Human Antimicrobial Use in Bangladesh: Five-Year Trend Analysis Including COVID-19 Pandemic Era
Abstract
1. Introduction
2. Results
2.1. National Antimicrobial Use Pattern of Bangladesh (2019 to 2023)
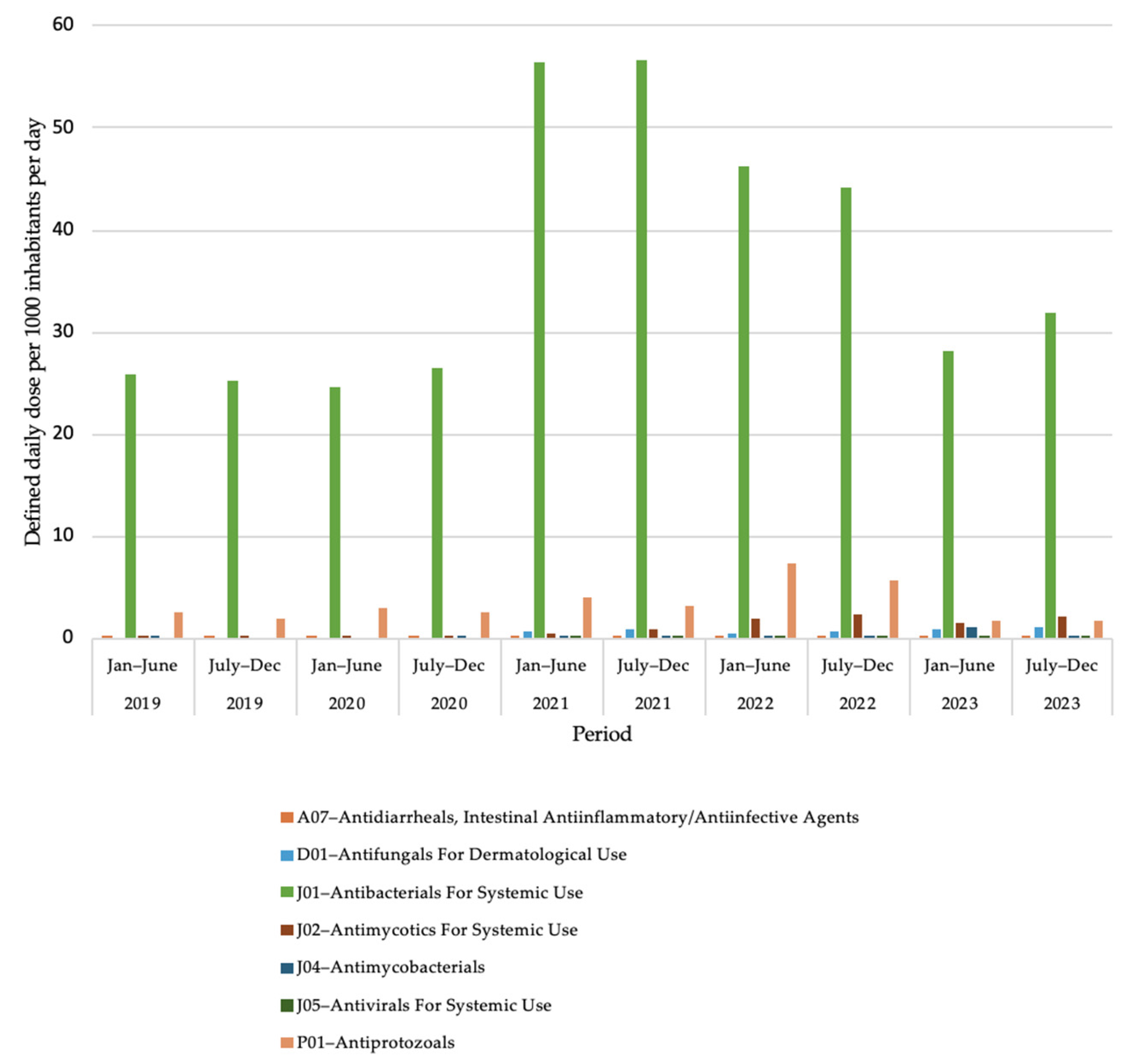
2.2. Antimicrobial Use at Second Level of Anatomical Therapeutic Chemical Classification
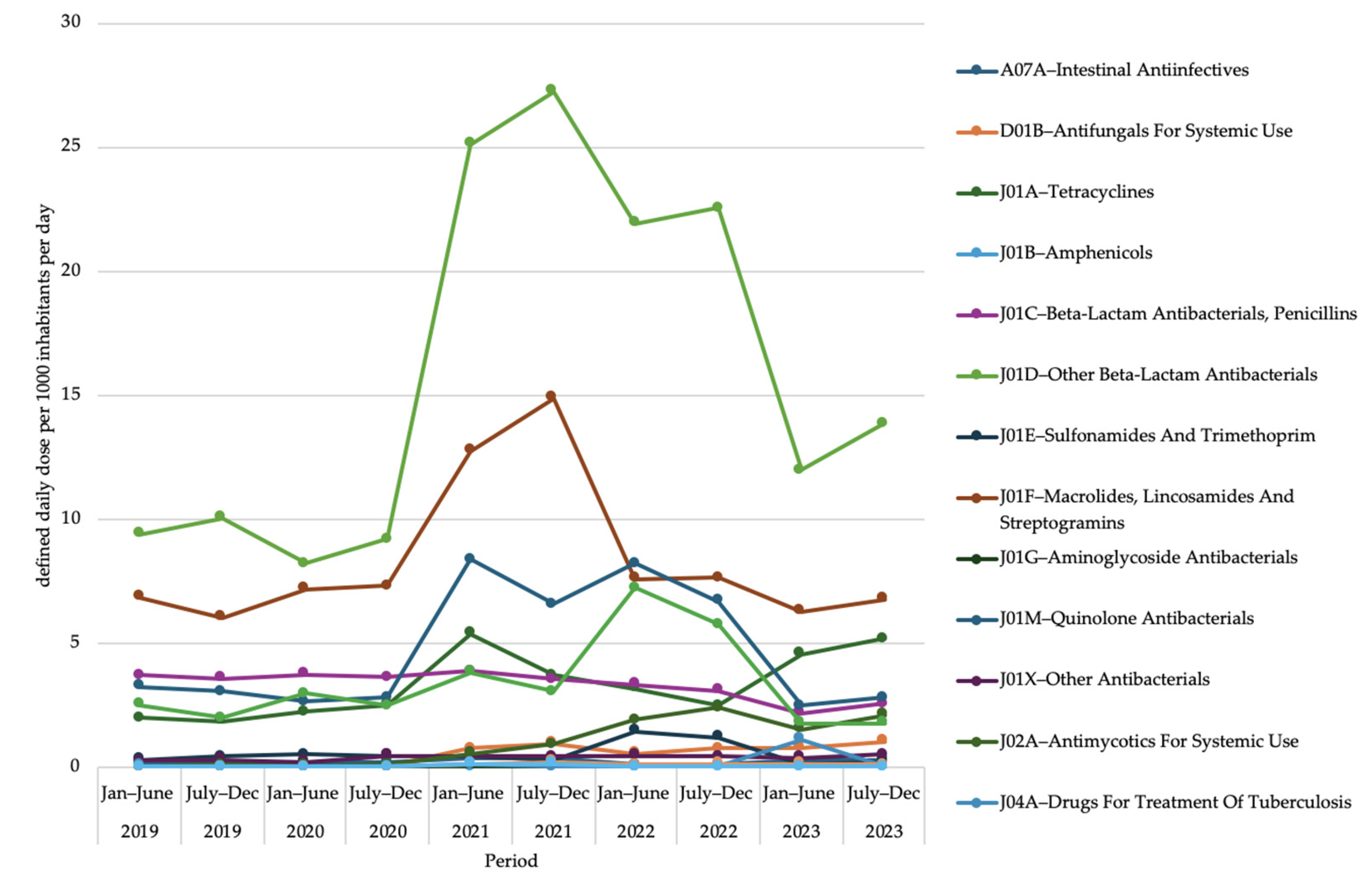
2.3. Antimicrobial Use at Third Level of Anatomical Therapeutic Chemical Classification
2.4. Drug Utilization 75% for Oral Dosage Form of Antimicrobials
2.5. Drug Utilization 75% for Parenteral Dosage Form of Antimicrobials
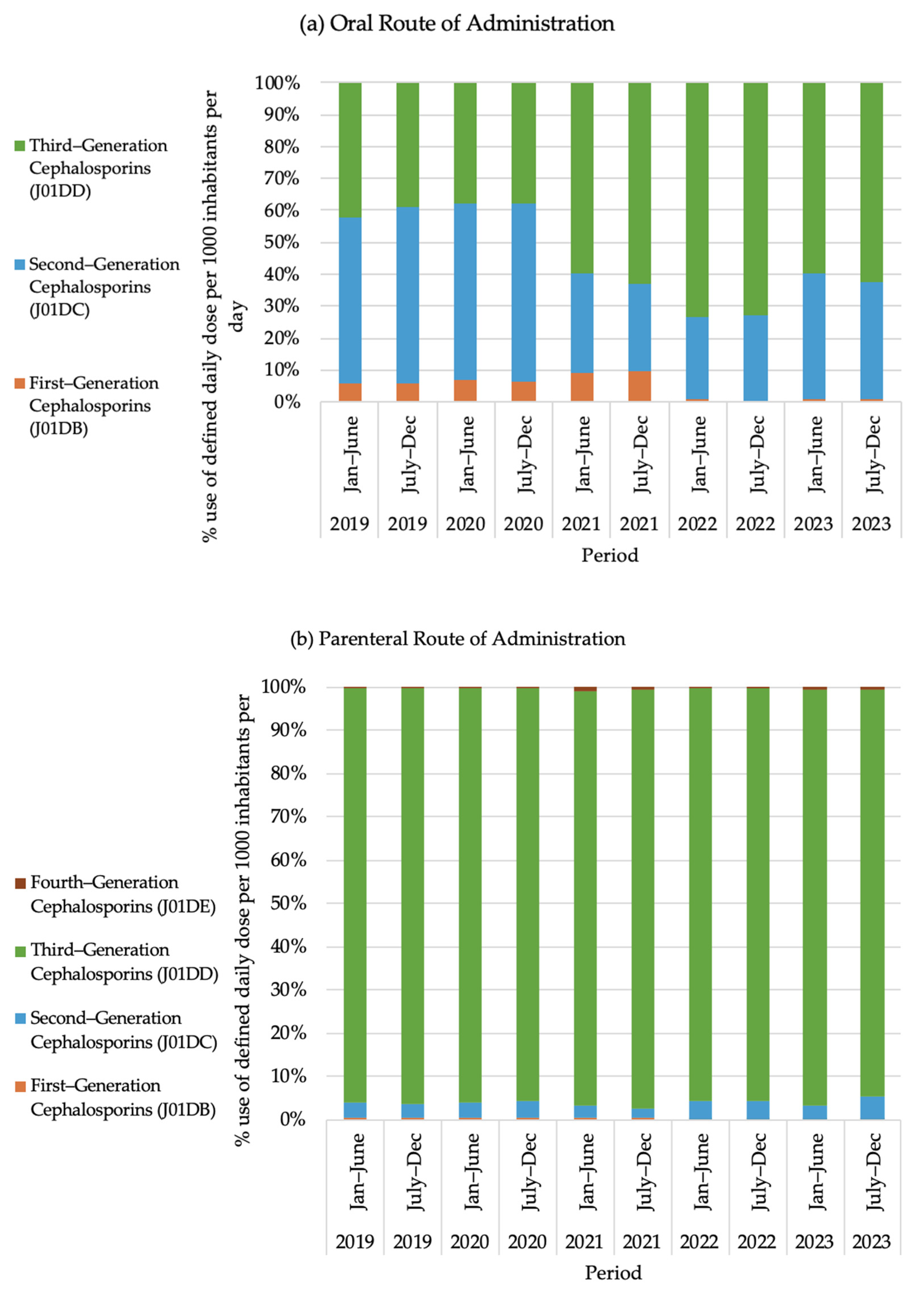
2.6. Use of Cephalosporin Group Antibiotics
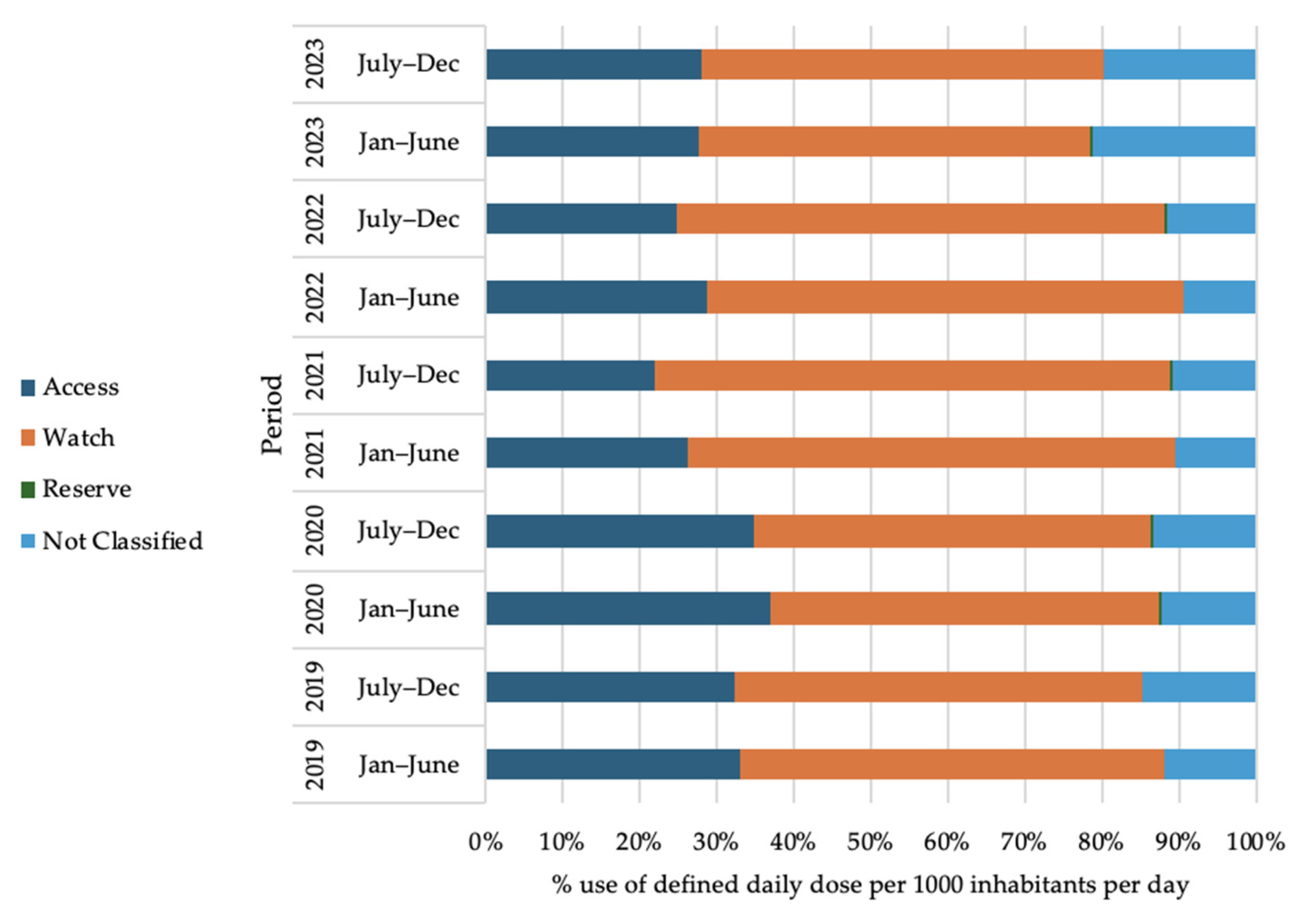
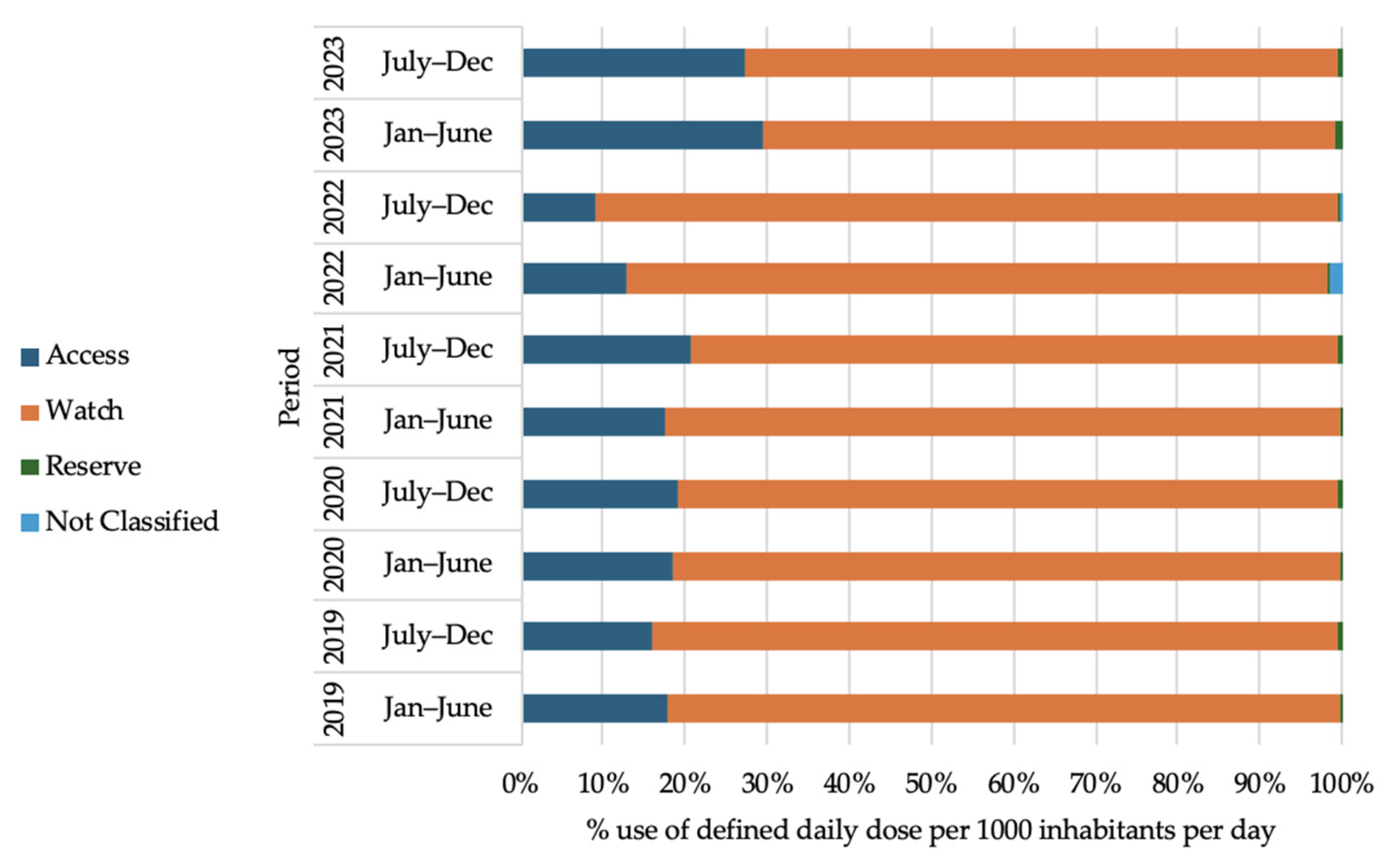
2.7. Antibiotic Use Trend According to WHO-AWaRe Classification of Antibiotics
3. Discussion
4. Materials and Methods
4.1. Data Sources
4.2. Methods
4.3. Population Data
4.4. Calculation of Defined Daily Dose (DDD) and Defined Daily Dose per 1000 Inhabitants per Day (DID) [13]
4.5. Analyzing Effect of COVID-19 Pandemic on National AMU
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMR | Antimicrobial resistance |
| AMU | Antimicrobial use |
| ASPs | Antimicrobial Stewardship Programs |
| ATC | Anatomical Therapeutic Chemical |
| AWaRe | Access, Watch, Reserve |
| COVID-19 | Coronavirus disease 2019 |
| DDD | Defined daily dose |
| DGDA | Directorate General of Drug Administration |
| DID | Defined daily dose per 1000 inhabitants per day |
| DU75 | Drug Utilization 75 percent |
| GLASS | Global Antimicrobial Resistance and Use Surveillance System |
| IPC | Infection prevention and control |
| LMICs | Low- and middle-income countries |
| OTC | Over the counter |
References
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Salam, M.A.; Al-Amin, M.Y.; Salam, M.T.; Pawar, J.S.; Akhter, N.; Rabaan, A.A.; Alqumber, M.A. Antimicrobial resistance: A growing serious threat for global public health. InHealthcare 2023, 11, 1946. [Google Scholar] [CrossRef] [PubMed]
- Chambial, P.; Thakur, N.; Bhukya, P.L.; Subbaiyan, A.; Kumar, U. Frontiers in superbug management: Innovating approaches to combat antimicrobial resistance. Arch. Microbiol. 2025, 207, 60. [Google Scholar] [CrossRef] [PubMed]
- Grau, S.; Bou, G.; Fondevilla, E.; Nicolás, J.; Rodríguez-Maresca, M.; Martínez-Martínez, L. How to measure and monitor antimicrobial consumption and resistance. Enfermedades Infecc. Microbiol. Clin. 2013, 31, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Sumon, S.A.; Sarker, S.; Chowdhury, A.A.; Abdullah, S.A.; Shahjahan, M.; Sharmin, S.; Harun, M.G. Antibiotic use in tertiary care hospitals in Bangladesh: Revealing the extent through a Point Prevalence Survey. Am. J. Infect. Control 2024, 52, 1052–1059. [Google Scholar] [CrossRef]
- Global Action Plan on Antimicrobial Resistance—World Health Organization. Available online: https://www.who.int/publications/i/item/9789241509763 (accessed on 25 May 2025).
- Global Strategy for Containment of Antimicrobial Resistance—World Health Organization. Available online: https://iris.who.int/bitstream/handle/10665/44812/9789241503181_eng.pdf (accessed on 25 May 2025).
- Directorate General of Health Services (DGHS), Bangladesh—Official Notice. Available online: https://dghs.portal.gov.bd/sites/default/files/files/dghs.portal.gov.bd/page/b6580681_7a63_4289_9ce8_b7586b2491f7/2025-04-09-09-02-44ab811f4ec3a7e3f3fa34082d3916e0.pdf (accessed on 25 May 2025).
- World Health Organization—Bangladesh. Available online: https://www.who.int/bangladesh (accessed on 25 May 2025).
- Bangladesh: Professional Fellowship—The Fleming Fund. Available online: https://www.flemingfund.org/grants/bangladesh-professional-fellowship/ (accessed on 25 May 2025).
- Statens Serum Institut. Available online: https://en.ssi.dk (accessed on 25 May 2025).
- International Livestock Research Institute. Available online: https://www.ilri.org/ (accessed on 25 May 2025).
- GLASS Manual on the Management of Antimicrobial Consumption Data—World Health Organization. Available online: https://www.who.int/publications/i/item/9789240010192 (accessed on 25 May 2025).
- The WHO AWaRe (Access, Watch, Reserve) Antibiotic Book—World Health Organization. Available online: https://www.who.int/publications/i/item/9789240062382 (accessed on 25 May 2025).
- COVID-19 Situation in the WHO European Region. Available online: https://www.who.int/europe/emergencies/situations/covid-19 (accessed on 25 May 2025).
- Sharma, S.; Singh, A.; Banerjee, T. Antibacterial agents used in COVID-19: A systematic review and meta-analysis. Environ. Sustain. 2021, 4, 503–513. [Google Scholar] [CrossRef]
- Fukushige, M.; Ngo, N.H.; Lukmanto, D.; Fukuda, S.; Ohneda, O. Effect of the COVID-19 pandemic on antibiotic consumption: A systematic review comparing 2019 and 2020 data. Front. Public Health 2022, 10, 946077. [Google Scholar] [CrossRef]
- Saleem, Z.; Mekonnen, B.A.; Orubu, E.S.; Islam, M.A.; Nguyen, T.T.; Ubaka, C.M.; Buma, D.; Thuy, N.D.; Sant, Y.; Sono, T.M.; et al. Current access, availability and use of antibiotics in primary care among key low-and middle-income countries and the policy implications. Expert Rev. Anti-Infect. Ther. 2025, 1–42. [Google Scholar] [CrossRef]
- Mondal, U.K.; Haque, T.; Biswas, M.A.; Satter, S.M.; Islam, M.S.; Alam, Z.; Shojon, M.; Debnath, S.; Islam, M.; Murshid, H.B.; et al. Antibiotic Prescribing practices for treating COVID-19 patients in Bangladesh. Antibiotics 2022, 11, 1350. [Google Scholar] [CrossRef]
- Bonna, A.S.; Pavel, S.R.; Ferdous, J.; Khan, S.A.; Ali, M. Antibiotic resistance: An increasingly threatening but neglected public health challenge in Bangladesh. Int. J. Surg. Open 2022, 49, 100581. [Google Scholar] [CrossRef]
- WHO Report on Surveillance of Antibiotic Consumption: 2016–2018 Early Implementation. Available online: https://www.who.int/publications/i/item/9789241514880 (accessed on 25 May 2025).
- Khan, S.; Hasan, S.S.; Bond, S.E.; Conway, B.R.; Aldeyab, M.A. Antimicrobial consumption in hospitalised COVID-19 patients: A systematic review and meta-analysis. Int. J. Pharm. Pract. 2022, 30 (Suppl. S1), i5–i6. [Google Scholar] [CrossRef]
- Pierce, J.; Stevens, M.P. COVID-19 and antimicrobial stewardship: Lessons learned, best practices, and future implications. Int. J. Infect. Dis. 2021, 113, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.S.; Mundra, S. Increasing consumption of antibiotics during the COVID-19 pandemic: Implications for patient health and emerging anti-microbial resistance. Antibiotics 2022, 12, 45. [Google Scholar] [CrossRef]
- Mah-E-Muneer, S.; Hassan, M.Z.; Biswas, M.A.A.J.; Rahman, F.; Akhtar, Z.; Das, P.; Islam, M.A.; Chowdhury, F. Use of antimicrobials among suspected COVID-19 patients at selected hospitals, Bangladesh: Findings from the first wave of COVID-19 pandemic. Antibiotics 2021, 10, 738. [Google Scholar] [CrossRef]
- Sumon, S.A.; Anwar, M.M.; Akther, F.M.; Priyanka, A.S.; Tamanna, T.; Rahman, A.; Islam, M.S.; Harun, M.D. Perceptions of antibiotic stewardship programmes and determinants of antibiotic prescribing patterns among physicians in tertiary hospitals in Bangladesh: Implications for future policy and practice. J. Hosp. Infect. 2024, 144, 56–65. [Google Scholar] [CrossRef]
- Khan, S.; Hasan, S.S.; Bond, S.E.; Conway, B.R.; Aldeyab, M.A. Antimicrobial consumption in patients with COVID-19: A systematic review and meta-analysis. Expert Rev. Anti-Infect. Ther. 2022, 20, 749–772. [Google Scholar] [CrossRef]
- National Guidelines on Clinical Management of Coronavirus Disease 2019 (COVID-19), Directorate General of Health Services, Bangladesh. Available online: https://file.portal.gov.bd/files/cs.sylhet.gov.bd/notices/245c5244_369b_4305_958d_1f70d99d6681/4c021caf6cf2915278aa3598e74f5c09.pdf (accessed on 25 May 2025).
- Kalam, A.; Shano, S.; Khan, M.A.; Islam, A.; Warren, N.; Hassan, M.M.; Rahman, M.; Saifullah, M.; Iqbal, A.; Howlader, R.; et al. Understanding the social drivers of antibiotic use during COVID-19 in Bangladesh: Implications for the reduction of antimicrobial resistance. PLoS ONE 2021, 16, e0261368. [Google Scholar] [CrossRef]
- Knight, G.M.; Glover, R.E.; McQuaid, C.F.; Olaru, I.D.; Gallandat, K.; Leclerc, Q.J.; Fuller, N.M.; Willcocks, S.J.; Hasan, R.; Van Kleef, E.; et al. Antimicrobial resistance and COVID-19: Intersections and implications. eLife 2021, 10, e64139. [Google Scholar] [CrossRef]
- Parveen, M.; Molla, M.M.; Yeasmin, M.; Nafisa, T.; Barna, A.A.; Ghosh, A.K. Evidences on irrational anti-microbial prescribing and consumption among COVID-19 positive patients and possible mitigation strategies: A descriptive cross sectional study. Bangladesh J. Infect. Dis. 2020, 7, S3. [Google Scholar] [CrossRef]
- Al Masud, A.; Walpola, R.L.; Sarker, M.; Kabir, A.; Asaduzzaman, M.; Islam, M.S.; Mostafa, A.T.; Akhtar, Z.; Barua, M.; Seale, H. Understanding antibiotic purchasing practices in community pharmacies: A potential driver of emerging antimicrobial resistance. Explor. Res. Clin. Soc. Pharm. 2024, 15, 100485. [Google Scholar] [CrossRef] [PubMed]
- Political Declaration of the High-level Meeting on Antimicrobial Resistance. Available online: https://www.un.org/pga/wp-content/uploads/sites/108/2024/09/FINAL-Text-AMR-to-PGA.pdf (accessed on 25 May 2025).
- Klein, E.Y.; Milkowska-Shibata, M.; Tseng, K.K.; Sharland, M.; Gandra, S.; Pulcini, C.; Laxminarayan, R. Assessment of WHO antibiotic consumption and access targets in 76 countries, 2000–2015: An analysis of pharmaceutical sales data. Lancet Infect. Dis. 2021, 21, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Robertson, J.; Iwamoto, K.; Hoxha, I.; Ghazaryan, L.; Abilova, V.; Cvijanovic, A.; Pyshnik, H.; Darakhvelidze, M.; Makalkina, L.; Jakupi, A.; et al. Antimicrobial medicines consumption in Eastern Europe and Central Asia—An updated cross-national study and assessment of quantitative metrics for policy action. Front. Pharmacol. 2019, 9, 1156. [Google Scholar] [CrossRef] [PubMed]
- List of Allopathic Drug Manufacturers—Directorate General of Drug Administration (DGDA), Bangladesh. Available online: http://dgda.gov.bd/ (accessed on 25 May 2025).
- GLASS Methodology for Surveillance of National Antimicrobial Consumption–World Health Organization. Available online: https://iris.who.int/bitstream/handle/10665/336215/9789240012639-eng.pdf?sequence=1 (accessed on 14 July 2025).
- Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report, Antibiotic Use Data for 2022–World Health Organization. Available online: https://iris.who.int/bitstream/handle/10665/381094/9789240108127-eng.pdf?sequence=1 (accessed on 14 July 2025).
- Mott MacDonald. Website: Mott MacDonald [Internet]. Available online: https://www.mottmac.com/ (accessed on 25 May 2025).
| Antimicrobial Medicine Use (Defined Daily Dose per 1000 Inhabitants per Day) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre–COVID-19 Period | During COVID–19 Period | After COVID–19 Period | ||||||||
| Dosage Form | 2019 | 2020 | 2021 | 2022 | 2023 | |||||
| Jan–June | July–Dec | Jan–June | July–Dec | Jan–June | July–Dec | Jan–June | July–Dec | Jan–June | July–Dec | |
| Oral | 28.02 | 26.97 | 27.45 | 28.74 | 60.91 | 61.30 | 54.67 | 52.13 | 33.01 | 36.49 |
| Parenteral | 0.55 | 0.58 | 0.46 | 0.46 | 1.38 | 1.06 | 1.48 | 1.16 | 0.73 | 0.88 |
| Total | 28.57 | 27.55 | 27.91 | 29.20 | 62.28 | 62.36 | 56.14 | 53.29 | 33.73 | 37.37 |
| Percentage of Defined Daily Dose per 1000 Inhabitants per Day (Rank in Parentheses) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Antimicrobial Agent (ATC Code) | 2019 | 2020 | 2021 | 2022 | 2023 | |||||
| Jan–June | July–Dec | Jan–June | July–Dec | Jan–June | July–Dec | Jan–June | July–Dec | Jan–June | July–Dec | |
| Cefixime (J01DD08) | 12.7 (2) | 13.17 (3) | 10.24 (4) | 11.18 (3) | 21.48 (1) | 24.57 (1) | 27.66(1) | 29.48 (1) | 20.46(1) | 22.26 (1) |
| Azithromycin (J01FA10) | 23.31 (1) | 21.22 (1) | 25.24 (1) | 24.57 (1) | 19.91 (2) | 23.18 (2) | 12.72 (3) | 13.24 (2) | 18.01(2) | 17.64 (2) |
| Cefuroxime and beta-lactamase inhibitor (J01DC52) | 11.18 (3) | 13.89 (2) | 11.78 (2) | 12.85 (2) | 7.4 (5) | 6.92 (4) | 4.58 (6) | 5.31 (5) | 9.82 (4) | 9.85 (4) |
| Ciprofloxacin (J01MA02) | 6.66 (5) | 6.62 (5) | 5.65 (7) | 5.62 (6) | 10.56 (3) | 7.83 (3) | 11.98 (4) | 10.3 (4) | 4.09 (7) | 4.42 (6) |
| Metronidazole (P01AB01) | 8.77 (4) | 7.06 (4) | 10.59 (3) | 8.45 (4) | 6.22 (6) | 4.88 (6) | 13.12 (2) | 10.9 (3) | 5.26 (5) | 4.72 (5) |
| Doxycycline (J01AA02) | 6.39 (6) | 6.05 (6) | 7.28 (5) | 7.78 (5) | 8.68 (4) | 5.92 (5) | 5.45 (5) | 4.41 (6) | 13.38(3) | 13.72 (3) |
| Cefuroxime (J01DC02) | 5.14 (8) | 5.29 (7) | 3.73 (7) | |||||||
| Flucloxacillin (J01CF05) | 5.28 (8) | 5.47 (7) | 4.13 (6) | 4.17 (7) | ||||||
| Amoxicillin (J01CA04) | 5.41 (7) | 5.97 (6) | ||||||||
| Cefradine (J01DB09) | 3.66 (7) | 4.09 (7) | ||||||||
| No of DU75 antimicrobials | N = 8 | N = 8 | N = 7 | N = 7 | N = 7 | N = 7 | N = 6 | N = 7 | N = 7 | N = 7 |
| Percentage of Defined Daily Dose per 1000 Inhabitants per Day (Rank in Parentheses) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Antibacterial Agent (ATC Code) | 2019 | 2020 | 2021 | 2022 | 2023 | |||||
| Jan–June | July–Dec | Jan–June | July–Dec | Jan–June | July–Dec | Jan–June | July–Dec | Jan–June | July–Dec | |
| Ceftriaxone (J01DD04) | 69.57 (1) | 71.9 (1) | 68.55 (1) | 66 (1) | 28.83 (2) | 40.72 (1) | 41.57 (1) | 63.57 (1) | 57.4 (1) | 59.4 (1) |
| Moxifloxacin (J01MA14) | 42.13 (1) | 25.88 (2) | 31.08 (2) | 12.19 (2) | ||||||
| Metronidazole (J01XD01) | 7.84 (2) | 6.88 (2) | 8.92 (2) | 8.16 (2) | 9.31 (3) | 10.44 (3) | 7.68 (3) | 5.47 (3) | 4.97 (3) | |
| Ciprofloxacin (J01MA02) | 5.02 (4) | |||||||||
| Amikacin (J01GB06) | 2.93 (3) | 3.67 (3) | ||||||||
| Amoxicillin (J01CA04) | 12.12 (2) | 11.73 (2) | ||||||||
| No of DU75 antibiotics | N = 2 | N = 3 | N = 3 | N = 3 | N = 4 | N = 3 | N = 3 | N = 2 | N = 3 | N = 3 |
| 96 | |
| 1.1 Single-dose antibiotics | Total number | 87 |
| Access | 29 | |
| Watch | 44 | |
| Reserve | 10 | |
| Not recommended | 4 | |
| 1.2 Fixed-dose combination antibiotics | Total number | 9 |
| Access | 3 | |
| Watch | 2 | |
| Reserve | 0 | |
| Not recommended | 4 | |
| Total number | 17 |
| 2.1 Single-dose antivirals | 13 | |
| 2.2 Fixed-dose antiviral combinations | 4 | |
| Single-dose | 10 |
| Total number | 9 |
| 4.1 Single-dose antiparasitics | 7 | |
| 4.2 Fixed-dose antiparasitics | 2 | |
| 132 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yesmin, S.M.S.; Chakma, P.; Habiba, U.; Rhod Larsen, A.; Tino Fusire, T.; Wangmo, S.; Sarkar, S.; Attauabi, M. Human Antimicrobial Use in Bangladesh: Five-Year Trend Analysis Including COVID-19 Pandemic Era. Antibiotics 2025, 14, 868. https://doi.org/10.3390/antibiotics14090868
Yesmin SMS, Chakma P, Habiba U, Rhod Larsen A, Tino Fusire T, Wangmo S, Sarkar S, Attauabi M. Human Antimicrobial Use in Bangladesh: Five-Year Trend Analysis Including COVID-19 Pandemic Era. Antibiotics. 2025; 14(9):868. https://doi.org/10.3390/antibiotics14090868
Chicago/Turabian StyleYesmin, S. M. Sabrina, Paritosh Chakma, Umme Habiba, Anders Rhod Larsen, Terence Tino Fusire, Sangay Wangmo, Shila Sarkar, and Majda Attauabi. 2025. "Human Antimicrobial Use in Bangladesh: Five-Year Trend Analysis Including COVID-19 Pandemic Era" Antibiotics 14, no. 9: 868. https://doi.org/10.3390/antibiotics14090868
APA StyleYesmin, S. M. S., Chakma, P., Habiba, U., Rhod Larsen, A., Tino Fusire, T., Wangmo, S., Sarkar, S., & Attauabi, M. (2025). Human Antimicrobial Use in Bangladesh: Five-Year Trend Analysis Including COVID-19 Pandemic Era. Antibiotics, 14(9), 868. https://doi.org/10.3390/antibiotics14090868







