Impact of Obesity on Serum Concentrations of Vancomycin Administered as Continuous Infusion and on Clinical Outcomes in Critically Ill Patients—A Retrospective Observational Study
Abstract
1. Introduction
2. Results
2.1. Study Population
2.2. Vancomycin Treatment Course and TDM Results
2.3. Bacterial Species
2.4. Disease Severity and Outcome Measures
2.5. Impact of RRT and BMI on Vancomycin Serum Concentrations
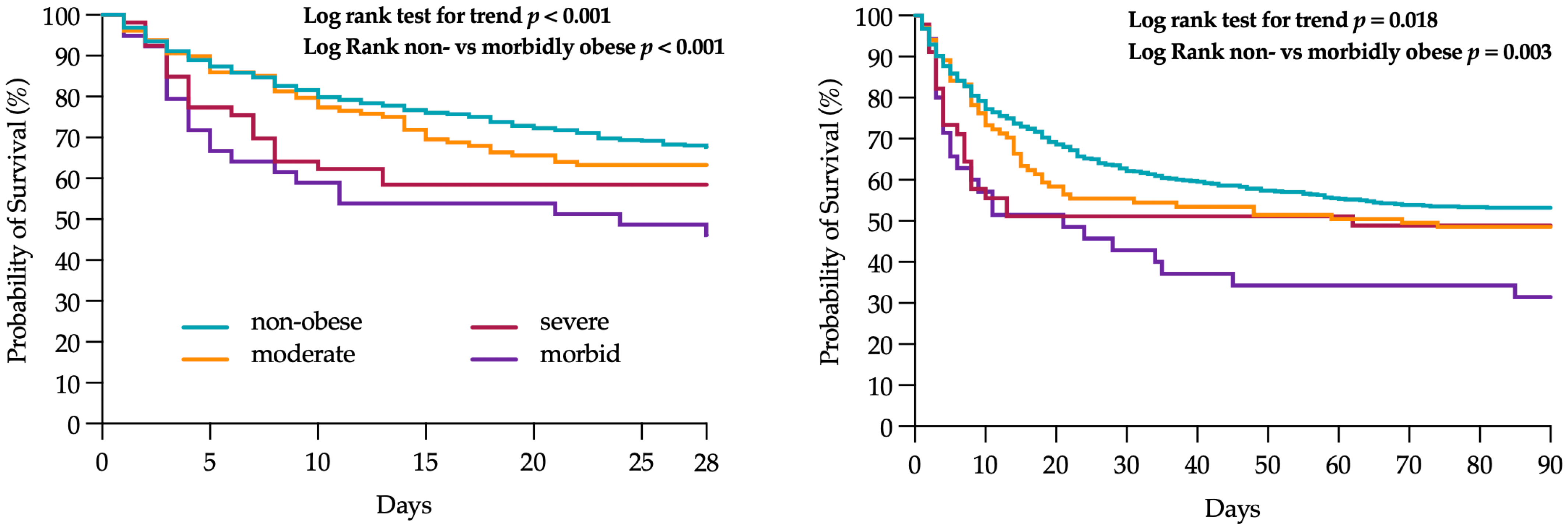
2.6. 28- and 90-Day Mortality Stratified by BMI Groups
2.7. Post Hoc Analysis of Outcomes by Vancomycin Concentrations
3. Discussion
4. Materials and Methods
4.1. Study Design and Patient Population
4.2. Vancomycin Dosing Regimen and TDM
4.3. Study Endpoints
4.4. Data Collection
4.5. Bioanalytical Assay
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PK | Pharmacokinetics |
| TDM | Therapeutic drug monitoring |
| CI | Continuous infusion |
| II | Intermittent infusion |
| MIC | Minimum inhibitory concentration |
| MRSA | Methicillin resistant Staphylococcus aureus |
| Vd | Volume of distribution |
| AKI | Acute kidney injury |
| AKIN | Acute Kidney Injury Network Classification |
| TBW | Total body weight |
| SOFA | Sequential Organ Failure Assessment Score |
| SAPS | Simplified Acute Physiology Score, Version II |
| RRT | Renal replacement therapy |
| ICU | Intensive care unit |
| WHO | World Health Organization |
| AUC | Area under the concentration-time curve |
| IBW | Ideal body weight |
| g | gram |
References
- Falagas, M.E.; Kompoti, M. Obesity and infection. Lancet Infect. Dis. 2006, 6, 438–446. [Google Scholar] [CrossRef]
- Pugliese, G.; Liccardi, A.; Graziadio, C.; Barrea, L.; Muscogiuri, G.; Colao, A. Obesity and infectious diseases: Pathophysiology and epidemiology of a double pandemic condition. Int. J. Obes. 2022, 46, 449–465. [Google Scholar] [CrossRef] [PubMed]
- Hanley, M.J.; Abernethy, D.R.; Greenblatt, D.J. Effect of obesity on the pharmacokinetics of drugs in humans. Clin. Pharmacokinet. 2010, 49, 71–87. [Google Scholar] [CrossRef] [PubMed]
- Knibbe, C.A.; Brill, M.J.; van Rongen, A.; Diepstraten, J.; van der Graaf, P.H.; Danhof, M. Drug disposition in obesity: Toward evidence-based dosing. Annu. Rev. Pharmacol. Toxicol. 2015, 55, 149–167. [Google Scholar] [CrossRef] [PubMed]
- Brill, M.J.; Diepstraten, J.; van Rongen, A.; van Kralingen, S.; van den Anker, J.N.; Knibbe, C.A. Impact of obesity on drug metabolism and elimination in adults and children. Clin. Pharmacokinet. 2012, 51, 277–304. [Google Scholar] [CrossRef]
- Masich, A.M.; Kalaria, S.N.; Gonzales, J.P.; Heil, E.L.; Tata, A.L.; Claeys, K.C.; Patel, D.; Gopalakrishnan, M. Vancomycin Pharmacokinetics in Obese Patients with Sepsis or Septic Shock. Pharmacotherapy 2020, 40, 211–220. [Google Scholar] [CrossRef]
- Álvarez, R.; López Cortés, L.E.; Molina, J.; Cisneros, J.M.; Pachón, J. Optimizing the Clinical Use of Vancomycin. Antimicrob. Agents Chemother. 2016, 60, 2601–2609. [Google Scholar] [CrossRef]
- Katip, W.; Jaruratanasirikul, S.; Pattharachayakul, S.; Wongpoowarak, W.; Jitsurong, A.; Lucksiri, A. The pharmacokinetics of vancomycin during the initial loading dose in patients with septic shock. Infect. Drug Resist. 2016, 9, 253–260. [Google Scholar] [CrossRef]
- Flannery, A.H.; Bissell, B.D.; Bastin, M.T.; Morris, P.E.; Neyra, J.A. Continuous Versus Intermittent Infusion of Vancomycin and the Risk of Acute Kidney Injury in Critically Ill Adults: A Systematic Review and Meta-Analysis. Crit. Care Med. 2020, 48, 912–918. [Google Scholar] [CrossRef]
- Tafelski, S.; Nachtigall, I.; Troeger, U.; Deja, M.; Krannich, A.; Gunzel, K.; Spies, C.; Group, A.B.S. Observational clinical study on the effects of different dosing regimens on vancomycin target levels in critically ill patients: Continuous versus intermittent application. J. Infect. Public Health 2015, 8, 355–363. [Google Scholar] [CrossRef]
- Hanrahan, T.; Whitehouse, T.; Lipman, J.; Roberts, J.A. Vancomycin-associated nephrotoxicity: A meta-analysis of administration by continuous versus intermittent infusion. Int. J. Antimicrob. Agents 2015, 46, 249–253. [Google Scholar] [CrossRef]
- Cataldo, M.A.; Tacconelli, E.; Grilli, E.; Pea, F.; Petrosillo, N. Continuous versus intermittent infusion of vancomycin for the treatment of Gram-positive infections: Systematic review and meta-analysis. J. Antimicrob. Chemother. 2012, 67, 17–24. [Google Scholar] [CrossRef]
- Rybak, M.J.; Le, J.; Lodise, T.P.; Levine, D.P.; Bradley, J.S.; Liu, C.; Mueller, B.A.; Pai, M.P.; Wong-Beringer, A.; Rotschafer, J.C.; et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: A revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am. J. Health Syst. Pharm. 2020, 77, 835–864. [Google Scholar] [CrossRef]
- Rybak, M.J.; Lomaestro, B.M.; Rotschafer, J.C.; Moellering, R.C.; Craig, W.A.; Billeter, M.; Dalovisio, J.R.; Levine, D.P. Vancomycin therapeutic guidelines: A summary of consensus recommendations from the infectious diseases Society of America, the American Society of Health-System Pharmacists, and the Society of Infectious Diseases Pharmacists. Clin. Infect. Dis. 2009, 49, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Lodise, T.P.; Lomaestro, B.; Graves, J.; Drusano, G.L. Larger vancomycin doses (at least four grams per day) are associated with an increased incidence of nephrotoxicity. Antimicrob. Agents Chemother. 2008, 52, 1330–1336. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.C.; Saw, S.; Soliman, D.; Bingham, A.L.; Pontiggia, L.; Hunter, K.; Chuang, L.; Siemianowski, L.A.; Ereshefsky, B.; Hollands, J.M. Intravenous Vancomycin Associated with the Development of Nephrotoxicity in Patients with Class III Obesity. Ann. Pharmacother. 2017, 51, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Wuerger, A.; Bowden, J.; Mitchell, A.; Marler, J. The Effect of Vancomycin and Piperacillin-Tazobactam on Incidence of Acute Kidney Injury in Patients with Obesity. Hosp. Pharm. 2023, 58, 605–613. [Google Scholar] [CrossRef]
- Reynolds, D.C.; Waite, L.H.; Alexander, D.P.; DeRyke, C.A. Performance of a vancomycin dosage regimen developed for obese patients. Am. J. Health Syst. Pharm. 2012, 69, 944–950. [Google Scholar] [CrossRef]
- Kubiak, D.W.; Alquwaizani, M.; Sansonetti, D.; Barra, M.E.; Calderwood, M.S. An Evaluation of Systemic Vancomycin Dosing in Obese Patients. Open Forum Infect. Dis. 2015, 2, ofv176. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Assadoon, M.S.; Pearson, J.C.; Kubiak, D.W.; Kovacevic, M.P.; Dionne, B.W. Evaluation of Vancomycin Accumulation in Patients with Obesity. Open Forum Infect. Dis. 2022, 9, ofac491. [Google Scholar] [CrossRef]
- Richardson, J.; Scheetz, M.; O’Donnell, E.P. The association of elevated trough serum vancomycin concentrations with obesity. J. Infect. Chemother. 2015, 21, 507–511. [Google Scholar] [CrossRef]
- Davies, S.W.; Efird, J.T.; Guidry, C.A.; Dietch, Z.C.; Willis, R.N.; Shah, P.M.; Hennessy, S.A.; Sawyer, R.G. Vancomycin-Associated Nephrotoxicity: The Obesity Factor. Surg. Infect. 2015, 16, 684–693. [Google Scholar] [CrossRef] [PubMed]
- Bruniera, F.R.; Ferreira, F.M.; Saviolli, L.R.; Bacci, M.R.; Feder, D.; da Luz Goncalves Pedreira, M.; Sorgini Peterlini, M.A.; Azzalis, L.A.; Campos Junqueira, V.B.; Fonseca, F.L. The use of vancomycin with its therapeutic and adverse effects: A review. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 694–700. [Google Scholar] [PubMed]
- Hotchkiss, R.S.; Moldawer, L.L.; Opal, S.M.; Reinhart, K.; Turnbull, I.R.; Vincent, J.L. Sepsis and septic shock. Nat. Rev. Dis. Primers 2016, 2, 16045. [Google Scholar] [CrossRef] [PubMed]
- Jarczak, D.; Kluge, S.; Nierhaus, A. Sepsis-Pathophysiology and Therapeutic Concepts. Front. Med. 2021, 8, 628302. [Google Scholar] [CrossRef]
- Rodriguez, A.; Martin-Loeches, I.; Moreno, G.; Diaz, E.; Ferre, C.; Salgado, M.; Marin-Corral, J.; Estella, A.; Sole-Violan, J.; Trefler, S.; et al. Association of obesity on the outcome of critically ill patients affected by COVID-19. Med. Intensiva 2024, 48, 142–154. [Google Scholar] [CrossRef]
- Global, B.M.I.M.C.; Di Angelantonio, E.; Bhupathiraju Sh, N.; Wormser, D.; Gao, P.; Kaptoge, S.; Berrington de Gonzalez, A.; Cairns, B.J.; Huxley, R.; Jackson Ch, L.; et al. Body-mass index and all-cause mortality: Individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet 2016, 388, 776–786. [Google Scholar] [CrossRef]
- Pepper, D.J.; Sun, J.; Welsh, J.; Cui, X.; Suffredini, A.F.; Eichacker, P.Q. Increased body mass index and adjusted mortality in ICU patients with sepsis or septic shock: A systematic review and meta-analysis. Crit. Care 2016, 20, 181. [Google Scholar] [CrossRef]
- Schetz, M.; De Jong, A.; Deane, A.M.; Druml, W.; Hemelaar, P.; Pelosi, P.; Pickkers, P.; Reintam-Blaser, A.; Roberts, J.; Sakr, Y.; et al. Obesity in the critically ill: A narrative review. Intensive Care Med. 2019, 45, 757–769. [Google Scholar] [CrossRef]
- Hogue, C.W., Jr.; Stearns, J.D.; Colantuoni, E.; Robinson, K.A.; Stierer, T.; Mitter, N.; Pronovost, P.J.; Needham, D.M. The impact of obesity on outcomes after critical illness: A meta-analysis. Intensive Care Med. 2009, 35, 1152–1170. [Google Scholar] [CrossRef]
- Casapao, A.M.; Lodise, T.P.; Davis, S.L.; Claeys, K.C.; Kullar, R.; Levine, D.P.; Rybak, M.J. Association between vancomycin day 1 exposure profile and outcomes among patients with methicillin-resistant Staphylococcus aureus infective endocarditis. Antimicrob. Agents Chemother. 2015, 59, 2978–2985. [Google Scholar] [CrossRef]
- Lodise, T.P.; Rosenkranz, S.L.; Finnemeyer, M.; Evans, S.; Sims, M.; Zervos, M.J.; Creech, C.B.; Patel, P.C.; Keefer, M.; Riska, P.; et al. The Emperor’s New Clothes: Prospective Observational Evaluation of the Association Between Initial VancomycIn Exposure and Failure Rates Among Adult Hospitalized Patients with Methicillin-resistant Staphylococcus aureus Bloodstream Infections (PROVIDE). Clin. Infect. Dis. 2020, 70, 1536–1545. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Obesity and Overweight 2016; World Health Organization (WHO): Geneva, Switzerland, 2016. [Google Scholar]
- Kluge, H.H.P. WHO European Regional Obesity Report 2022; World Health Organization: Copenhagen, Denmark, 2022; p. 206. [Google Scholar]

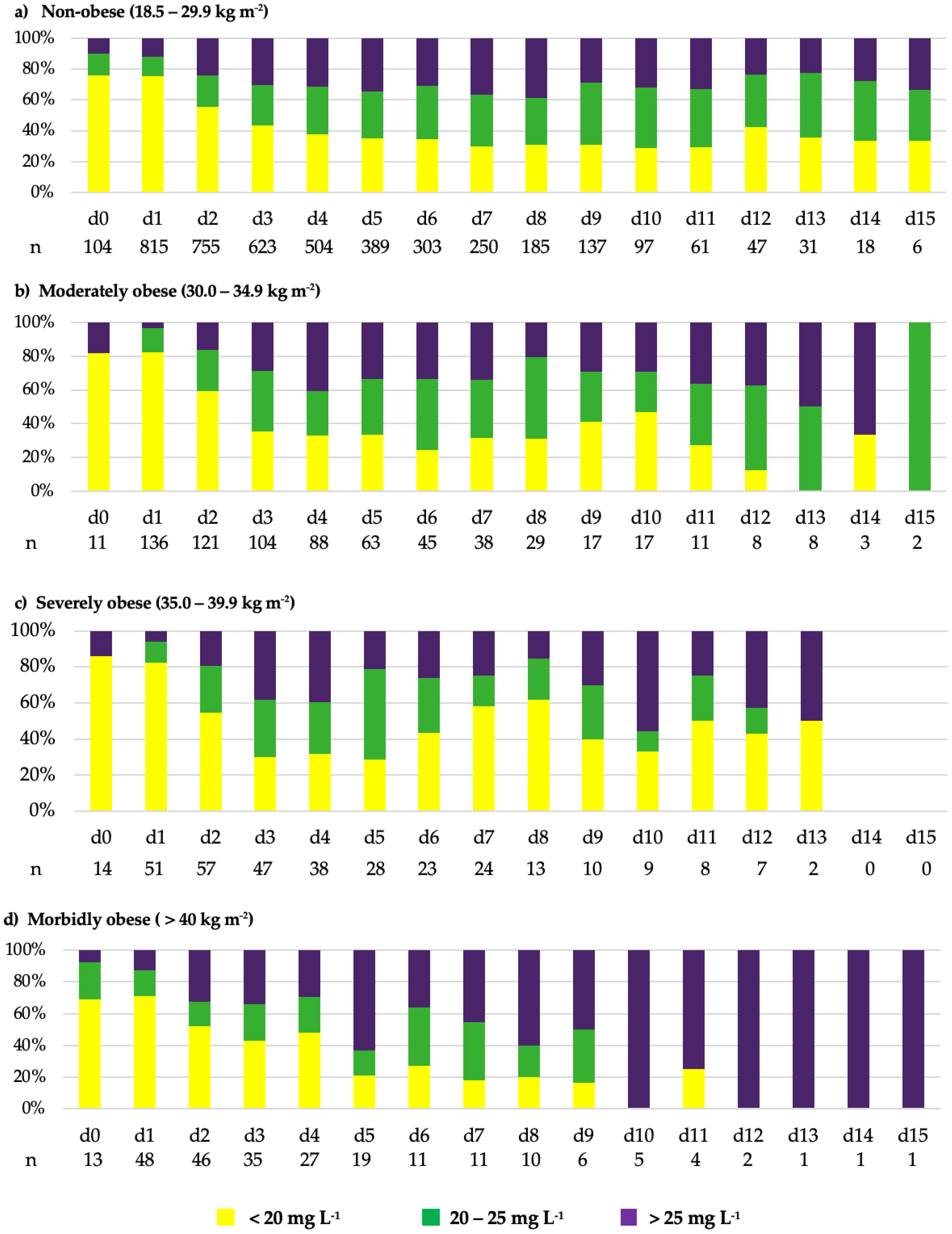

| BMI (kg m−2) | Total | 18.5–29.9 | 30.0–34.9 | 35.0–39.9 | >40 | p-Value |
|---|---|---|---|---|---|---|
| Patients | 1066 | 829 | 135 | 54 | 44 | |
| Sex, male | 699 (65.6) | 562 (67.5) | 81 (60.0) | 29 (52.7) | 27 (61.4) | 0.050 |
| Age (years) | 62 (51; 73) | 61 (50; 73) | 64 (56; 75) | 63 (56; 71) | 58 (50; 67) | 0.031 |
| TBW (kg) | 80 (70; 90) | 75 (65; 82) | 95 (85; 100) | 110 (100; 120) | 138 (122; 156) | <0.001 |
| IBW (kg) | 72 (60; 80) | 72 (63; 80) | 70 (59; 80) | 70 (54; 80) | 69 (59; 75) | 0.098 |
| BMI (kg m−2) | 26.1 (23.6; 29.4) | 24.7 (22.9; 27.1) | 31.6 (30.9; 33.1) | 37.0 (35.5; 38.7) | 46.7 (42.5; 53.4) | <0.001 |
| SOFA score | 6 (4; 10) | 6 (4; 10) | 6 (4; 10) | 7 (5; 11) | 6 (4; 12) | 0.792 |
| SAPS-II score | 43 (28; 57) | 43 (28; 57) | 46 (31; 59) | 49 (33; 63) | 39 (28; 57) | 0.143 |
| Necessity of RRT | 234 (22.0) | 163 (19.7) | 37 (26.6) | 19 (32.8) | 15 (34.9) | <0.001 |
| Necessity of MV | 790 (74.1) | 608 (73.6) | 97 (69.8) | 47 (81.0) | 38 (88.4) | 0.061 |
| BMI (kg m−2) | Total (n = 1066) | 18.5–29.9 (n = 829) | 30.0–34.9 (n = 135) | 35.0–39.9 (n = 54) | >40 (n = 44) | p-Value |
|---|---|---|---|---|---|---|
| Vancomycin therapy (days) | 4 (2; 7) | 4 (2; 7) | 4 (3; 7) | 4 (3; 8) | 3 (2; 6) | 0.723 |
| No. of TDM measurements obtained from each patient | 4 (2; 6) | 4 (2; 6) | 4 (2; 6) | 4 (2; 7) | 3 (2; 5) | 0.446 |
| TDM measurements (cum.) * | 4699 | 3633 | 627 | 260 | 179 | |
| <20 mg L−1 | 2247 (47.8) | 1760 (48.4) | 297 (47.4) | 121 (46.5) | 69 (38.6) | 0.073 |
| 20–25 mg L−1 | 1218 (25.9) | 931 (25.6) | 177 (28.2) | 75 (28.9) | 35 (19.6) | 0.077 |
| >25 mg L−1 | 1234 (26.3) | 942 (25.9) | 153 (24.4) | 64 (24.6) | 75 (41.9) | <0.001 |
| Days to target concentrations | 3 (2; 4) | 3 (2; 4) | 3 (2; 4) | 3 (2; 4) | 3 (1; 4) | 0.835 |
| Therapy days < 20 mg L−1 | 2 (1; 3) | 2 (1; 3) | 2 (1; 3) | 1 (1; 3) | 1 (1; 2) | 0.159 |
| % of therapy days | 50.0 (25.0; 100) | 50.0 (25.0; 100) | 50.0 (25.0; 100) | 50.0 (25.0; 75.0) | 50.0 (17.0; 100) | 0.757 |
| Therapy days 20–25 mg L−1 | 1 (0; 2) | 1 (0; 2) | 1 (0; 2) | 1 (0; 2) | 1 (0; 1) | 0.189 |
| % of therapy days | 16.7 (0.0; 37.5) | 14.3 (0.0; 37.5) | 25.0 (0.0; 43.7) | 25.0 (0.0; 40.7) | 35.7 (0.0; 33.3) | 0.190 |
| Therapy days > 25 mg L−1 | 1 (0; 2) | 0 (0; 2) | 0 (0; 2) | 1 (0; 2) | 0 (0; 3) | 0.579 |
| % of therapy days | 9.1 (0.0; 40.0) | 0.0 (0.0; 42.9) | 0.0 (0.0; 33.3) | 23.1 (0.0; 40.0) | 0.0 (0.0; 50.0) | 0.529 |
| Loading dose (g) | 1.0 (1.0; 1.0) | 1.0 (1.0; 1.0) | 1.0 (1.0; 1.0) | 1.0 (1.0; 1.0) | 1.0 (1.0; 1.0) | 0.180 |
| Vancomycin cum. dose (g) | 8.0 (5.4; 12.5) | 8.1 (5.4; 12.7) | 7.6 (5.5; 11.8) | 8.0 (5.9; 12.6) | 8.8 (4.5; 12.9) | 0.919 |
| Vancomycin dose per day/IBW (mg kg−1) | 31.1 (22.6; 42.3) | 31.0 (22.7; 41.5) | 30.3 (21.8; 42.8) | 31.3 (21.9; 44.1) | 36.4 (24.5; 50.3) | 0.259 |
| BMI (kg m−2) | Total | 18.5–29.9 | 30.0–34.9 | 35.0–39.9 | >40 | p-Value |
|---|---|---|---|---|---|---|
| Median SOFA * | 7 (3; 11) | 7 (3; 10) | 6 (3; 10) | 9 (4; 12) | 10 (3; 12) | 0.024 |
| Highest SOFA * | 9 (6; 13) | 9 (6; 13) | 9 (5; 13) | 12 (5; 15) | 12 (6; 14) | 0.122 |
| Median SAPS-II * | 43 (31; 55) | 43 (30; 55) | 43 (33; 55) | 50 (36; 64) | 48 (33; 62) | 0.035 |
| Highest SAPS-II * | 57 (40; 71) | 48 (33; 62) | 57 (44; 73) | 61 (50; 81) | 57 (42; 74) | 0.066 |
| Mechanical ventilation ** | 865 (81.1) | 664 (80.4) | 112 (80.6) | 49 (84.5) | 40 (93.0) | 0.193 |
| Ventilated days | 3 (1; 6) | 3 (1; 6) | 4 (1; 6) | 4 (3; 8) | 4 (2; 7) | 0.228 |
| AKI (highest stage) | 0.805 | |||||
| AKIN stage 1 | 256 (27.2) | 196 (26.7) | 35 (30.2) | 13 (26.0) | 12 (30.0) | |
| AKIN stage 2 | 172 (18.3) | 134 (18.3) | 23 (19.8) | 8 (16.0) | 7 (17.5) | |
| AKIN stage 3 | 207 (22.0) | 156 (21.3) | 26 (22.4) | 16 (32.0) | 9 (22.5) | |
| RRT ** | 375 (35.2) | 269 (32.6) | 50 (36.0) | 31 (53.4) | 25 (58.1) | <0.001 |
| RRT days (cum.) | 4 (2; 6) | 4 (2; 6) | 4 (2; 7) | 4 (3; 6) | 2 (2; 4) | 0.187 |
| Length of stay, ICU | 22 (9; 37) | 21 (9; 36) | 23 (9, 42) | 23 (11; 38) | 17 (7; 50) | 0.783 |
| Length of stay, hospital | 35 (22; 60) | 34 (22; 60) | 41 (21; 60) | 34 (22; 60) | 39 (14; 71) | 0.940 |
| ICU mortality | 343 (32.1) | 255 (30.8) | 41 (29.5) | 22 (37.9) | 25 (58.1) | 0.002 |
| In-hospital mortality | 408 (38.0) | 305 (36.9) | 52 (37.4) | 23 (39.7) | 25 (58.1) | 0.047 |
| 28-day mortality | 331 (34.3) | 241 (32.4) | 47 (36.7) | 22 (41.5) | 21 (53.8) | 0.025 |
| 90-day mortality | 418 (50.0) | 314 (48.2) | 56 (54.4) | 23 (51.1) | 25 (69.4) | 0.068 |
| Supratherapeutic Vancomycin Concentrations at Any Point (>25 mg L−1) | p-Value | ||
|---|---|---|---|
| No | Yes | ||
| 28-day mortality | 160 (33.9) | 172 (34.9) | 0.746 |
| 90-day mortality | 186 (44.7) | 233 (55.3) | 0.002 |
| ICU mortality | 153 (28.8) | 190 (35.3) | 0.022 |
| In-hospital mortality | 175 (33.0) | 231 (42.9) | <0.001 |
| No | Yes | ||
| Highest SOFA score | 52 (37; 66) | 61 (44; 74) | <0.001 |
| Highest SAPS score | 8 (5; 12) | 11 (6;15) | <0.001 |
| Length of stay, ICU | 18 (6; 30) | 25 (13; 44) | <0.001 |
| Length of stay, hospital | 31 (19; 55) | 41 (24; 66) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nothofer, S.; Angeli, R.; Weiss, M.; Dumps, C.; Berger, F.; Eckert, J.; Girrbach, F.; Scheidt, N.; Menzel, S.; Lange, M.; et al. Impact of Obesity on Serum Concentrations of Vancomycin Administered as Continuous Infusion and on Clinical Outcomes in Critically Ill Patients—A Retrospective Observational Study. Antibiotics 2025, 14, 895. https://doi.org/10.3390/antibiotics14090895
Nothofer S, Angeli R, Weiss M, Dumps C, Berger F, Eckert J, Girrbach F, Scheidt N, Menzel S, Lange M, et al. Impact of Obesity on Serum Concentrations of Vancomycin Administered as Continuous Infusion and on Clinical Outcomes in Critically Ill Patients—A Retrospective Observational Study. Antibiotics. 2025; 14(9):895. https://doi.org/10.3390/antibiotics14090895
Chicago/Turabian StyleNothofer, Stefanie, Rico Angeli, Manfred Weiss, Christian Dumps, Felix Berger, Josephin Eckert, Felix Girrbach, Nadin Scheidt, Susan Menzel, Mirko Lange, and et al. 2025. "Impact of Obesity on Serum Concentrations of Vancomycin Administered as Continuous Infusion and on Clinical Outcomes in Critically Ill Patients—A Retrospective Observational Study" Antibiotics 14, no. 9: 895. https://doi.org/10.3390/antibiotics14090895
APA StyleNothofer, S., Angeli, R., Weiss, M., Dumps, C., Berger, F., Eckert, J., Girrbach, F., Scheidt, N., Menzel, S., Lange, M., Wrigge, H., & Simon, P. (2025). Impact of Obesity on Serum Concentrations of Vancomycin Administered as Continuous Infusion and on Clinical Outcomes in Critically Ill Patients—A Retrospective Observational Study. Antibiotics, 14(9), 895. https://doi.org/10.3390/antibiotics14090895








