfish Immunol. 2013, 35, 480–488. [Google Scholar] [CrossRef]
- Shabir, U.; Ali, S.; Magray, A.R.; Ganai, B.A.; Firdous, P.; Hassan, T.; Nazir, R. Fish Antimicrobial Peptides (AMP’s) as Essential and Promising Molecular Therapeutic Agents: A Review. Microb. Pathog. 2018, 114, 50–56. [Google Scholar] [CrossRef]
- Shan, Z.; Yang, Y.; Guan, N.; Xia, X.; Liu, W. NKL-24: A Novel Antimicrobial Peptide Derived from Zebrafish NK-Lysin That Inhibits Bacterial Growth and Enhances Resistance against Vibrio Parahaemolyticus Infection in Yesso Scallop, Patinopecten yessoensis. Fish Shellfish Immunol. 2020, 106, 431–440. [Google Scholar] [CrossRef]
- Liu, H.; Lei, M.; Du, X.; Cui, P.; Zhang, S. Identification of a Novel Antimicrobial Peptide from Amphioxus Branchiostoma Japonicum by in Silico and Functional Analyses. Sci. Rep. 2015, 5, 18355. [Google Scholar] [CrossRef]
- Nam, J.; Yun, H.; Rajasekaran, G.; Kumar, S.D.; Kim, J.I.; Min, H.J.; Shin, S.Y.; Lee, C.W. Structural and Functional Assessment of MBjAMP1, an Antimicrobial Peptide from Branchiostoma japonicum, Revealed a Novel α-Hairpinin-like Scaffold with Membrane Permeable and DNA Binding Activity. J. Med. Chem. 2018, 61, 11101–11113. [Google Scholar] [CrossRef]
- Sultana, A.; Luo, H.; Ramakrishna, S. Antimicrobial Peptides and Their Applications in Biomedical Sector. Antibiotics 2021, 10, 1094. [Google Scholar] [CrossRef] [PubMed]
- Szymczak, P.; Możejko, M.; Grzegorzek, T.; Jurczak, R.; Bauer, M.; Neubauer, D.; Sikora, K.; Michalski, M.; Sroka, J.; Setny, P.; et al. Discovering Highly Potent Antimicrobial Peptides with Deep Generative Model HydrAMP. Nat. Commun. 2023, 14, 1453. [Google Scholar] [CrossRef] [PubMed]
- Szymczak, P.; Zarzecki, W.; Wang, J.; Duan, Y.; Wang, J.; Coelho, L.P.; de la Fuente-Nunez, C.; Szczurek, E. AI-Driven Antimicrobial Peptide Discovery: Mining and Generation. Acc. Chem. Res. 2025, 58, 1831–1846. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kang, H.K.; Choi, M.-C.; Chae, J.D.; Son, B.K.; Chong, Y.P.; Seo, C.H.; Park, Y. Antibacterial Activity and Mechanism of Action of Analogues Derived from the Antimicrobial Peptide MBjAMP1 Isolated from Branchiostoma japonicum. J. Antimicrob. Chemother. 2018, 73, 2054–2063. [Google Scholar] [CrossRef]
- Lee, I.H.; Cho, Y.; Lehrer, R.I. Styelins, Broad-Spectrum Antimicrobial Peptides from the Solitary Tunicate, Styela Clava. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 1997, 118, 515–521. [Google Scholar] [CrossRef]
- In, I.-H.; Zhao, C.; Nguyen, T.; Menzel, L.; Waring, A.J.; Lehrer, R.I.; Sherman, M.A. Clavaspirin, an Antibacterial and Haemolytic Peptide from Styela clava. J. Pept. Res. 2001, 58, 445–456. [Google Scholar] [CrossRef]
- Zhao, C.; Liaw, L.; Lee, I.H.; Lehrer, R.I. CDNA Cloning of Three Cecropin-like Antimicrobial Peptides (Styelins) from the Tunicate, Styela clava. FEBS Lett. 1997, 412, 144–148. [Google Scholar] [CrossRef]
- Jang, W.S.; Kim, K.N.; Lee, Y.S.; Nam, M.H.; Lee, I.H. Halocidin: A New Antimicrobial Peptide from Hemocytes of the Solitary Tunicate, Halocynthia aurantium. FEBS Lett. 2002, 521, 81–86. [Google Scholar] [CrossRef]
- Jang, W.S.; Kim, H.K.; Lee, K.Y.; Kim, S.A.; Han, Y.S.; Lee, I.H. Antifungal Activity of Synthetic Peptide Derived from Halocidin, Antimicrobial Peptide from the Tunicate, Halocynthia aurantium. FEBS Lett. 2006, 580, 1490–1496. [Google Scholar] [CrossRef]
- Lee, I.H.; Lee, Y.S.; Kim, C.H.; Kim, C.R.; Hong, T.; Menzel, L.; Boo, L.M.; Pohl, J.; Sherman, M.A.; Waring, A.; et al. Dicynthaurin: An Antimicrobial Peptide from Hemocytes of the Solitary Tunicate, Halocynthia aurantium. Biochim. Biophys. Acta (BBA) Gen. Subj. 2001, 1527, 141–148. [Google Scholar] [CrossRef]
- Galinier, R.; Roger, E.; Sautiere, P.; Aumelas, A.; Banaigs, B.; Mitta, G. Halocyntin and Papillosin, Two New Antimicrobial Peptides Isolated from Hemocytes of the Solitary Tunicate, Halocynthia papillosa. J. Pept. Sci. 2009, 15, 48–55. [Google Scholar] [CrossRef]
- Fedders, H.; Leippe, M. A Reverse Search for Antimicrobial Peptides in Ciona Intestinalis: Identification of a Gene Family Expressed in Hemocytes and Evaluation of Activity. Dev. Comp. Immunol. 2008, 32, 286–298. [Google Scholar] [CrossRef] [PubMed]
- Tessera, V.; Guida, F.; Juretić, D.; Tossi, A. Identification of Antimicrobial Peptides from Teleosts and Anurans in Expressed Sequence Tag Databases Using Conserved Signal Sequences. FEBS J. 2012, 279, 724–736. [Google Scholar] [CrossRef] [PubMed]
- Tessler, M.; Neumann, J.S.; Kamm, K.; Osigus, H.-J.; Eshel, G.; Narechania, A.; Burns, J.A.; DeSalle, R.; Schierwater, B. Phylogenomics and the First Higher Taxonomy of Placozoa, an Ancient and Enigmatic Animal Phylum. Front. Ecol. Evol. 2022, 10, 1016357. [Google Scholar] [CrossRef]
- Lu, Y.; Zhuang, Y.; Liu, J. Mining Antimicrobial Peptides from Small Open Reading Frames in Ciona intestinalis. J. Pept. Sci. 2013, 20, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Hansen, I.K.Ø.; Lövdahl, T.; Simonovic, D.; Hansen, K.Ø.; Andersen, A.J.C.; Devold, H.; Richard, C.S.M.; Andersen, J.H.; Strøm, M.B.; Haug, T. Antimicrobial Activity of Small Synthetic Peptides Based on the Marine Peptide Turgencin A: Prediction of Antimicrobial Peptide Sequences in a Natural Peptide and Strategy for Optimization of Potency. Int. J. Mol. Sci. 2020, 21, 5460. [Google Scholar] [CrossRef]
- Daly, M.; Brugler, M.R.; Cartwright, P.; Collins, A.G.; Dawson, M.N.; Fautin, D.G.; France, S.; McFadden, C.S.; Opresko, D.M.; Rodriguez, E.; et al. The phylum Cnidaria: A review of phylogenetic patterns and diversity 300 years after Linnaeus. Zootaxa 2007, 1668, 127–182. [Google Scholar] [CrossRef]
- Fautin, D.G. Structural Diversity, Systematics, and Evolution of Cnidae. Toxicon 2009, 54, 1054–1064. [Google Scholar] [CrossRef]
- Rodrigues, T.; Domínguez-Pérez, D.; Almeida, D.; Matos, A.; Antunes, A. Medusozoans Reported in Portugal and Its Ecological and Economical Relevance. Reg. Stud. Mar. Sci. 2020, 35, 101230. [Google Scholar] [CrossRef]
- Rodrigues, T.; Almeida, D.; Guardiola, F.A.; Borges, P.A.V.; Antunes, A. A Comprehensive Compilation of Iberian Medusozoan Data: Diversity, Ecology, and Omics Insights. Reg. Stud. Mar. Sci. 2024, 73, 103462. [Google Scholar] [CrossRef]
- Hore, G.; Ghosh, S.; Banerjee, D. Cnidaria Immune System and Nanoparticles. Immune Syst. Anim. 2022, 2, 13–48. [Google Scholar]
- Brown, T.; Rodriguez-Lanetty, M. Defending against Pathogens—Immunological Priming and Its Molecular Basis in a Sea Anemone, Cnidarian. Sci. Rep. 2015, 5, 17425. [Google Scholar] [CrossRef]
- Kayal, E.; Bentlage, B.; Sabrina Pankey, M.; Ohdera, A.H.; Medina, M.; Plachetzki, D.C.; Collins, A.G.; Ryan, J.F. Phylogenomics Provides a Robust Topology of the Major Cnidarian Lineages and Insights on the Origins of Key Organismal Traits. BMC Evol. Biol. 2018, 18, 68. [Google Scholar] [CrossRef]
- Vidal-Dupiol, J.; Ladrière, O.; Destoumieux-Garzón, D.; Sautière, P.-E.; Meistertzheim, A.-L.; Tambutté, E.; Tambutté, S.; Duval, D.; Fouré, L.; Adjeroud, M.; et al. Innate Immune Responses of a Scleractinian Coral to Vibriosis. J. Biol. Chem. 2011, 286, 22688–22698. [Google Scholar] [CrossRef]
- Mason, B.; Cooke, I.; Moya, A.; Augustin, R.; Lin, M.-F.; Satoh, N.; Bosch, T.C.G.; Bourne, D.G.; Hayward, D.C.; Andrade, N.; et al. AmAMP1 from Acropora millepora and Damicornin Define a Family of Coral-Specific Antimicrobial Peptides Related to the Shk Toxins of Sea Anemones. Dev. Comp. Immunol. 2021, 114, 103866. [Google Scholar] [CrossRef]
- Lima, L.; Migliolo, L.; Castro, C.; Pires, D.; Lopez-Abarrategui, C.; Goncalves, E.; Vasconcelos, I.; Oliveira, J.; Otero-Gonzalez, A.; Franco, O.; et al. Identification of a Novel Antimicrobial Peptide from Brazilian Coast Coral Phyllogorgia Dilatata. Protein Pept. Lett. 2013, 20, 1153–1158. [Google Scholar] [CrossRef]
- Kim, C.; Lee, Y.J.; Go, H.; Oh, H.Y.; Lee, T.K.; Park, J.B.; Park, N.G. Defensin-neurotoxin Dyad in a Basally Branching Metazoan Sea Anemone. FEBS J. 2017, 284, 3320–3338. [Google Scholar] [CrossRef]
- La Corte, C.; Catania, V.; Dara, M.; Parrinello, D.; Staropoli, M.; Trapani, M.R.; Cammarata, M.; Parisi, M.G. Equinins as Novel Broad-Spectrum Antimicrobial Peptides Isolated from the Cnidarian Actinia equina (Linnaeus, 1758). Mar. Drugs 2024, 22, 172. [Google Scholar] [CrossRef]
- Ovchinnikova, T.V.; Balandin, S.V.; Aleshina, G.M.; Tagaev, A.A.; Leonova, Y.F.; Krasnodembsky, E.D.; Men’shenin, A.V.; Kokryakov, V.N. Aurelin, a Novel Antimicrobial Peptide from Jellyfish Aurelia Aurita with Structural Features of Defensins and Channel-Blocking Toxins. Biochem. Biophys. Res. Commun. 2006, 348, 514–523. [Google Scholar] [CrossRef]
- Shenkarev, Z.O.; Panteleev, P.V.; Balandin, S.V.; Gizatullina, A.K.; Altukhov, D.A.; Finkina, E.I.; Kokryakov, V.N.; Arseniev, A.S.; Ovchinnikova, T. V Recombinant Expression and Solution Structure of Antimicrobial Peptide Aurelin from Jellyfish Aurelia aurita. Biochem. Biophys. Res. Commun. 2012, 429, 63–69. [Google Scholar] [CrossRef]
- Augustin, R.; Anton-Erxleben, F.; Jungnickel, S.; Hemmrich, G.; Spudy, B.; Podschun, R.; Bosch, T.C.G. Activity of the Novel Peptide Arminin against Multiresistant Human Pathogens Shows the Considerable Potential of Phylogenetically Ancient Organisms as Drug Sources. Antimicrob. Agents Chemother. 2009, 53, 5245–5250. [Google Scholar] [CrossRef]
- Liang, X.; Wang, R.; Dou, W.; Zhao, L.; Zhou, L.; Zhu, J.; Wang, K.; Yan, J. Arminin 1a-C, a Novel Antimicrobial Peptide from Ancient Metazoan Hydra, Shows Potent Antileukemia Activity against Drug-Sensitive and Drug-Resistant Leukemia Cells. Drug Des. Dev. Ther. 2018, 12, 3691–3703. [Google Scholar] [CrossRef]
- Joo, M.-S.; Choi, K.-M.; Cho, D.-H.; Choi, H.-S.; Min, E.Y.; Han, H.-J.; Cho, M.Y.; Bae, J.-S.; Park, C.-I. The Molecular Characterization, Expression Analysis and Antimicrobial Activity of Theromacin from Asian Polychaeta (Perinereis linea). Dev. Comp. Immunol. 2020, 112, 103773. [Google Scholar] [CrossRef]
- Fraune, S.; Augustin, R.; Anton-Erxleben, F.; Wittlieb, J.; Gelhaus, C.; Klimovich, V.B.; Samoilovich, M.P.; Bosch, T.C.G. In an Early Branching Metazoan, Bacterial Colonization of the Embryo Is Controlled by Maternal Antimicrobial Peptides. Proc. Natl. Acad. Sci. USA 2010, 107, 18067–18072. [Google Scholar] [CrossRef]
- Turner, R.L.; Meyer, C.E. Salinity Tolerance of the Brackish-Water Echinoderm Ophiophragmus filograneus (Ophiuroidea). Mar. Ecol. Prog. Ser. 1980, 2, 249–256. [Google Scholar] [CrossRef]
- Talbot, T.D.; Lawrence, J.M. The Effect of Salinity on Respiration, Excretion, Regeneration and Production in Ophiophragmus filograneus (Echinodermata: Ophiuroidea). J. Exp. Mar. Biol. Ecol. 2002, 275, 1–14. [Google Scholar] [CrossRef]
- Arizza, V.; Schillaci, D. Echinoderm Antimicrobial Peptides: The Ancient Arms of the Deuterostome Innate Immune System; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Li, C.; Blencke, H.-M.; Haug, T.; Stensvåg, K. Antimicrobial Peptides in Echinoderm Host Defense. Dev. Comp. Immunol. 2015, 49, 190–197. [Google Scholar] [CrossRef]
- Magalhães, W.F.; Hutchings, P.; Oceguera-Figueroa, A.; Martin, P.; Schmelz, R.M.; Wetzel, M.J.; Wiklund, H.; Maciolek, N.J.; Kawauchi, G.Y.; Williams, J.D. Segmented worms (Phylum Annelida): A celebration of twenty years of progress through Zootaxa and call for action on the taxonomic work that remains. Zootaxa 2021, 4979, 190–211. [Google Scholar] [CrossRef]
- Kim, C.-H.; Go, H.-J.; Oh, H.Y.; Park, J.B.; Lee, T.K.; Seo, J.-K.; Elphick, M.R.; Park, N.G. Identification of a Novel Antimicrobial Peptide from the Sea Star Patiria pectinifera. Dev. Comp. Immunol. 2018, 86, 203–213. [Google Scholar] [CrossRef]
- Li, C.; Blencke, H.-M.; Smith, L.C.; Karp, M.T.; Stensvåg, K. Two Recombinant Peptides, SpStrongylocins 1 and 2, from Strongylocentrotus Purpuratus, Show Antimicrobial Activity against Gram-Positive and Gram-Negative Bacteria. Dev. Comp. Immunol. 2010, 34, 286–292. [Google Scholar] [CrossRef]
- Li, C.; Haug, T.; Styrvold, O.B.; Jørgensen, T.Ø.; Stensvåg, K. Strongylocins, Novel Antimicrobial Peptides from the Green Sea Urchin, Strongylocentrotus droebachiensis. Dev. Comp. Immunol. 2008, 32, 1430–1440. [Google Scholar] [CrossRef]
- Solstad, R.G.; Li, C.; Isaksson, J.; Johansen, J.; Svenson, J.; Stensvåg, K.; Haug, T. Novel Antimicrobial Peptides EeCentrocins 1, 2 and EeStrongylocin 2 from the Edible Sea Urchin Echinus esculentus Have 6-Br-Trp Post-Translational Modifications. PLoS ONE 2016, 11, e0151820. [Google Scholar] [CrossRef]
- Schillaci, D.; Cusimano, M.; Cunsolo, V.; Saletti, R.; Russo, D.; Vazzana, M.; Vitale, M.; Arizza, V. Immune Mediators of Sea-Cucumber Holothuria tubulosa (Echinodermata) as Source of Novel Antimicrobial and Anti-Staphylococcal Biofilm Agents. AMB Express 2013, 3, 35. [Google Scholar] [CrossRef]
- Cusimano, M.G.; Spinello, A.; Barone, G.; Schillaci, D.; Cascioferro, S.; Magistrato, A.; Parrino, B.; Arizza, V.; Vitale, M. A Synthetic Derivative of Antimicrobial Peptide Holothuroidin 2 from Mediterranean Sea Cucumber (Holothuria tubulosa) in the Control of Listeria monocytogenes. Mar. Drugs 2019, 17, 159. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Patocka, J.; Nepovimova, E.; Oleksak, P.; Valis, M.; Wu, W.; Kuca, K. Marine Invertebrate Peptides: Antimicrobial Peptides. Front. Microbiol. 2021, 12, 785085. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.L.; Shafee, T.; Papenfuss, A.T.; Norton, R.S. Evolution of Cnidarian Trans-defensins: Sequence, Structure and Exploration of Chemical Space. Proteins Struct. Funct. Bioinform. 2019, 87, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Xue, R.; Han, Y.; Li, F.; Chen, L.; Yang, D.; Zhao, J. Identification, Antibacterial Activities and Action Mode of Two Macins from Manila Clam Venerupis philippinarum. Fish Shellfish Immunol. 2021, 118, 411–420. [Google Scholar] [CrossRef]
- Leoni, G.; De Poli, A.; Mardirossian, M.; Gambato, S.; Florian, F.; Venier, P.; Wilson, D.; Tossi, A.; Pallavicini, A.; Gerdol, M. Myticalins: A Novel Multigenic Family of Linear, Cationic Antimicrobial Peptides from Marine Mussels (Mytilus spp.). Mar. Drugs 2017, 15, 261. [Google Scholar] [CrossRef]
- Mao, F.; Bao, Y.; Wong, N.-K.; Huang, M.; Liu, K.; Zhang, X.; Yang, Z.; Yi, W.; Shu, X.; Xiang, Z.; et al. Large-Scale Plasma Peptidomic Profiling Reveals a Novel, Nontoxic, Crassostrea hongkongensis-Derived Antimicrobial Peptide against Foodborne Pathogens. Mar. Drugs 2021, 19, 420. [Google Scholar] [CrossRef]
- Maselli, V.; Galdiero, E.; Salzano, A.M.; Scaloni, A.; Maione, A.; Falanga, A.; Naviglio, D.; Guida, M.; Di Cosmo, A.; Galdiero, S. OctoPartenopin: Identification and Preliminary Characterization of a Novel Antimicrobial Peptide from the Suckers of Octopus vulgaris. Mar. Drugs 2020, 18, 380. [Google Scholar] [CrossRef]
- Nikapitiya, C.; Dananjaya, S.H.S.; Chandrarathna, H.P.S.U.; De Zoysa, M.; Whang, I. Octominin: A Novel Synthetic Anticandidal Peptide Derived from Defense Protein of Octopus minor. Mar. Drugs 2020, 18, 56. [Google Scholar] [CrossRef]
- Jayasinghe, J.N.C.; Whang, I.; De Zoysa, M. Antifungal Efficacy of Antimicrobial Peptide Octominin II against Candida albicans. Int. J. Mol. Sci. 2023, 24, 14053. [Google Scholar] [CrossRef]
- López-Abarrategui, C.; Alba, A.; Silva, O.N.; Reyes-Acosta, O.; Vasconcelos, I.M.; Oliveira, J.T.A.; Migliolo, L.; Costa, M.P.; Costa, C.R.; Silva, M.R.R.; et al. Functional Characterization of a Synthetic Hydrophilic Antifungal Peptide Derived from the Marine Snail Cenchritis muricatus. Biochimie 2012, 94, 968–974. [Google Scholar] [CrossRef]
- López-Abarrategui, C.; McBeth, C.; Mandal, S.M.; Sun, Z.J.; Heffron, G.; Alba-Menéndez, A.; Migliolo, L.; Reyes-Acosta, O.; García-Villarino, M.; Nolasco, D.O.; et al. Cm-P5: An Antifungal Hydrophilic Peptide Derived from the Coastal Mollusk Cenchritis muricatus (Gastropoda: Littorinidae). FASEB J. 2015, 29, 3315–3325. [Google Scholar] [CrossRef]
- González García, M.; Rodríguez, A.; Alba, A.; Vázquez, A.A.; Morales Vicente, F.E.; Pérez-Erviti, J.; Spellerberg, B.; Stenger, S.; Grieshober, M.; Conzelmann, C.; et al. New Antibacterial Peptides from the Freshwater Mollusk Pomacea poeyana (Pilsbry, 1927). Biomolecules 2020, 10, 1473. [Google Scholar] [CrossRef]
- Iijima, R.; Kisugi, J.; Yamazaki, M. A Novel Antimicrobial Peptide from the Sea Hare Dolabella Auricularia. Dev. Comp. Immunol. 2003, 27, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Mitta, G.; Vandenbulcke, F.; Hubert, F.; Salzet, M.; Roch, P. Involvement of Mytilins in Mussel Antimicrobial Defense. J. Biol. Chem. 2000, 275, 12954–12962. [Google Scholar] [CrossRef] [PubMed]
- Sonthi, M.; Toubiana, M.; Pallavicini, A.; Venier, P.; Roch, P. Diversity of Coding Sequences and Gene Structures of the Antifungal Peptide Mytimycin (MytM) from the Mediterranean Mussel, Mytilus galloprovincialis. Mar. Biotechnol. 2011, 13, 857–867. [Google Scholar] [CrossRef] [PubMed]
- Novoa, B.; Romero, A.; Álvarez, Á.L.; Moreira, R.; Pereiro, P.; Costa, M.M.; Dios, S.; Estepa, A.; Parra, F.; Figueras, A. Antiviral Activity of Myticin C Peptide from Mussel: An Ancient Defense against Herpesviruses. J. Virol. 2016, 90, 7692–7702. [Google Scholar] [CrossRef]
- Liao, Z.; Wang, X.; Liu, H.; Fan, M.; Sun, J.; Shen, W. Molecular Characterization of a Novel Antimicrobial Peptide from Mytilus coruscus. Fish Shellfish Immunol. 2013, 34, 610–616. [Google Scholar] [CrossRef]
- Oh, R.; Lee, M.J.; Kim, Y.-O.; Nam, B.-H.; Kong, H.J.; Kim, J.-W.; Park, J.; Seo, J.-K.; Kim, D.-G. Myticusin-Beta, Antimicrobial Peptide from the Marine Bivalve, Mytilus coruscus. Fish Shellfish Immunol. 2020, 99, 342–352. [Google Scholar] [CrossRef]
- Pacor, S.; Benincasa, M.; Musso, M.V.; Krce, L.; Aviani, I.; Pallavicini, A.; Scocchi, M.; Gerdol, M.; Mardirossian, M. The Proline-Rich Myticalins from Mytilus galloprovincialis Display a Membrane-Permeabilizing Antimicrobial Mode of Action. Peptides 2021, 143, 170594. [Google Scholar] [CrossRef]
- Liu, L.; Yang, J.; Yang, Z.; He, J.; Zhang, X.; He, M. Molecular Characterization of Two Novel Antimicrobial Peptides Myticalin and Mytimacin from Mytilus coruscus. Acta Hydrobiol. Sin. 2022, 46, 1888–1899. [Google Scholar] [CrossRef]
- Arenas, G.; Guzmán, F.; Cárdenas, C.; Mercado, L.; Marshall, S.H. A Novel Antifungal Peptide Designed from the Primary Structure of a Natural Antimicrobial Peptide Purified from Argopecten purpuratus Hemocytes. Peptides 2009, 30, 1405–1411. [Google Scholar] [CrossRef] [PubMed]
- Gueguen, Y.; Bernard, R.; Julie, F.; Paulina, S.; Delphine, D.-G.; Franck, V.; Philippe, B.; Evelyne, B. Oyster Hemocytes Express a Proline-Rich Peptide Displaying Synergistic Antimicrobial Activity with a Defensin. Mol. Immunol. 2009, 46, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.-Y.; Kim, D.-G.; Kim, Y.-O.; Park, J.Y.; Seo, J.-K.; Nam, B.-H.; Hong, Y.-K. Purification and CDNA Cloning of the Antimicrobial Peptide ApMolluscidin from the Pen Shell, Atrina pectinata. Fish Shellfish Immunol. 2018, 81, 408–415. [Google Scholar] [CrossRef]
- Jayathilaka, E.H.T.T.; Rajapaksha, D.C.; Nikapitiya, C.; Lee, J.; De Zoysa, M.; Whang, I. Novel Antimicrobial Peptide “Octoprohibitin” against Multidrug Resistant Acinetobacter baumannii. Pharmaceuticals 2022, 15, 928. [Google Scholar] [CrossRef]
- Houyvet, B.; Zanuttini, B.; Corre, E.; Le Corguillé, G.; Henry, J.; Zatylny-Gaudin, C. Design of Antimicrobial Peptides from a Cuttlefish Database. Amino Acids 2018, 50, 1573–1582. [Google Scholar] [CrossRef]
- Benoist, L.; Houyvet, B.; Henry, J.; Corre, E.; Zanuttini, B.; Zatylny-Gaudin, C. In-Depth In Silico Search for Cuttlefish (Sepia Officinalis) Antimicrobial Peptides Following Bacterial Challenge of Haemocytes. Mar. Drugs 2020, 18, 439. [Google Scholar] [CrossRef]
- Dolashka, P.; Moshtanska, V.; Borisova, V.; Dolashki, A.; Stevanovic, S.; Dimanov, T.; Voelter, W. Antimicrobial Proline-Rich Peptides from the Hemolymph of Marine Snail Rapana venosa. Peptides 2011, 32, 1477–1483. [Google Scholar] [CrossRef]
- Gauri, S.S.; Mandal, S.M.; Pati, B.R.; Dey, S. Purification and Structural Characterization of a Novel Antibacterial Peptide from Bellamya Bengalensis: Activity against Ampicillin and Chloramphenicol Resistant Staphylococcus epidermidis. Peptides 2011, 32, 691–696. [Google Scholar] [CrossRef]
- Bitaab, M.A.; Siadat, S.O.; Pazooki, J.; Sefidbakht, Y. Antibacterial and Molecular Dynamics Study of the Dolabellanin B2 Isolated from Sea Slug, Peronia peronii. Biosci. Biotechnol. Res. Asia 2015, 12, 2023–2035. [Google Scholar] [CrossRef][Green Version]
- Mladineo, I.; Rončević, T.; Gerdol, M.; Tossi, A. Helminthic Host Defense Peptides: Using the Parasite to Defend the Host. Trends Parasitol. 2023, 39, 345–357. [Google Scholar] [CrossRef]
- Rončević, T.; Gerdol, M.; Mardirossian, M.; Maleš, M.; Cvjetan, S.; Benincasa, M.; Maravić, A.; Gajski, G.; Krce, L.; Aviani, I.; et al. Anisaxins, Helical Antimicrobial Peptides from Marine Parasites, Kill Resistant Bacteria by Lipid Extraction and Membrane Disruption. Acta Biomater. 2022, 146, 131–144. [Google Scholar] [CrossRef]
- Irvine, A.; McKenzie, D.; McCoy, C.J.; Graham, R.L.J.; Graham, C.; Huws, S.A.; Atkinson, L.E.; Mousley, A. Novel Integrated Computational AMP Discovery Approaches Highlight Diversity in the Helminth AMP Repertoire. PLoS Pathog. 2023, 19, e1011508. [Google Scholar] [CrossRef]
- Juretić, D.; Golemac, A.; Strand, D.E.; Chung, K.; Ilić, N.; Goić-Barišić, I.; Pellay, F.-X. The Spectrum of Design Solutions for Improving the Activity-Selectivity Product of Peptide Antibiotics against Multidrug-Resistant Bacteria and Prostate Cancer PC-3 Cells. Molecules 2020, 25, 3526. [Google Scholar] [CrossRef]
- Simunić, J.; Petrov, D.; Bouceba, T.; Kamech, N.; Benincasa, M.; Juretić, D. Trichoplaxin—A New Membrane-Active Antimicrobial Peptide from Placozoan CDNA. Biochim. Biophys. Acta (BBA) Biomembr. 2014, 1838, 1430–1438. [Google Scholar] [CrossRef] [PubMed]
- Eitel, M.; Schierwater, B. The Phylogeography of the Placozoa Suggests a Taxon-Rich Phylum in Tropical and Subtropical Waters. Mol. Ecol. 2010, 19, 2315–2327. [Google Scholar] [CrossRef] [PubMed]
- Kamm, K.; Schierwater, B.; DeSalle, R. Innate Immunity in the Simplest Animals—Placozoans. BMC Genom. 2019, 20, 5. [Google Scholar] [CrossRef] [PubMed]
- Chitsulo, L.; Loverde, P.; Engels, D. Focus: Schistosomiasis. Nat. Rev. Microbiol. 2004, 2, 12. [Google Scholar] [CrossRef]
- Shinn, A.P.; Pratoomyot, J.; Bron, J.E.; Paladini, G.; Brooker, E.E.; Brooker, A.J. Economic costs of protistan and metazoan parasites to global mariculture. Parasitology 2015, 142, 196–270. [Google Scholar] [CrossRef]
- Calado, R.; Mamede, R.; Cruz, S.; Leal, M.C. Updated Trends on the Biodiscovery of New Marine Natural Products from Invertebrates. Mar. Drugs 2022, 20, 389. [Google Scholar] [CrossRef]
- Laport, M.; Santos, O.; Muricy, G. Marine Sponges: Potential Sources of New Antimicrobial Drugs. Curr. Pharm. Biotechnol. 2009, 10, 86–105. [Google Scholar] [CrossRef] [PubMed]
- Wiens, M.; Schröder, H.C.; Korzhev, M.; Wang, X.-H.; Batel, R.; Müller, W.E.G. Inducible ASABF-Type Antimicrobial Peptide from the Sponge Suberites domuncula: Microbicidal and Hemolytic Activity In Vitro and Toxic Effect on Molluscs In Vivo. Mar. Drugs 2011, 9, 1969–1994. [Google Scholar] [CrossRef] [PubMed]
- Ebada, S.; Proksch, P. The Chemistry of Marine Sponges; Springer: Dordrecht, The Netherlands, 2012; pp. 191–293. [Google Scholar] [CrossRef]
- Rohde, S.; Nietzer, S.; Schupp, P.J. Prevalence and Mechanisms of Dynamic Chemical Defenses in Tropical Sponges. PLoS ONE 2015, 10, e0132236. [Google Scholar] [CrossRef] [PubMed]
- Vitali, A. Antimicrobial Peptides Derived from Marine Sponges. Am. J. Clin. Microbiol. Antimicrob. 2018, 1, 1006. [Google Scholar]
- Thoms, C.; Schupp, P.; Custódio, M.R.; Lôbo-Hajdu, G.; Hajdu, E.; Muricy, G. Chemical Defense Strategies in Sponges: A Review. Porifera Res. Biodivers. Innov. Sustain. 2007, 28, 627–637. [Google Scholar]
- Daletos, G.; Kalscheuer, R.; Koliwer-Brandl, H.; Hartmann, R.; de Voogd, N.J.; Wray, V.; Lin, W.; Proksch, P. Callyaerins from the Marine Sponge Callyspongia aerizusa: Cyclic Peptides with Antitubercular Activity. J. Nat. Prod. 2015, 78, 1910–1925. [Google Scholar] [CrossRef]
- Nishimura, S.; Arita, Y.; Honda, M.; Iwamoto, K.; Matsuyama, A.; Shirai, A.; Kawasaki, H.; Kakeya, H.; Kobayashi, T.; Matsunaga, S.; et al. Marine Antifungal Theonellamides Target 3β-Hydroxysterol to Activate Rho1 Signaling. Nat. Chem. Biol. 2010, 6, 519–526. [Google Scholar] [CrossRef]
- Shin, H.J.; Rashid, M.A.; Cartner, L.K.; Bokesch, H.R.; Wilson, J.A.; McMahon, J.B.; Gustafson, K.R. Stellettapeptins A and B, HIV-Inhibitory Cyclic Depsipeptides from the Marine Sponge Stelletta sp. Tetrahedron Lett. 2015, 56, 4215–4219. [Google Scholar] [CrossRef]
- Sjögren, M.; Göransson, U.; Johnson, A.-L.; Dahlström, M.; Andersson, R.; Bergman, J.; Jonsson, P.R.; Bohlin, L. Antifouling Activity of Brominated Cyclopeptides from the Marine Sponge Geodia barretti. J. Nat. Prod. 2004, 67, 368–372. [Google Scholar] [CrossRef]
- Sjögren, M.; Johnson, A.-L.; Hedner, E.; Dahlström, M.; Göransson, U.; Shirani, H.; Bergman, J.; Jonsson, P.R.; Bohlin, L. Antifouling Activity of Synthesized Peptide Analogs of the Sponge Metabolite Barettin. Peptides 2006, 27, 2058–2064. [Google Scholar] [CrossRef]
- Smith, D.; Buddie, A.G.; Goss, R.J.M.; Overmann, J.; Lepleux, C.; Brönstrup, M.; Kloareg, B.; Meiners, T.; Brennecke, P.; Ianora, A.; et al. Discovery Pipelines for Marine Resources: An Ocean of Opportunity for Biotechnology? World J. Microbiol. Biotechnol. 2019, 35, 107. [Google Scholar] [CrossRef]
- Cheung, R.C.F.; Ng, T.B.; Wong, J.H. Marine Peptides: Bioactivities and Applications. Mar. Drugs 2015, 13, 4006–4043. [Google Scholar] [CrossRef]
- Wang, X.; Yu, H.; Xing, R.; Li, P. Characterization, Preparation, and Purification of Marine Bioactive Peptides. Biomed. Res. Int. 2017, 2017, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Charlet, M.; Chernysh, S.; Philippe, H.; Hetru, C.; Hoffmann, J.A.; Bulet, P. Innate Immunity. J. Biol. Chem. 1996, 271, 21808–21813. [Google Scholar] [CrossRef]
- Zhao, J.; Li, C.; Chen, A.; Li, L.; Su, X.; Li, T. Molecular Characterization of a Novel Big Defensin from Clam Venerupis philippinarum. PLoS ONE 2010, 5, e13480. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, X.; Teng, D.; Zhang, Y.; Mao, R.; Xi, D.; Wang, J. Candidacidal Mechanism of the Arenicin-3-Derived Peptide NZ17074 from Arenicola marina. Appl. Microbiol. Biotechnol. 2014, 98, 7387–7398. [Google Scholar] [CrossRef] [PubMed]
- Jeyarajan, S.; Peter, A.S.; Sathyan, A.; Ranjith, S.; Kandasamy, I.; Duraisamy, S.; Chidambaram, P.; Kumarasamy, A. Expression and Purification of Epinecidin-1 Variant (Ac-Var-1) by Acid Cleavage. Appl. Microbiol. Biotechnol. 2024, 108, 176. [Google Scholar] [CrossRef]
- Shike, H.; Lauth, X.; Westerman, M.E.; Ostland, V.E.; Carlberg, J.M.; Van Olst, J.C.; Shimizu, C.; Bulet, P.; Burns, J.C. Bass Hepcidin Is a Novel Antimicrobial Peptide Induced by Bacterial Challenge. Eur. J. Biochem. 2002, 269, 2232–2237. [Google Scholar] [CrossRef]
- Li, Y. Carrier Proteins for Fusion Expression of Antimicrobial Peptides in Escherichia coli. Biotechnol. Appl. Biochem. 2009, 54, 1–9. [Google Scholar] [CrossRef]
- Li, Y.; Chang, Q.; Wu, M.; Zhao, X. Total Synthesis of Five Proline-Enriched Cyclic Heptapeptides from the Marine Sponge Stylissa carteri. Tetrahedron Lett. 2018, 59, 1828–1831. [Google Scholar] [CrossRef]
- Ingham, A.B.; Moore, R.J. Recombinant Production of Antimicrobial Peptides in Heterologous Microbial Systems. Biotechnol. Appl. Biochem. 2007, 47, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Cabral, K.M.S.; Almeida, M.S.; Valente, A.P.; Almeida, F.C.L.; Kurtenbach, E. Production of the Active Antifungal Pisum Sativum Defensin 1 (Psd1) in Pichia pastoris: Overcoming the Inefficiency of the STE13 Protease. Protein Expr. Purif. 2003, 31, 115–122. [Google Scholar] [CrossRef]
- Xu, F.; Meng, K.; Wang, Y.-R.; Luo, H.-Y.; Yang, P.-L.; Wu, N.-F.; Fan, Y.-L.; Yao, B. Eukaryotic Expression and Antimicrobial Spectrum Determination of the Peptide Tachyplesin II. Protein Expr. Purif. 2008, 58, 175–183. [Google Scholar] [CrossRef]
- Jeyarajan, S.; Sathyan, A.; Peter, A.S.; Ranjith, S.; Duraisamy, S.; Natarajaseenivasan, S.M.; Chidambaram, P.; Kumarasamy, A. Bioproduction and Characterization of Epinecidin-1 and Its Variants Against Multi Drug Resistant Bacteria Through In Silico and In Vitro Studies. Int. J. Pept. Res. Ther. 2023, 29, 66. [Google Scholar] [CrossRef]
- Zhou, L.; Li, G.; Jiao, Y.; Huang, D.; Li, A.; Chen, H.; Liu, Y.; Li, S.; Li, H.; Wang, C. Molecular and Antimicrobial Characterization of a Group G Anti-Lipopolysaccharide Factor (ALF) from Penaeus monodon. Fish Shellfish Immunol. 2019, 94, 149–156. [Google Scholar] [CrossRef]
- Chen, T.; Zhou, J.; Qu, Z.; Zou, Q.; Liu, X.; Su, J.; Fu, X.; Yuan, G. Administration of Dietary Recombinant Hepcidin on Grass Carp (Ctenopharyngodon idella) against Flavobacterium columnare Infection under Cage Aquaculture Conditions. Fish Shellfish Immunol. 2020, 99, 27–34. [Google Scholar] [CrossRef]
- Sperstad, S.V.; Haug, T.; Blencke, H.-M.; Styrvold, O.B.; Li, C.; Stensvåg, K. Antimicrobial Peptides from Marine Invertebrates: Challenges and Perspectives in Marine Antimicrobial Peptide Discovery. Biotechnol. Adv. 2011, 29, 519–530. [Google Scholar] [CrossRef]
- Bruni, N.; Capucchio, M.T.; Biasibetti, E.; Pessione, E.; Cirrincione, S.; Giraudo, L.; Corona, A.; Dosio, F. Antimicrobial Activity of Lactoferrin-Related Peptides and Applications in Human and Veterinary Medicine. Molecules 2016, 21, 752. [Google Scholar] [CrossRef]
- Nguyen Ngoc, D.; Latalski, M.; Danielewicz, A.; Szponder, T.; Wessely-Szponder, J.; Mazur, E. Application of Antimicrobial Peptides (AMPs) in Treatment of Osteomyelitis in Human and Veterinary Orthopedics. J. Funct. Biomater. 2025, 16, 90. [Google Scholar] [CrossRef]
- Wang, W.-F.; Cheng, C.-X.; Liu, H.; Chen, X.-L.; Wang, H.-L. 6His-Tatritin Promotes Antimicrobial Defense via Regulating Immune Ability and Intestinal Microbial Community in Grass Carp (Ctenopharyngodon idella). Fish Shellfish Immunol. 2023, 133, 108532. [Google Scholar] [CrossRef]
- Masters, E.A.; Trombetta, R.P.; de Mesy Bentley, K.L.; Boyce, B.F.; Gill, A.L.; Gill, S.R.; Nishitani, K.; Ishikawa, M.; Morita, Y.; Ito, H.; et al. Evolving Concepts in Bone Infection: Redefining “Biofilm”, “Acute vs. Chronic Osteomyelitis”, “the Immune Proteome” and “Local Antibiotic Therapy.”. Bone Res. 2019, 7, 20. [Google Scholar] [CrossRef]
- Wei, Y.; Wu, J.; Chen, Y.; Fan, K.; Yu, X.; Li, X.; Zhao, Y.; Li, Y.; Lv, G.; Song, G.; et al. Efficacy and Safety of PL-5 (Peceleganan) Spray for Wound Infections. Ann. Surg. 2022, 277, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Lai, Z.; Shan, A. Advances of Antimicrobial Peptide-Based Biomaterials for the Treatment of Bacterial Infections. Adv. Sci. 2023, 10, 2206602. [Google Scholar] [CrossRef] [PubMed]
- Lacasse, P.; Lauzon, K.; Diarra, M.S.; Petitclerc, D. Utilization of Lactoferrin to Fight Antibiotic-Resistant Mammary Gland Pathogens1,2. J. Anim. Sci. 2008, 86, 66–71. [Google Scholar] [CrossRef]
- Kawai, K.; Nagahata, H.; Lee, N.-Y.; Anri, A.; Shimazaki, K. Effect of Infusing Lactoferrin Hydrolysate into Bovine Mammary Glands with Subclinical Mastitis. Vet. Res. Commun. 2003, 27, 539–548. [Google Scholar] [CrossRef]
- Kawai, K.; Shimazaki, K.; Higuchi, H.; Nagahata, H. Antibacterial Activity of Bovine Lactoferrin Hydrolysate against Mastitis Pathogens and Its Effect on Superoxide Production of Bovine Neutrophils. Zoonoses Public Health 2007, 54, 160–164. [Google Scholar] [CrossRef]
- Zhang, J.X.; Zhang, S.F.; Wang, T.D.; Guo, X.J.; Hu, R.L. Mammary Gland Expression of Antibacterial Peptide Genes to Inhibit Bacterial Pathogens Causing Mastitis. J. Dairy. Sci. 2007, 90, 5218–5225. [Google Scholar] [CrossRef]
- Tang, Z.R.; Sun, Z.H.; Tang, X.S.; Feng, Z.M.; Zhou, D.; Xiao, D.F.; Zhang, B.; Li, L.L. Dietary Bovine Lactoferricin Improved Gut Microflora, Benefited Intestinal Mucosal Morphology and Inhibited Circular Cytokines Delivery in 21-Day Weaned Piglets. J. Appl. Anim. Res. 2011, 39, 153–157. [Google Scholar] [CrossRef]
- Tang, Z.; Yin, Y.; Zhang, Y.; Huang, R.; Sun, Z.; Li, T.; Chu, W.; Kong, X.; Li, L.; Geng, M.; et al. Effects of Dietary Supplementation with an Expressed Fusion Peptide Bovine Lactoferricin–Lactoferrampin on Performance, Immune Function and Intestinal Mucosal Morphology in Piglets Weaned at Age 21 d. Br. J. Nutr. 2008, 101, 998–1005. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.-S.; Shao, H.; Li, T.-J.; Tang, Z.-R.; Huang, R.-L.; Wang, S.-P.; Kong, X.-F.; Wu, X.; Yin, Y.-L. Dietary Supplementation with Bovine Lactoferrampin–Lactoferricin Produced by Pichia Pastoris Fed-Batch Fermentation Affects Intestinal Microflora in Weaned Piglets. Appl. Biochem. Biotechnol. 2012, 168, 887–898. [Google Scholar] [CrossRef] [PubMed]
- Hou, Z.P.; Wang, W.J.; Liu, J.X.; Liu, G.; Liu, Z.Q.; Souffrant, W.B.; Urubschurov, V.; Fan, J. Effects of Lactoferricin B and Cecropin P1 on Growth Performance, Faecal Score and Dry Matter in Weaned Piglets Orally Challenged with Enterotoxigenic Escherichia coli F4. J. Food Agric. Environ. 2011, 9, 275–280. [Google Scholar]
- Corona, A.; Vercelli, A.; Bruni, N.; Guidi, E.; Cornegliani, L. In Vitro Activity of Lactoferricin Solution against Malassezia pachydermatis from Otitis Externa in Dogs and Cats. Vet. Dermatol. 2021, 32, 316-e86. [Google Scholar] [CrossRef] [PubMed]
- Naiel, M.A.E.; Ghazanfar, S.; Negm, S.S.; Shukry, M.; Abdel-Latif, H.M.R. Applications of Antimicrobial Peptides (AMPs) as an Alternative to Antibiotic Use in Aquaculture—A Mini-Review. Ann. Anim. Sci. 2023, 23, 691–701. [Google Scholar] [CrossRef]
- Oeemig, J.S.; Lynggaard, C.; Knudsen, D.H.; Hansen, F.T.; Nørgaard, K.D.; Schneider, T.; Vad, B.S.; Sandvang, D.H.; Nielsen, L.A.; Neve, S.; et al. Eurocin, a New Fungal Defensin. J. Biol. Chem. 2012, 287, 42361–42372. [Google Scholar] [CrossRef]
- Zhu, S. Discovery of Six Families of Fungal Defensin-like Peptides Provides Insights into Origin and Evolution of the CSαβ Defensins. Mol. Immunol. 2008, 45, 828–838. [Google Scholar] [CrossRef]
- Thackray, P.D.; Moir, A. SigM, an Extracytoplasmic Function Sigma Factor of Bacillus subtilis, Is Activated in Response to Cell Wall Antibiotics, Ethanol, Heat, Acid, and Superoxide Stress. J. Bacteriol. 2003, 185, 3491–3498. [Google Scholar] [CrossRef]
- Zhou, Q.-J.; Wang, J.; Liu, M.; Qiao, Y.; Hong, W.-S.; Su, Y.-Q.; Han, K.-H.; Ke, Q.-Z.; Zheng, W.-Q. Identification, Expression and Antibacterial Activities of an Antimicrobial Peptide NK-Lysin from a Marine Fish Larimichthys crocea. Fish Shellfish Immunol. 2016, 55, 195–202. [Google Scholar] [CrossRef]
- Gyan, W.R.; Yang, Q.; Tan, B.; Jan, S.S.; Jiang, L.; Chi, S.; Dong, X.; Liu, H.; Shuang, Z. Effects of Antimicrobial Peptides on Growth, Feed Utilization, Serum Biochemical Indices and Disease Resistance of Juvenile Shrimp, Litopenaeus vannamei. Aquac. Res. 2020, 51, 1222–1231. [Google Scholar] [CrossRef]
- Edwards, M. Plankton and Global Change. Mar. Plankton A Pract. Guide Ecol. Methodol. Taxon. 2017, 1, 67–80. [Google Scholar]
- Amiss, A.S.; von Pein, J.B.; Webb, J.R.; Condon, N.D.; Harvey, P.J.; Phan, M.D.; Schembri, M.A.; Currie, B.J.; Sweet, M.J.; Craik, D.J.; et al. Modified Horseshoe Crab Peptides Target and Kill Bacteria inside Host Cells. Cell. Mol. Life Sci. 2021, 79, 38. [Google Scholar] [CrossRef]
- Balseiro, P.; Falcó, A.; Romero, A.; Dios, S.; Martínez-López, A.; Figueras, A.; Estepa, A.; Novoa, B. Mytilus galloprovincialis Myticin C: A Chemotactic Molecule with Antiviral Activity and Immunoregulatory Properties. PLoS ONE 2011, 6, e23140. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Jyoti, M.A.; Song, H.Y.; Jang, W.S. Antifungal Activity and Action Mechanism of Histatin 5-Halocidin Hybrid Peptides against Candida ssp. PLoS ONE 2016, 11, e0150196. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.E.; Kang, S.W.; Shin, Y.K.; Jun, J.C.; Kim, Y.-O.; Hur, Y.B.; Kim, J.-H.; Chae, S.-H.; Lee, J.-S.; Choi, I.H.; et al. Comparative Analysis of Expressed Sequence Tags (ESTs) between Normal Group and Softness Syndrome Group in Halocynthia roretzi. Mol. Cell. Toxicol. 2011, 7, 357–365. [Google Scholar] [CrossRef]
- Shin, S.H.; Lee, Y.S.; Shin, Y.P.; Kim, B.; Kim, M.H.; Chang, H.-R.; Jang, W.S.; Lee, I.H. Therapeutic Efficacy of Halocidin-Derived Peptide HG1 in a Mouse Model of Candida albicans Oral Infection. J. Antimicrob. Chemother. 2013, 68, 1152–1160. [Google Scholar] [CrossRef]
- Guryanova, S.V.; Ovchinnikova, T. V Multifaceted Marine Peptides and Their Therapeutic Potential. Mar. Drugs 2025, 23, 288. [Google Scholar] [CrossRef]
- Agüero-Chapin, G.; Domínguez-Pérez, D.; Marrero-Ponce, Y.; Castillo-Mendieta, K.; Antunes, A. Unveiling Encrypted Antimicrobial Peptides from Cephalopods’ Salivary Glands: A Proteolysis-Driven Virtual Approach. ACS Omega 2024, 9, 43353–43367. [Google Scholar] [CrossRef]
- Barroso, R.A.; Agüero-Chapin, G.; Sousa, R.; Marrero-Ponce, Y.; Antunes, A. Unlocking Antimicrobial Peptides: In Silico Proteolysis and Artificial Intelligence-Driven Discovery from Cnidarian Omics. Molecules 2025, 30, 550. [Google Scholar] [CrossRef]
- Klimovich, A.; Bosch, T.C.G. Novel Technologies Uncover Novel ‘Anti’-Microbial Peptides in Hydra Shaping the Species-Specific Microbiome. Philos. Trans. R. Soc. B Biol. Sci. 2024, 379, 20230058. [Google Scholar] [CrossRef]
- Aronica, P.G.A.; Reid, L.M.; Desai, N.; Li, J.; Fox, S.J.; Yadahalli, S.; Essex, J.W.; Verma, C.S. Computational Methods and Tools in Antimicrobial Peptide Research. J. Chem. Inf. Model. 2021, 61, 3172–3196. [Google Scholar] [CrossRef] [PubMed]
- Wan, F.; Torres, M.D.T.; Peng, J.; de la Fuente-Nunez, C. Deep-Learning-Enabled Antibiotic Discovery through Molecular de-Extinction. Nat. Biomed. Eng. 2024, 8, 854–871. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Feng, J.; Kang, Y.; Pan, P.; Ge, J.; Wang, Y.; Wang, M.; Wu, Z.; Zhang, X.; Yu, J.; et al. Discovery of Antimicrobial Peptides with Notable Antibacterial Potency by an LLM-Based Foundation Model. Sci. Adv. 2025, 11, eads8932. [Google Scholar] [CrossRef] [PubMed]
- Santos-Júnior, C.D.; Pan, S.; Zhao, X.-M.; Coelho, L.P. Macrel: Antimicrobial Peptide Screening in Genomes and Metagenomes. PeerJ 2020, 8, e10555. [Google Scholar] [CrossRef]
- Asif, F.; Zaman, S.U.; Arnab, M.K.H.; Hasan, M.; Islam, M.M. Antimicrobial Peptides as Therapeutics: Confronting Delivery Challenges to Optimize Efficacy. Microbe 2024, 2, 100051. [Google Scholar] [CrossRef]
- Jiang, Z.; Feng, J.; Wang, F.; Wang, J.; Wang, N.; Zhang, M.; Hsieh, C.; Hou, T.; Cui, W.; Ma, L. AI-Guided Design of Antimicrobial Peptide Hydrogels for Precise Treatment of Drug-resistant Bacterial Infections. Adv. Mater. 2025, 37, 2500043. [Google Scholar] [CrossRef]
- Silver, L.L. Challenges of Antibacterial Discovery. Clin. Microbiol. Rev. 2011, 24, 71–109. [Google Scholar] [CrossRef]
- Chen, P.; Ye, T.; Li, C.; Praveen, P.; Hu, Z.; Li, W.; Shang, C. Embracing the Era of Antimicrobial Peptides with Marine Organisms. Nat. Prod. Rep. 2024, 41, 331–346. [Google Scholar] [CrossRef]
- Kanaujia, K.A.; Wagh, S.; Pandey, G.; Phatale, V.; Khairnar, P.; Kolipaka, T.; Rajinikanth, P.S.; Saraf, S.A.; Srivastava, S.; Kumar, S. Harnessing Marine Antimicrobial Peptides for Novel Therapeutics: A Deep Dive into Ocean-Derived Bioactives. Int. J. Biol. Macromol. 2025, 307, 142158. [Google Scholar] [CrossRef]
- Ahmed, I.; Asgher, M.; Sher, F.; Hussain, S.; Nazish, N.; Joshi, N.; Sharma, A.; Parra-Saldívar, R.; Bilal, M.; Iqbal, H. Exploring Marine as a Rich Source of Bioactive Peptides: Challenges and Opportunities from Marine Pharmacology. Mar. Drugs 2022, 20, 208. [Google Scholar] [CrossRef]
- Carpenter, A.L.; McGuire, J.A.; Nemergut, E.C.; Bauer, K.M.; Navalgund, Y.A.; Scalzo, D.C.; Vaglienti, R.M.; Layne-Stuart, C.M. Dilution of Ziconotide for Intrathecal Trial: The Effect of Dilution on the Incidence of Side Effects and Pain Relief: A Single-Center Retrospective Case-Control Study. Pain Physician 2025, 28, 147–154. [Google Scholar] [CrossRef]
- Zhang, T.; Ouyang, Z.; Zhang, Y.; Sun, H.; Kong, L.; Xu, Q.; Qu, J.; Sun, Y. Marine Natural Products in Inflammation-Related Diseases: Opportunities and Challenges. Med. Res. Rev. 2025, 45, 1375–1406. [Google Scholar] [CrossRef] [PubMed]
- Holzer, S.; Rzechorzek, N.J.; Short, I.R.; Jenkyn-Bedford, M.; Pellegrini, L.; Kilkenny, M.L. Structural Basis for Inhibition of Human Primase by Arabinofuranosyl Nucleoside Analogues Fludarabine and Vidarabine. ACS Chem. Biol. 2019, 14, 1904–1912. [Google Scholar] [CrossRef] [PubMed]
- Min, K.H.; Kim, K.H.; Ki, M.-R.; Pack, S.P. Antimicrobial Peptides and Their Biomedical Applications: A Review. Antibiotics 2024, 13, 794. [Google Scholar] [CrossRef] [PubMed]
- Luong, H.X.; Thanh, T.T.; Tran, T.H. Antimicrobial Peptides—Advances in Development of Therapeutic Applications. Life Sci. 2020, 260, 118407. [Google Scholar] [CrossRef]
- Brookwell, A.; Oza, J.P.; Caschera, F. Biotechnology Applications of Cell-Free Expression Systems. Life 2021, 11, 1367. [Google Scholar] [CrossRef]
- Chen, Q.; Yang, Z.; Liu, H.; Man, J.; Oladejo, A.O.; Ibrahim, S.; Wang, S.; Hao, B. Novel Drug Delivery Systems: An Important Direction for Drug Innovation Research and Development. Pharmaceutics 2024, 16, 674. [Google Scholar] [CrossRef]
- Dubey, A.; Vahabi, H.; Kumaravel, V. Antimicrobial and Biodegradable 3D Printed Scaffolds for Orthopedic Infections. ACS Biomater. Sci. Eng. 2023, 9, 4020–4044. [Google Scholar] [CrossRef]

| Group | Peptide Name | Source Organism | Structure/Class | Key Features | Antimicrobial Activity | Reference(s) |
|---|---|---|---|---|---|---|
| Clitellata | Lumbricin | Lumbricus rubellus, Hirudo medicinalis | Proline-rich | Immune response, CNS regeneration | Broad-spectrum (bacteria); D. nishinomiyaensis | [43,44,45,46] |
| Polychaeta | Theromyzin | Theromyzon tessulatum | Anionic, α-helical | First anionic AMP in invertebrates | Not specified | [47] |
| Arenicin | Arenicola marina | β-hairpin, disulfide-stabilized | Extremophile; synthetic analogs developed | Broad-spectrum (bacteria) | [48,49,50] | |
| Abarenicin | Abarenicola pacifica | BRICHOS-related | Extremophile; BRICHOS domain | Not specified | [51] | |
| UuBRI-21 | Urechis unicinctus | BRICHOS-related | β-sheet; thermal adaptation | Not specified | [51] | |
| Nicomicin | Nicomache minor | α-helical | Anticancer activity | Antibacterial, anticancer | [52] | |
| Alvinellacin | Alvinella pompejana | β-sheet, disulfide-stabilized | Deep-sea AMP; heat-tolerant species | Antibacterial | [53,54] | |
| Capitellacin | Capitella teleta | β-sheet, disulfide-stabilized | Homolog of alvinellacin | Antibacterial | [54] | |
| Polaricin | Amphitritides sp. | Not specified | Antarctic AMP | Antibacterial | [55] | |
| HfBRI-25/28 | Heteromastus filiformis | β-hairpin (HfBRI-25), α-helical (HfBRI-28) | Polar origin; disulfide-stabilized | Not specified | [55,56,57] | |
| AmBRI-44a | Arenicola marina | Defensin-like, disulfide-stabilized | Four disulfide bridges | Not specified | [57] | |
| Hedistin | Hediste diversicolor | Cationic α-helical | Brominated tryptophan residues | Not specified | [39] | |
| Perinerin | Perinereis aibuhitensis | Cationic α-helical | Two possible intramolecular disulfide bridges | Not specified | [58] |
| Group | Peptide Name | Source Organism | Structure/Class | Key Features | Antimicrobial Activity | Reference(s) |
|---|---|---|---|---|---|---|
| Cephalochordata | BjAMP1 | Branchiostoma japonicum | Two α-helices connected by a reverse turn (amphipathic) | Penetrates membranes without disrupting structure; binds LPS/LTA; may bind DNA/RNA; non-toxic to mammalian cells | Broad-spectrum antibacterial activity | [112,113,114,115,116] |
| mBjAMP1 (analogs) | Synthetic analogs | Modified α-helical peptides | Enhanced antimicrobial and antibiofilm activity through amino acid modifications | Improved broad-spectrum activity | [115,117] | |
| Tunicata | Styelins | Styela clava | Phenylalanine-rich | Identified from ascidians | Active against Gram-positive, Gram-negative bacteria, and fungi | [118,119,120] |
| Clavanins | Styela clava | α-helical | Histidine-rich variants | Broad-spectrum antimicrobial activity | [118,119,120] | |
| Clavaspirin | Styela clava | Histidine-rich | Functions not fully described | Broad-spectrum antimicrobial activity | [118,119,120] | |
| Halocidin | Halocynthia aurantium | Two amphipathic α-helices with disulfide bond | Two monomers (18 and 15 amino acids); 18-residue monomer more active; synthetic analogs (e.g., di-K19Hc) show improved activity | Broad-spectrum antibacterial activity | [39,47,53,121,122,123] | |
| Dicynthaurin | Halocynthia aurantium | Dimer of two 30-aa amphipathic α-helical peptides | Linked by single cysteine disulfide bond; α-helical conformation | Broad-spectrum antibacterial activity | [121,123] | |
| Halocyntin, Papillosin | Halocynthia papillosa | Not detailed | Identified from hemocytes | Broad-spectrum antibacterial activity | [124] | |
| Ci-MAM-A24, Ci-PAP-A22 | Ciona intestinalis | Not specified (from EST database) | Early AMP identification from EST screening | Antibacterial activity | [125,126,127] | |
| P-02 to P-10 | Ciona intestinalis | Short ORF-derived peptides | Predicted from sORFs; 5 out of 10 tested peptides showed activity | Antibacterial activity | [128] | |
| Turgencins (AMox1, etc.) | Synoicum turgens | Cysteine-rich, unusual disulfide bridges, amidated C-end | Methionine oxidation variants; AMox1 most potent; active against melanoma and fibroblast cells | Broad-spectrum antibacterial and anticancer activity | [129] | |
| StAMP-1 to StAMP-11 | Synthetic (based on turgencin) | Synthetic analogs | StAMP-9 most potent; selective antimicrobial with low hemolysis and cytotoxicity | Broad-spectrum antibacterial, antifungal, and non-cytotoxic | [129] |
| Group | Peptide Name | Source Organism | Structure/Class | Key Features | Antimicrobial Activity | Reference(s) |
|---|---|---|---|---|---|---|
| Anthozoa | Damicornin | Pocillopora damicornis | Cysteine-rich (6 cysteines) | Gene expression repressed by Vibrio coralliilyticus | Gram-positive bacteria, Fusarium oxysporum (fungus) | [137] |
| AmAMP1 | Acropora millepora | Cysteine-rich (6 cysteines) | Expressed in ectodermal cells during coral development | Broad-spectrum: Gram-positive and Gram-negative bacteria | [138] | |
| Pd-AMP1 | Phyllogorgia dilatata | β-hairpin structure | Soft coral-derived peptide | Gram-positive bacteria | [139] | |
| Crassicorin | Urticina crassicornis | Double β-hairpin, 6 cysteines | Sea anemone-derived, structurally stable | Antibacterial activity | [140] | |
| Equinins | Actinia equina | Not specified | No hemolysis on human cells; low antibacterial activity | Weak antibacterial activity | [141] | |
| Medusozoa | Aurelin | Aurelia aurita | 6 cysteines; 2 helices + coil | Isolated from jellyfish mesoglea | Antibacterial activity | [142,143] |
| Arminin 1a-C | Hydra | α-helical peptide | Selective anticancer effects; no hemolysis; C-terminal domain of Arminin 1a | Strong antibacterial; anti-leukemia cell viability | [144,145] | |
| Periculin-1 | Hydra | Anionic N-term, cationic C-term; 8 cysteines | Expressed in female germline; controls bacterial colonization during embryogenesis | Potent antimicrobial activity | [146,147] |
| Group | Peptide Name | Source Organism | Structure/Class | Key Features | Antimicrobial Activity | Reference(s) |
|---|---|---|---|---|---|---|
| Asteroidea | PpCrAMP | Patiria pectinifera | β-hairpin | Contains two β-strands linked by a random coil | Antibacterial activity | [153] |
| Echinoidea | Strongylocins | Strongylocentrotus droebachiensis, S. purpuratus, Echinus esculentus | Cysteine-rich, cationic peptide | Six-cysteine motif; brominated tryptophan | Strong antibacterial activity | [154,155,156] |
| Echinoidea | Centrocins | Strongylocentrotus droebachiensis, Echinus esculentus | Heterodimeric peptide | 30-residue heavy chain + 12-residue light chain linked by disulfide bond; brominated tryptophan | Strong antibacterial and some antifungal activity | [154,155,156] |
| Holothuroidea | Holothuroidins | Holothuria tubulosa | Cationic α-helical peptide | Small peptides; weak antibacterial potency but notable antibiofilm properties | Weak antibacterial, antibiofilm activity | [157,158] |
| Group | Peptide Name | Source Organism | Structure/Class | Key Features | Antimicrobial Activity | Reference(s) |
|---|---|---|---|---|---|---|
| Bivalvia | Mytilins | Mytilus spp. | Cysteine-rich | Signal peptide + mature region + C-terminal extension | Bacteria, fungi | [160,171] |
| Mytimycins | Mytilus spp. | Cysteine-rich | Similar structure; precursor-based | Bacteria, fungi | [94,160,172] | |
| Myticins | Mytilus spp. | Cysteine-rich | Precursor-derived; conserved motif | Bacteria, fungi, viruses | [73,95,173] | |
| Myticusins | Mytilus spp. | Cysteine-rich | Target parasites in addition to microbes | Bacteria, fungi, parasites | [161,174,175] | |
| Myticalins | Mytilus spp. | Cationic, α-helical + random coil | Broad-spectrum, gene-encoded | Gram-positive and Gram-negative bacteria | [162,176,177] | |
| Mytichitins | Mytilus spp. | Chitin-binding domain | Targets chitin; enhances antimicrobial defense | Bacteria, fungi, parasites | [81] | |
| Ap | Argopecten purpuratus | Proline-rich, cationic | Non-mussel species; strong antifungal effect | Fungi | [178] | |
| Cg-Prp | Crassostrea gigas | Proline-rich | Limited direct effect; synergistic with Cg-Def | Synergistic enhancement with defensin | [102,179] | |
| URP20 | Crassostrea hongkongensis | α-helical, cationic | Potent activity; non-toxic to mammalian cells | Bacteria, fungi | [163] | |
| Molluscidin | Crassostrea gigas, Atrina pectinata, Haliotis discus | Non-amphipathic; alternating α-helix and random coil | Contains repeated dibasic residues; isolated from gill tissue; low cytotoxicity | Active against Gram-positive and Gram-negative bacteria | [106,180] | |
| Cephalopoda | Octopartenopin | Octopus vulgaris | Random coil, pentapeptide | Derived from sucker tissue | Antibacterial | [164] |
| Octominins | Octopus minor | Cationic, α-helical | Well-characterized peptide family | Antibacterial | [165,166] | |
| Octopromycin | Octopus minor | Cationic, α-helical | Novel structure | Antibacterial | [99] | |
| Octoprohibitin | Octopus minor | Cationic, α-helical | Immunomodulatory potential | Antibacterial | [181] | |
| KT19, VA20, GR21 | Sepia officinalis | Putative peptides | Identified via peptide screening | Antimicrobial potential | [182] | |
| NF19, AV19, GK28 | Sepia officinalis | Putative peptides | Active peptide variants | Antimicrobial potential | [183] | |
| Gastropoda | Cm-p1 | Cenchritis muricatus | α-helical | Antifungal without mammalian toxicity | Fungi | [167,168] |
| Peptide 4 | Rapana venosa | Random coil, proline-rich | Invasive species; Gram-negative targeting | Gram-negative bacteria | [184] | |
| Peptide 7 | Rapana venosa | α-helical | Broad-spectrum potential | Gram-negative bacteria | [184] | |
| Bb-AMP4 | Filopaludina bengalensis | Not defined | Freshwater snail origin | Gram-positive bacteria | [185] | |
| Pom-1 (Closticin 574) | Pomacea poeyana | α-helical | Antiviral and antibacterial activity | Bacteria, viruses (e.g., ZIKV) | [169] | |
| Pom-2 (Cecropin D-like) | Pomacea poeyana | α-helical | Narrower spectrum | Antibacterial | [169] | |
| Dolabellanin B2 | Dolabella auricularia, Peronia peronii | α-helical | Found in sea hare and sea slug | Antibacterial, antifungal | [170,186] |
| Source | Peptides | Activity | References |
|---|---|---|---|
| Porifera peptides | Callyaerin A and B | Antimicrobial | [203] |
| Theonellamide F | Antimicrobial | [204] | |
| Theonellamide G | Antimicrobial | [204] | |
| Koshikamides F and H | Antiviral | [79] | |
| Celebesides A–C | Antiviral | [79] | |
| Mirabamides A–D | Antiviral | [205] | |
| Mirabamides E–H | Antiviral | [205] | |
| Stellettapeptins A and B | Antiviral | [205] | |
| Barettin and 8,9-dihydrobarettin | Antifouling | [206] | |
| Barrettides A and B | Antifouling | [207] |
| Animal Model | Condition | Peptide Used | Pathogen(s) | Delivery Method | Key Outcomes | Reference(s) |
|---|---|---|---|---|---|---|
| Dairy cattle (cow) | Bovine mastitis | Lf + Penicillin G | Staphylococcus aureus (β-lactam-resistant) | Intramammary infusion | Cure rate improved from 12.5% to 33%; β-lactamase activity reduced | [231] |
| Dairy cattle (cow) | Subclinical mastitis | Lf Hydrolysate (LFH) | E. coli, Staphylococcus spp. | Intramammary infusion | Bacterial load reduced on Day 1; full recovery in 14 days | [232] |
| Dairy cattle (cow) | Protothecal/fungal mastitis | bLfcin | Prototheca zopfii, yeast spp. | In vitro | Demonstrated antimicrobial activity (in vitro only) | [233] |
| Goat | Preventive udder infection | bLfcin | S. aureus, E. coli | Recombinant plasmid vector | Peptide persisted in milk up to 6 days; milk showed antibacterial activity | [234] |
| Piglets | Enterotoxigenic E. coli | cipB-Lfcin | E. coli | Dietary supplementation (100 mg/kg) | Improved gut morphology, lower cytokine levels, reduced E. coli count | [235] |
| Piglets | Enterotoxigenic E. coli | cipB-Lfcin-Lframpin | E. coli | Dietary supplementation (100 mg/kg) | Enhanced growth, gut health similar to colistin treatment | [236] |
| Piglets | Enterotoxigenic E. coli | bLfcin/Lfampin | E. coli | Recombinant yeast (P. pastoris) | Improved growth and gut health | [237] |
| Piglets | Enterotoxigenic E. coli F4 | bLfcin | E. coli F4 | Oral administration | Reduced diarrhea and maintained healthy weight; no toxicity | [238] |
| Dogs | Fungal and bacterial otitis | bLfcin + verbascoside | Malassezia spp., others | Topical emulsion | Reduced microbial load, clinical improvement in 7 days | [239] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawsar, M.A.; Zhao, C.; Mao, F.; Yu, Z.; Zhang, Y. Unlocking Antimicrobial Peptides from Marine Invertebrates: A Comprehensive Review of Antimicrobial Discovery. Antibiotics 2025, 14, 924. https://doi.org/10.3390/antibiotics14090924
Kawsar MA, Zhao C, Mao F, Yu Z, Zhang Y. Unlocking Antimicrobial Peptides from Marine Invertebrates: A Comprehensive Review of Antimicrobial Discovery. Antibiotics. 2025; 14(9):924. https://doi.org/10.3390/antibiotics14090924
Chicago/Turabian StyleKawsar, Md. Abu, Chengqing Zhao, Fan Mao, Ziniu Yu, and Yang Zhang. 2025. "Unlocking Antimicrobial Peptides from Marine Invertebrates: A Comprehensive Review of Antimicrobial Discovery" Antibiotics 14, no. 9: 924. https://doi.org/10.3390/antibiotics14090924
APA StyleKawsar, M. A., Zhao, C., Mao, F., Yu, Z., & Zhang, Y. (2025). Unlocking Antimicrobial Peptides from Marine Invertebrates: A Comprehensive Review of Antimicrobial Discovery. Antibiotics, 14(9), 924. https://doi.org/10.3390/antibiotics14090924







