Electron Beam Irradiation for Efficient Antibiotic Degradation in Aqueous Solutions
Abstract
1. Introduction
2. Results and Discussion
2.1. The Impact of Accelerated Electrons on Antibiotics in Aqueous Solutions
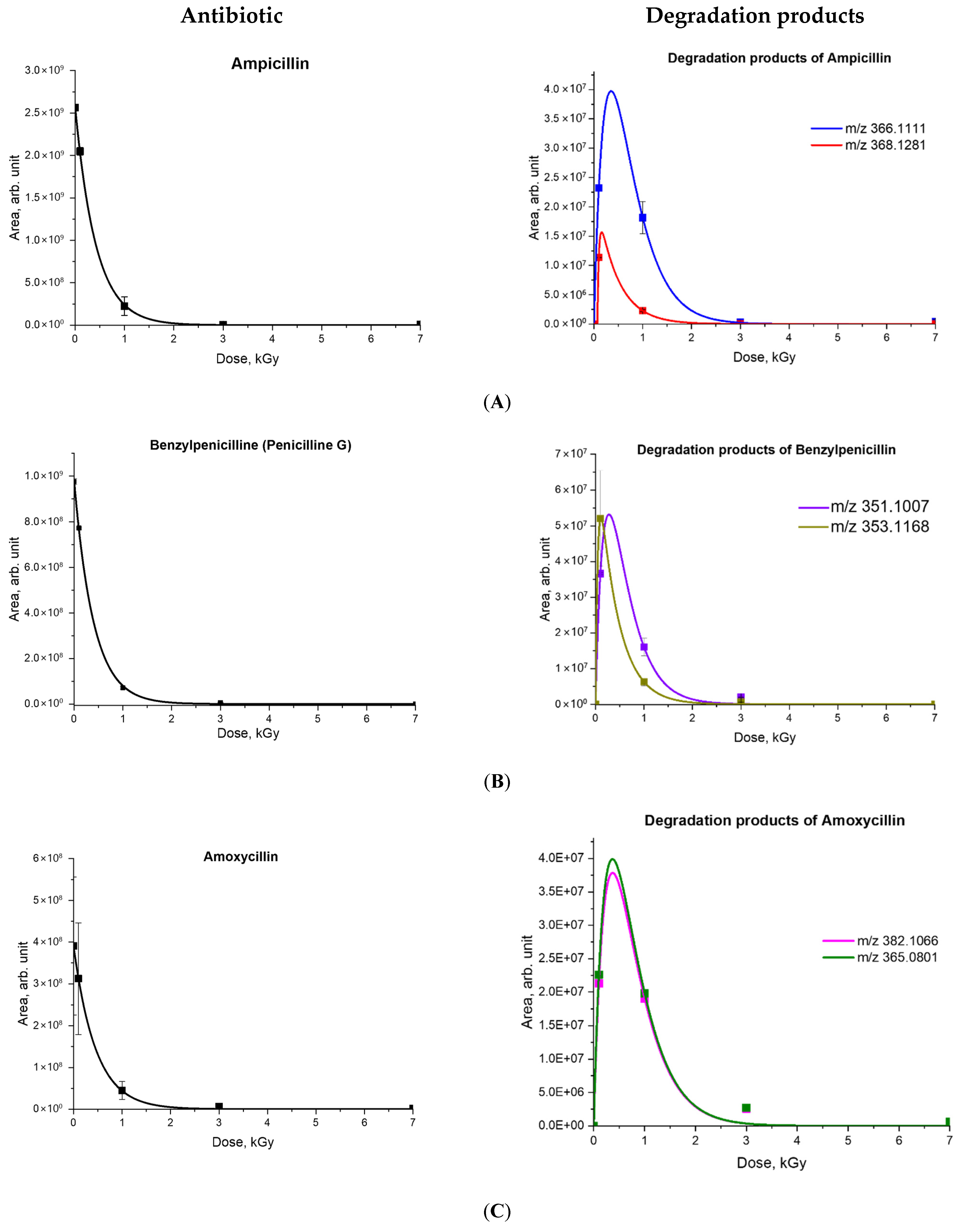
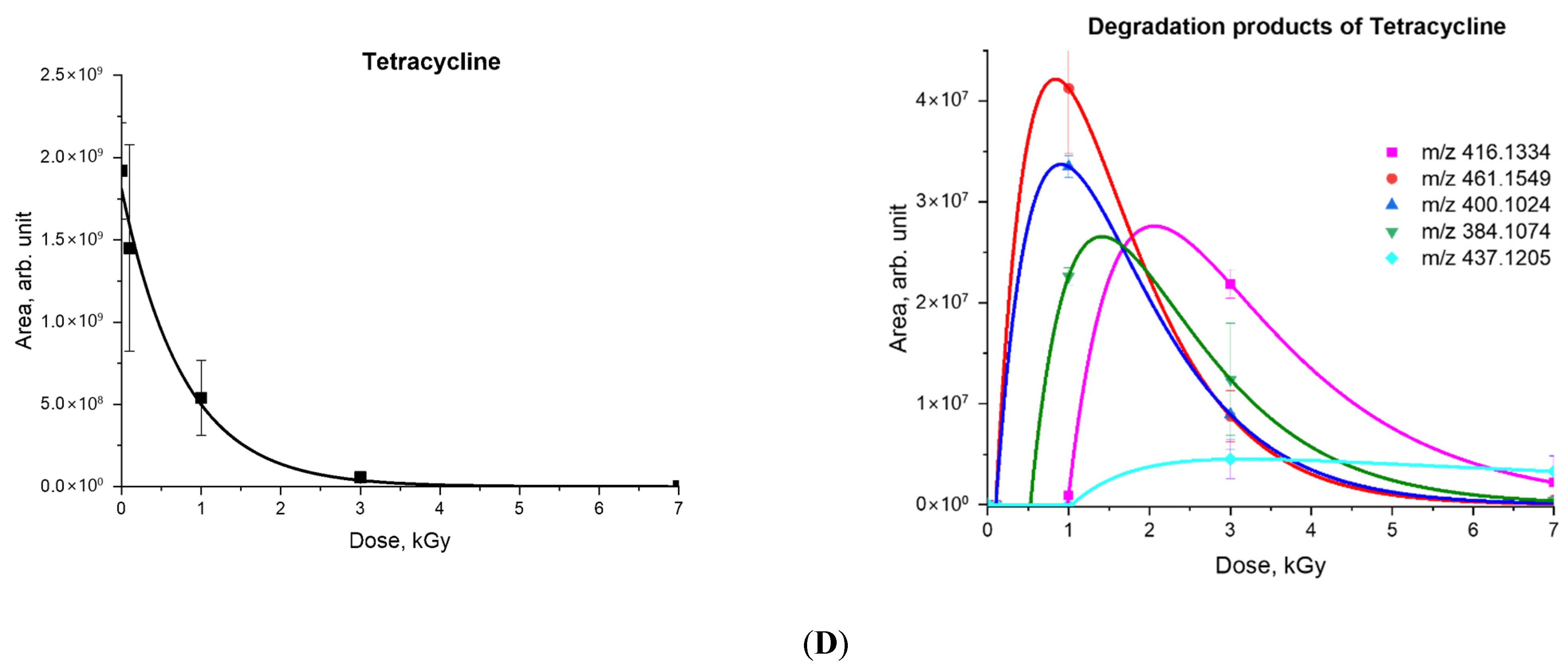
2.2. A Mathematical Model Describing the Dependency of the Concentrations of Antibiotics and Degradation Products on Irradiation Dose
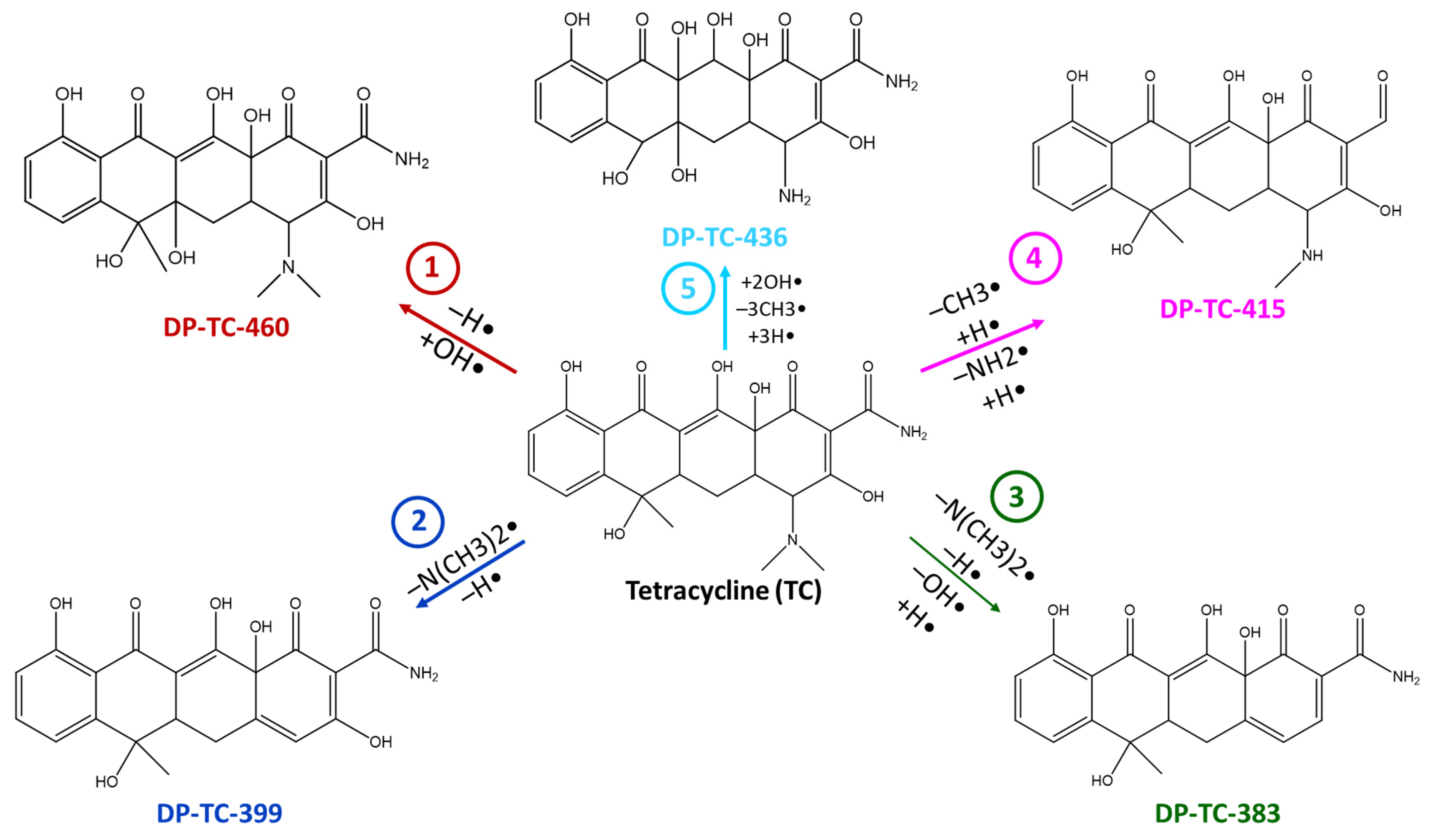
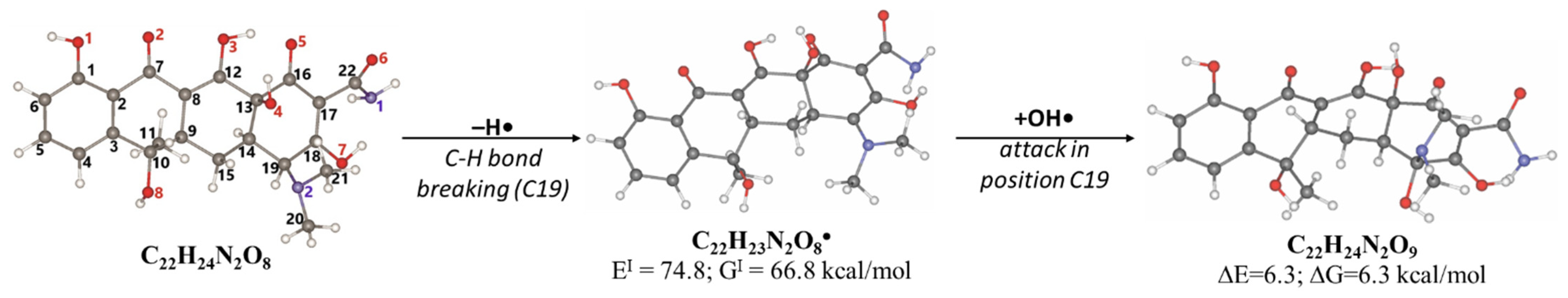
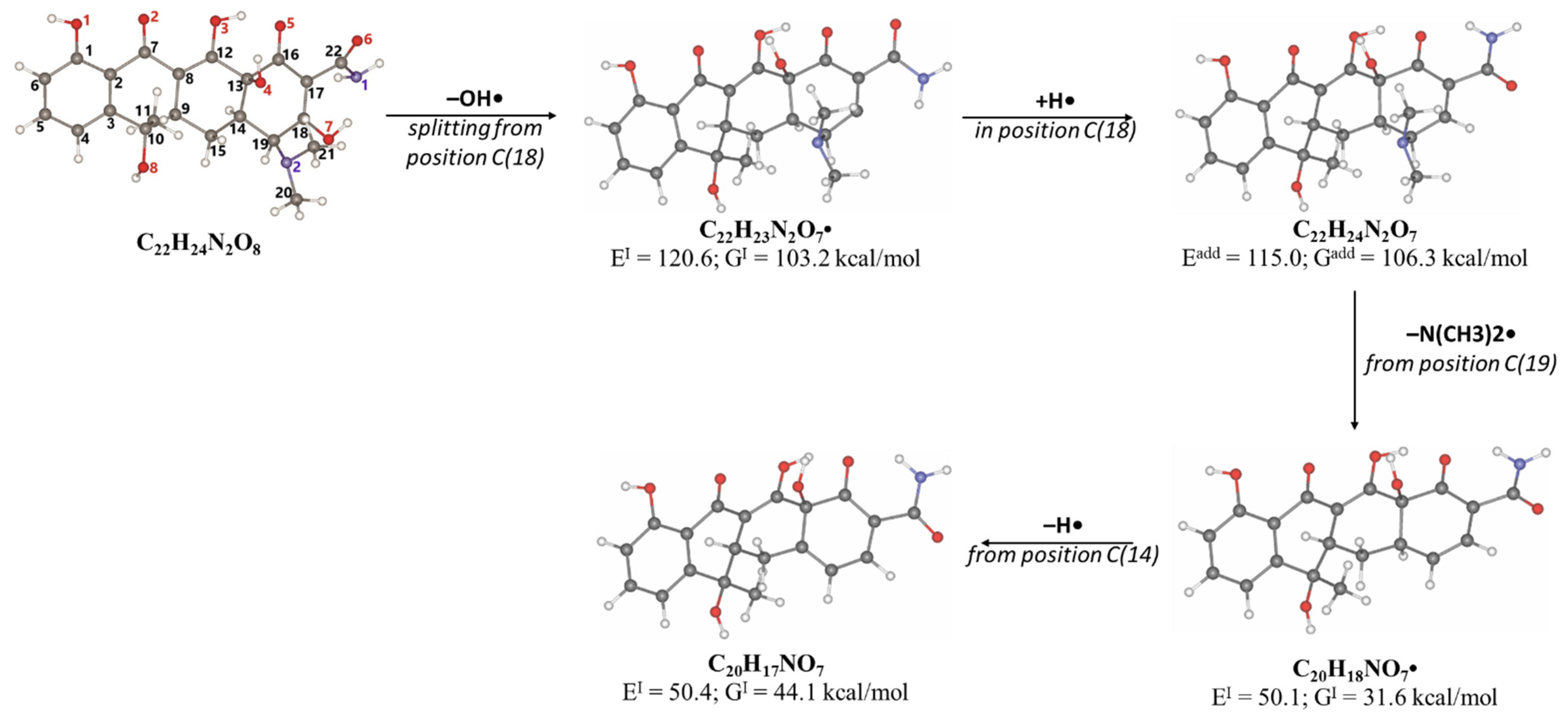
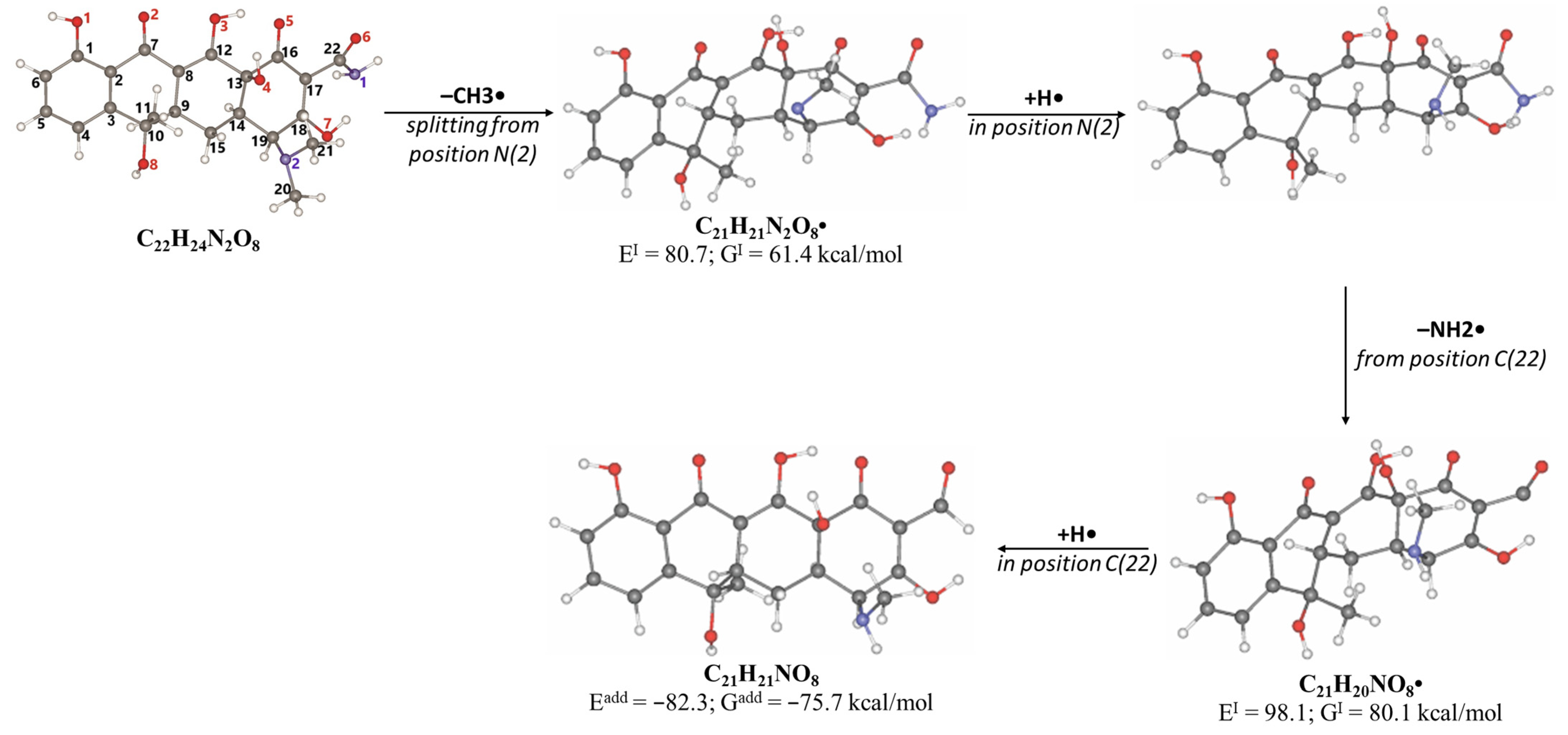
2.3. Antibiotic Degradation Pathways in Water Under E-Beam Irradiation Using Tetracycline as an Example
3. Materials and Methods
3.1. Research Stages
3.2. Reagents, Equipment, Reference Materials and Sample Preparation for Analysis
- Reagents
- Equipment
- Sample Preparation
3.3. E-Beam Irradiation of Aqueous Solutions
3.4. Dosimetry Control
3.5. HPLC-MS Analysis
3.6. Density Functional Theory (DFT) Calculation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HPLC-HRMS | High-performance liquid chromatography combined with high-resolution mass spectrometry |
| DFT | Density functional theory |
| TC | Tetracycline |
| DOX | Doxycycline |
| AMP | Ampicillin |
| AMO | Amoxicillin |
| PENG | Penicillin G (Benzylpenicillin) |
| STR | Streptomycin |
| CAP | Chloramphenicol |
| DPs | Degradation products |
Appendix A
| HPLC-MS Parameters | Value |
|---|---|
| HPLC | |
| Column | Thermo RS LC HPLC (150 mm × 2.1 mm, 2.2 µm particle size) |
| Mobile phases | Mobile phase A: 0.1% formic acid in water Mobile phase B: 100% acetonitrile |
| Flow rate | 0.3 mL/min |
| Column temperature | 35 °C |
| Injection volume | 20 µL |
| Gradient elution mode | 0–2 min—5% B; 2–15 min—95% B; 15–18 min—95% B; 18–19 min—5% B; 19–24 min—5% B. |
| MS | |
| Ionization method | Electrospray ionization (ESI) |
| Ion generation mode | Both polarities (+ and −) |
| Ion source temperature | 325 °C |
| Drying gas pressure | 344.7 kPa |
| Nebulizing gas pressure | 69 kPa |
| Capillary voltage | +3500; −2500 V |
| Mass scanning range | 100–1000 m/z |
References
- Barathe, P.; Kaur, K.; Reddy, S.; Shriram, V.; Kumar, V. Antibiotic pollution and associated antimicrobial resistance in the environment. J. Hazard. Mater. Lett. 2024, 5, 100105. [Google Scholar] [CrossRef]
- Brüssow, H. The antibiotic resistance crisis and the development of new antibiotics. Microb. Biotechnol. 2024, 17, e14510. [Google Scholar] [CrossRef]
- Browne, A.J.; Chipeta, M.G.; Haines-Woodhouse, G.; Kumaran, E.P.; Hamadani, B.H.K.; Zaraa, S.; Henry, N.J.; Deshpande, A.; Reiner, R.C.; Day, N.P.J.; et al. Global antibiotic consumption and usage in humans, 2000–18: A spatial modelling study. Lancet Planet. Health 2021, 5, e893–e904. [Google Scholar] [CrossRef]
- Klein, E.Y.; Van Boeckel, T.P.; Martinez, E.M.; Pant, S.; Gandra, S.; Levin, S.A.; Goossens, H.; Laxminarayan, R. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc. Natl. Acad. Sci. USA 2018, 115, E3463–E3470. [Google Scholar] [CrossRef]
- de Ilurdoz, M.S.; Sadhwani, J.J.; Reboso, J.V. Antibiotic removal processes from water & wastewater for the protection of the aquatic environment-a review. J. Water Process Eng. 2022, 45, 102474. [Google Scholar] [CrossRef]
- Bacanli, M.; Başaran, N. Importance of antibiotic residues in animal food. Food Chem. Toxicol. 2019, 125, 462–466. [Google Scholar] [CrossRef] [PubMed]
- Hosain, M.Z.; Kabir, S.L.; Kamal, M.M. Antimicrobial uses for livestock production in developing countries. Vet. World 2021, 14, 210. [Google Scholar] [CrossRef]
- Yaman, H.; Baig, M.T.; Kayan, A. Synthesis and characterization of tetrasubstituted porphyrin tin (IV) complexes and their adsorption properties over tetracycline antibiotics. Reactions 2025, 6, 12. [Google Scholar] [CrossRef]
- Farrukh, M.; Munawar, A.; Nawaz, Z.; Hussain, N.; Hafeez, A.B.; Szweda, P. Antibiotic resistance and preventive strategies in foodborne pathogenic bacteria: A comprehensive review. Food Sci. Biotechnol. 2025, 34, 2101–2129. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, R.; Mahajan, P.; Pandiya, S.; Bajaj, A.; Verma, S.K.; Yadav, P.; Kharat, A.S.; Khan, A.U.; Dua, M.; Johri, A.K. Antibiotic resistance: A global crisis, problems and solutions. Crit. Rev. Microbiol. 2024, 50, 896–921. [Google Scholar] [CrossRef]
- World Health Organization. Antimicrobial Resistance (2021). Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 21 March 2023).
- Zhu, L.; Lin, X.; Di, Z.; Cheng, F.; Xu, J. Occurrence, risks, and removal methods of antibiotics in urban wastewater treatment systems: A review. Water 2024, 16, 3428. [Google Scholar] [CrossRef]
- Yang, Q.; Gao, Y.; Ke, J.; Show, P.L.; Ge, Y.; Liu, Y.; Guo, R.; Chen, J. Antibiotics: An overview on the environmental occurrence, toxicity, degradation, and removal methods. Bioengineered 2021, 12, 7376–7416. [Google Scholar] [CrossRef]
- Nasrollahi, N.; Vatanpour, V.; Khataee, A. Removal of antibiotics from wastewaters by membrane technology: Limitations, successes, and future improvements. Sci. Total Environ. 2022, 838, 156010. [Google Scholar] [CrossRef]
- Mahdi, M.H.; Mohammed, T.J.; Al-Najar, J.A. Advanced Oxidation Processes (AOPs) for treatment of antibiotics in wastewater: A review. IOP Conf. Ser. Earth Environ. Sci. 2021, 779, 012109. [Google Scholar] [CrossRef]
- Pandis, P.K.; Kalogirou, C.; Kanellou, E.; Vaitsis, C.; Savvidou, M.G.; Sourkouni, G.; Zorpas, A.A.; Argirusis, C. Key points of advanced oxidation processes (AOPs) for wastewater, organic pollutants and pharmaceutical waste treatment: A mini review. ChemEngineering. 2022, 6, 8. [Google Scholar] [CrossRef]
- Li, S.; Wu, Y.; Zheng, H.; Li, H.; Zheng, Y.; Nan, J.; Ma, J.; Nagarajan, D.; Chang, J.S. Antibiotics degradation by advanced oxidation process (AOPs): Recent advances in ecotoxicity and antibiotic-resistance genes induction of degradation products. Chemosphere 2023, 311, 136977. [Google Scholar] [CrossRef]
- Ribeiro, A.R.; Nunes, O.C.; Pereira, M.F.; Silva, A.M. An overview on the advanced oxidation processes applied for the treatment of water pollutants defined in the recently launched Directive 2013/39/EU. Environ. Int. 2015, 75, 33–51. [Google Scholar] [CrossRef]
- Wojnárovits, L.; Takács, E. Wastewater treatment with ionizing radiation. J. Radioanal. Nucl. Chem. 2017, 311, 973–981. [Google Scholar] [CrossRef]
- Trojanowicz, M.; Bojanowska-Czajka, A.; Capodaglio, A.G. Can radiation chemistry supply a highly efficient AO (R) P process for organics removal from drinking and waste water? A review. Environ. Sci. Pollut. Res. 2017, 24, 20187–20208. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, L.; Zhang, G.; Pang, T.; Zhang, X.; Cai, D.; Wu, Z. In situ degradation of antibiotic residues in medical intravenous infusion bottles using high energy electron beam irradiation. Sci. Rep. 2017, 7, 39928. [Google Scholar] [CrossRef]
- Zhu, F.; Pan, J.; Zou, Q.; Wu, M.; Wang, H.; Xu, G. Electron beam irradiation of typical sulfonamide antibiotics in the aquatic environment: Kinetics, removal mechanisms, degradation products and toxicity assessment. Chemosphere 2021, 274, 129713. [Google Scholar] [CrossRef]
- Wang, J.; Zhuan, R.; Chu, L. The occurrence, distribution and degradation of antibiotics by ionizing radiation: An overview. Sci. Total Environ. 2019, 646, 1385–1397. [Google Scholar] [CrossRef]
- Tominaga, F.K.; Boiani, N.F.; Silva, T.T.; dos Santos, J.G.; Lebre, D.T.; Leo, P.; Borrely, S.I. Electron beam irradiation applied for the detoxification and degradation of single ciprofloxacin aqueous solution and multiclass pharmaceutical quaternary mixture. Sep. Purif. Technol. 2023, 307, 122818. [Google Scholar] [CrossRef]
- Cho, J.Y. Evaluation of degradation of antibiotic tetracycline in pig manure by electron beam irradiation. Bull. Environ. Contam. Toxicol. 2010, 84, 450–453. [Google Scholar] [CrossRef]
- Pugajeva, I.; Rusko, J.; Perkons, I.; Lundanes, E.; Bartkevics, V. Determination of pharmaceutical residues in wastewater using high performance liquid chromatography coupled to quadrupole-Orbitrap mass spectrometry. J. Pharm. Biomed. Anal. 2017, 133, 64–74. [Google Scholar] [CrossRef]
- Gavage, M.; Delahaut, P.; Gillard, N. Suitability of high-resolution mass spectrometry for routine analysis of small molecules in food, feed and water for safety and authenticity purposes: A review. Foods 2021, 10, 601. [Google Scholar] [CrossRef]
- Balsalobre, L.; Blanco, A.; Alarcón, T. Beta-lactams. In Antibiotic Drug Resistance; Wiley: Hoboken, NJ, USA, 2019; pp. 57–72. [Google Scholar] [CrossRef]
- Han, C.H.; Park, H.D.; Kim, S.B.; Yargeau, V.; Choi, J.W.; Lee, S.H.; Park, J.A. Oxidation of tetracycline and oxytetracycline for the photo-Fenton process: Their transformation products and toxicity assessment. Water Res. 2020, 172, 115514. [Google Scholar] [CrossRef]
- Xu, Z.; Song, X.; Li, Y.; Li, G.; Luo, W. Removal of antibiotics by sequencing-batch membrane bioreactor for swine wastewater treatment. Sci. Total Environ. 2019, 684, 23–30. [Google Scholar] [CrossRef]
- Gorito, A.M.; Ribeiro, A.R.L.; Rodrigues, P.; Pereira, M.F.R.; Guimarães, L.; Almeida, C.M.R.; Silva, A.M. Antibiotics removal from aquaculture effluents by ozonation: Chemical and toxicity descriptors. Water Res. 2022, 218, 118497. [Google Scholar] [CrossRef] [PubMed]
- Datta, J.; Mala, A. Removal of antibiotics from the aquatic environment using adsorption technologies: An overview. Water Sci. Technol. 2020, 82, 401–426. [Google Scholar] [CrossRef]
- Vinayagam, R.; Varadavenkatesan, T.; Selvaraja, R. Tetracycline Adsorption Research (2015–2025): A bibliometric analysis of trends, problems, and directions for the future. Results Eng. 2025, 27, 106383. [Google Scholar] [CrossRef]
- Ma, J.; Chen, Y.; Zhou, G.; Ge, H.; Liu, H. Recent advances in photocatalytic degradation of tetracycline antibiotics. Catalysts 2024, 14, 762. [Google Scholar] [CrossRef]
- Krakkó, D.; Heieren, B.T.; Illés, Á.; Kvamme, K.; Dóbé, S.; Záray, G. (V) UV degradation of the antibiotic tetracycline: Kinetics, transformation products and pathway. Process Saf. Environ. Prot. 2022, 163, 395–404. [Google Scholar] [CrossRef]
- Kozlova, E.; Bliznyuk, U.; Chernyaev, A.; Borshchegovskaya, P.; Braun, A.; Ipatova, V.; Zolotov, S.; Nikitchenko, A.; Chulikova, N.; Malyuga, A.; et al. Optimization Function for Determining Optimal Dose Range for Beef and Seed Potato Irradiation. Foods 2024, 13, 3729. [Google Scholar] [CrossRef]
- Spectrophotometer UV-3000. Available online: https://istina.msu.ru/equipment/card/615320740/ (accessed on 29 September 2024).
- Mulmule, M.D.; Shimmy, S.M.; Bambole, V.; Jamdar, S.N.; Rawat, K.P.; Sarma, K.S.S. Combination of electron beam irradiation and thermal treatment to enhance the shelf-life of traditional Indian fermented food (Idli). Radiat. Phys. Chem. 2017, 131, 95–99. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Laikov, D.N. Optimization of atomic density-fitting basis functions for molecular two-electron integral approximations. J. Chem. Phys. 2020, 153, 114121. [Google Scholar] [CrossRef] [PubMed]
- Laikov, D.N.; Ustynyuk, Y.A. PRIRODA-04: A quantum-chemical program suite. New possibilities in the study of molecular systems with the application of parallel computing. Russ. Chem. Bull. 2005, 54, 820–826. [Google Scholar] [CrossRef]
- Pal, S.; Ahamed, Z.; Pal, P. Removal of antibiotics and pharmaceutically active compounds from water Environment: Experiments towards industrial scale up. Sep. Purif. Technol. 2022, 295, 121249. [Google Scholar] [CrossRef]
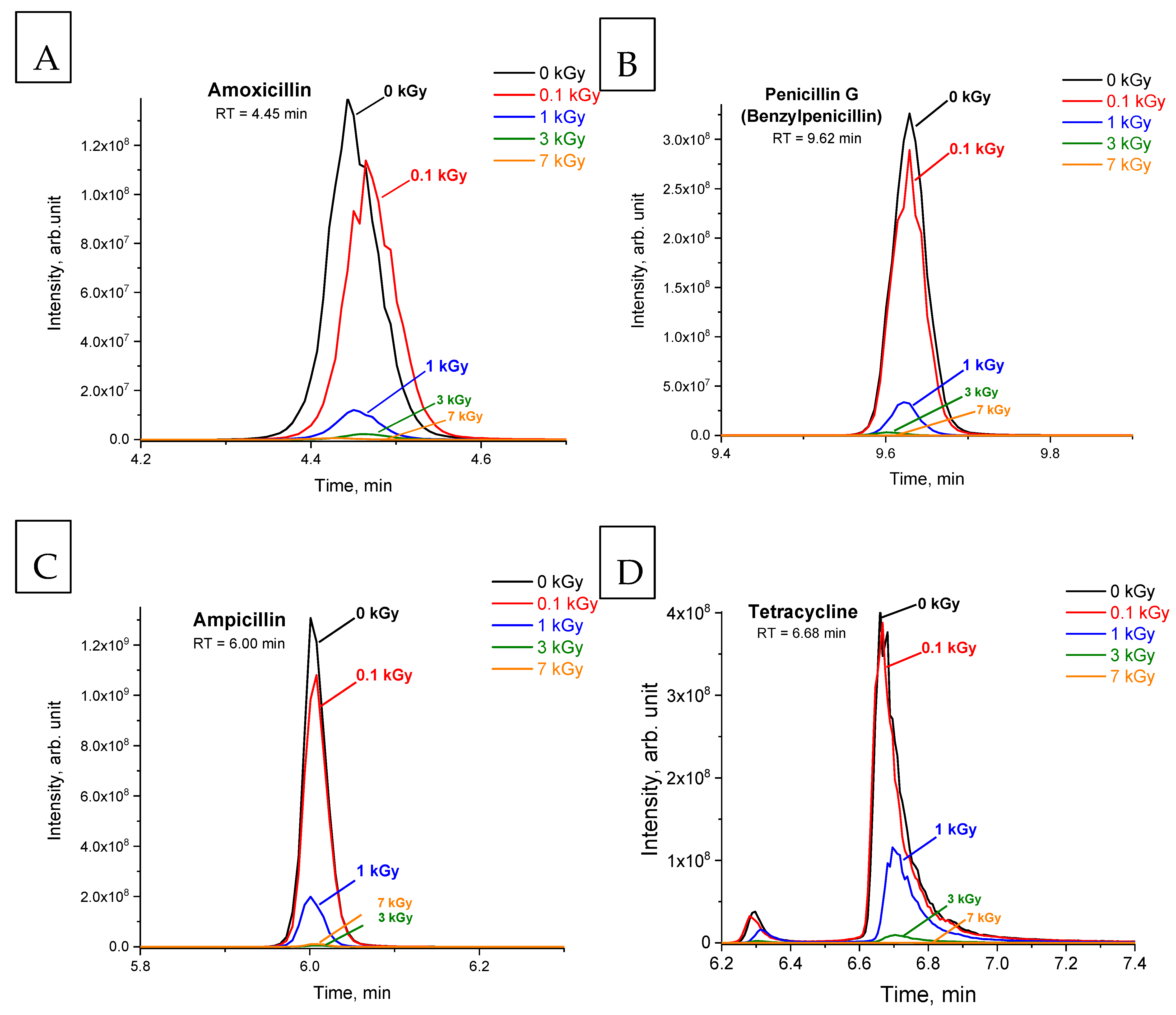

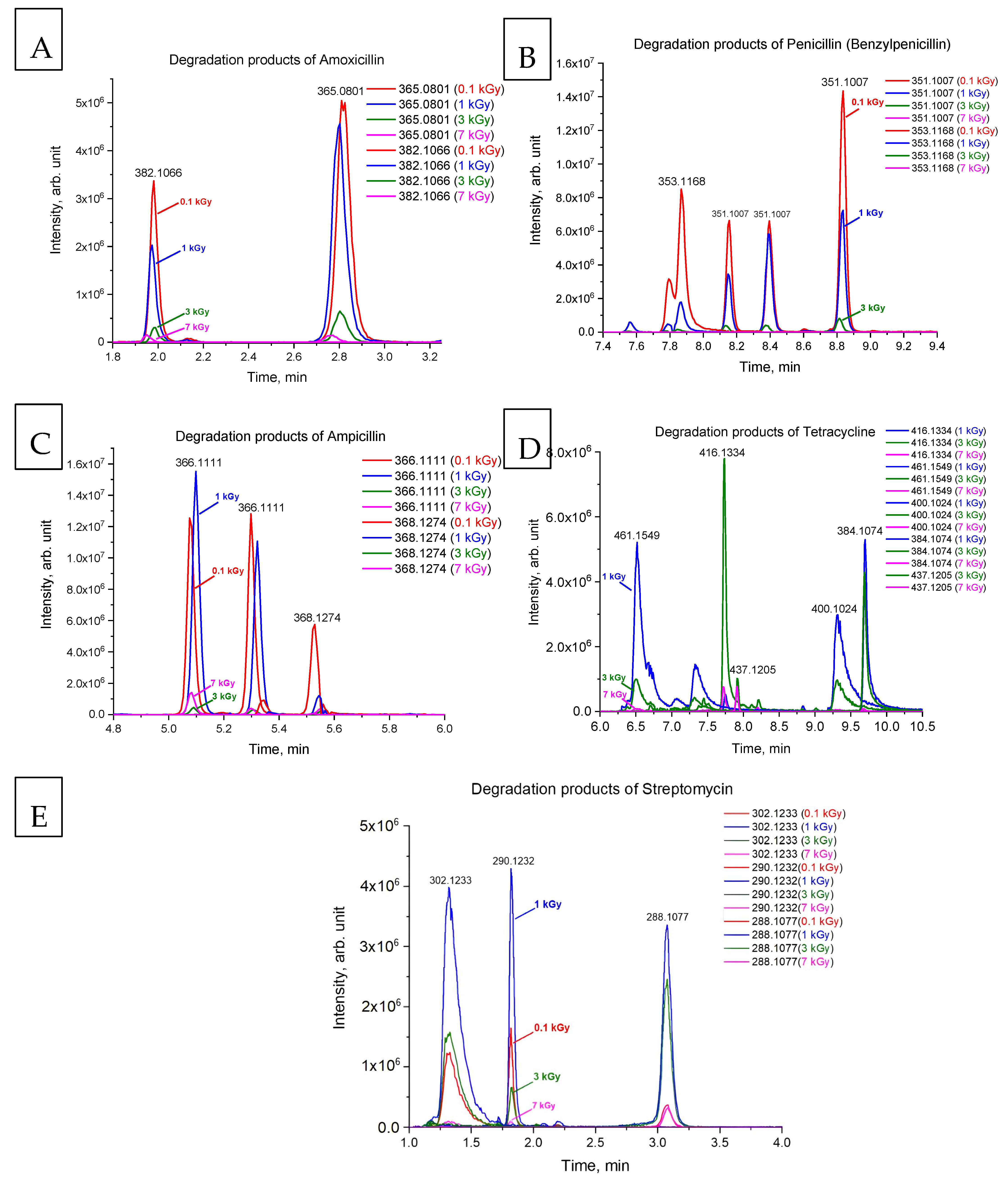



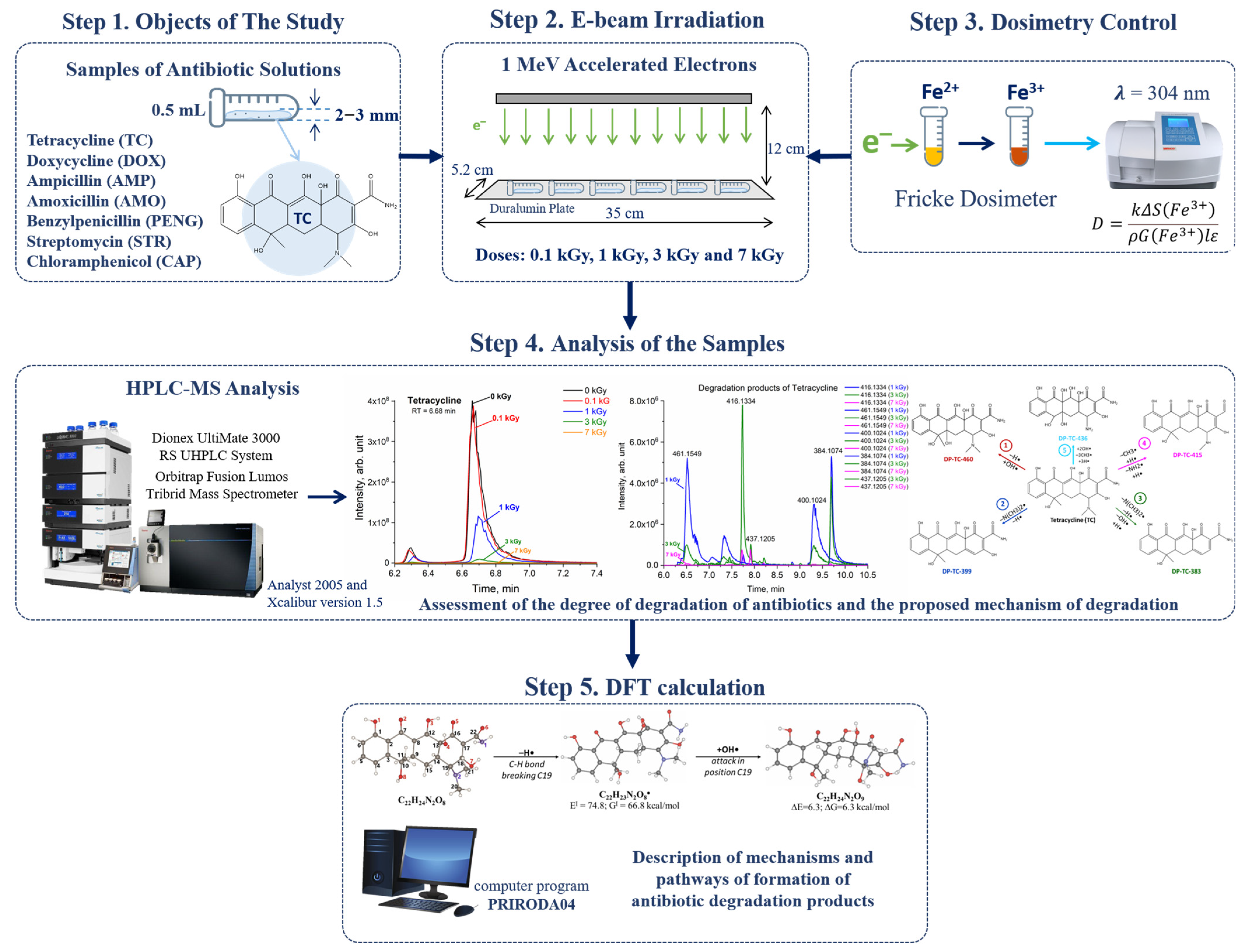
| Antibiotic | m/z | Polarity | Retention Time (RT), min | Peak Area (0 kGy), arb. unit. |
|---|---|---|---|---|
| Amoxicillin (Figure 1A) | 366.1110 | [M+H]+ | 4.45 | 3.7 × 108 |
| Penicillin G (Figure 1B) | 335.1056 | [M+H]+ | 9.62 | 9.7 × 108 |
| Ampicillin (Figure 1C) | 350.1163 | [M+H]+ | 6.00 | 2.5 × 109 |
| Tetracycline (Figure 1D) | 445.1592 | [M+H]+ | 6.68 | 1.9 × 109 |
| Doxycycline (Figure 1E) | 445.1594 | [M+H]+ | 7.70 | 4.5 × 109 |
| Streptomycin (Figure 1F) | 582.2725 | [M+H]+ | 0.86 | 7.4 × 107 |
| Chloramphenicol (Figure 1G) | 321.0050 | [M+H]− | 8.73 | 2.4 × 109 |
| Antibiotic | Removal, % | ||||
|---|---|---|---|---|---|
| 0 kGy | 0.1 kGy | 1 kGy | 3 kGy | 7 kGy | |
| Amoxicillin (Figure 1A) | 0 | 21.5 ± 3.1 | 88.4 ± 8.6 | 97.8 ± 0.8 | 98.9 ± 0.4 |
| Penicillin G (Figure 1B) | 0 | 20.8 ± 4.7 | 92.6 ± 3.3 | 99.3 ± 0.6 | 100 1 |
| Ampicillin (Figure 1C) | 0 | 20.1 ± 9.9 | 91.2 ± 18.4 | 99.4 ± 1.9 | 99.6 ± 1.0 |
| Tetracycline (Figure 1D) | 0 | 6.2 ± 2.4 | 65.3 ± 5.5 | 97.9 ± 3.3 | 99.9 ± 0.1 |
| Doxycycline (Figure 1E) | 0 | 8.6 ± 1.8 | 65.2 ± 14.8 | 99.4 ± 0.8 | 99.9 ± 0.1 |
| Streptomycin (Figure 1F) | 0 | 10.1 ± 2.5 | 61.6 ± 14.4 | 99.8 ± 0.3 | 100 1 |
| Chloramphenicol (Figure 1G) | 0 | 2.4 ± 0.5 | 35.1 ± 8.1 | 82.8 ± 3.8 | 98.8 ± 3.4 |
| Antibiotic | C0 | α | Rcorr | Degradation Products | β | D0 | k |
|---|---|---|---|---|---|---|---|
| Tetracycline | 100 | 1.28 ± 0.16 | 0.99 | 461.1549 | 0.65 ± 0.08 | 0.98 ± 0.12 | 0.14 ± 0.02 |
| 400.1024 | 1.44 ± 0.18 | 0.09 ± 0.01 | 0.10 ± 0.01 | ||||
| 384.1074 | 1.20 ± 0.15 | 0.09 ± 0.01 | 0.07 ± 0.01 | ||||
| 416.1334 | 0.98 ± 0.12 | 0.53 ± 0.07 | 0.09 ± 0.01 | ||||
| 437.1205 | 0.10 ± 0.01 | 1.04 ± 0.13 | 0.015 ± 0.002 | ||||
| Ampicillin | 100 | 2.38 ± 0.24 | 0.98 | 366.1111 | 3.38 ± 0.34 | 2.33 ± 0.24 | 0.12 ± 0.01 |
| 368.1274 | 32.12 ± 3.25 | 0.07 ± 0.01 | 0.28 ± 0.03 | ||||
| Amoxicillin | 100 | 2.18 ± 0.84 | 0.99 | 365.0801 | 3.29 ± 1.27 | 6.01 ± 2.32 | 0.72 ± 0.26 |
| 382.1066 | 3.34 ± 1.30 | 3.08 ± 1.19 | 0.76 ± 0.29 | ||||
| Benzylpenicillin | 100 | 2.51 ± 0.12 | 0.99 | 351.1007 | 4.97 ± 0.23 | 3.86 ± 0.18 | 0.54 ± 0.02 |
| 353.1168 | 22.77 ± 1.05 | 0.0022 ± 0.0001 | 1.60 ± 0.07 | ||||
| Streptomycin | 100 | 1.02 ± 0.50 | 0.99 | 302.1233 | 1.12 ± 0.56 | 2.39 ± 1.19 | 0.96 ± 0.48 |
| 290.1232 | 3.28 ± 1.65 | 0.05 ± 0.03 | 0.74 + 0.37 | ||||
| 288.1077 | 0.68 ± 0.34 | 0.05 ± 0.03 | 0.38 ± 0.19 | ||||
| Doxycycline | 100 | 0.56 ± 0.04 | 0.89 | Not detected | |||
| Chloramphenicol | 100 | 1.49 ± 0.08 | 0.98 | Not detected | |||
| Antibiotic | Name and Molecular Formula [M+H]+ | Retention Time, min | Dose, kGy | Accurate Mass [M+H]+ | Mass Accuracy, ppm | Structure |
|---|---|---|---|---|---|---|
| Tetracycline | DP-TC-460 C22H25N2O9 | 6.51 и 7.31 | 1 | 461.1549 | −1.31 | 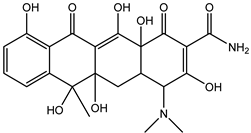 |
| DP-TC-399 C20H18NO8 | 9.33 | 1 | 400.1024 | −0.77 | 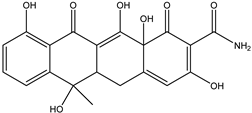 | |
| DP-TC-383 C20H18NO7 | 9.68 | 1 | 384.1074 | −0.37 | 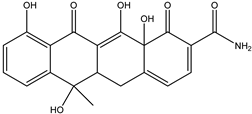 | |
| DP-TC-415 C21H22NO8 | 7.74 | 1 | 416.1334 | −1.44 |  | |
| DP-TC-436 C19H21N2O10 | 7.92 | 3 | 437.1205 | 0.31 | 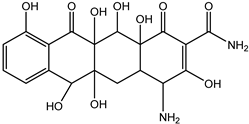 | |
| Amoxicillin | DP-AMO-364 C16H17N2SO6 | 2.81 | 0.1 | 365.0801 | 0.07 | 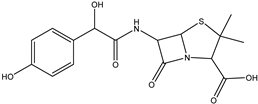 |
| DP-AMO-381 C16H20N3SO6 | 1.97 | 0.1 | 382.1066 | −0.33 | 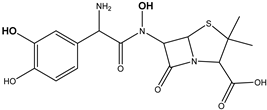 | |
| Ampicillin | DP-AMP-365 C16H20N3SO5 | 5.12 | 0.1 | 366.1111 | −0.37 | 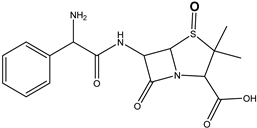 |
| DP-AMP-367 C16H22N3SO5 | 5.60 | 0.1 | 368.1274 | 0.82 | 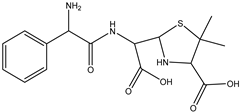 | |
| Benzylpenicillin | DP-PENG-350 C16H19N2SO5 | 8.16; 8.40; 8.83 | 0.1 | 351.1007 | −0.34 | 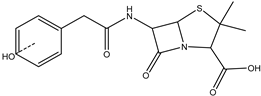 |
| DP-PENG-352 C16H21N2SO5 | 7.83 | 0.1 | 353.1168 | 0.54 | 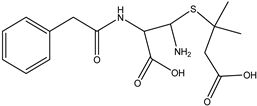 | |
| DP-PENG-308 C15H21N2SO3 | 7.83 | 0.1 | 309.1276 | −0.2 | 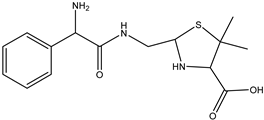 | |
| Streptomycin | DP-STR-301 C13H20O7N | 1.31 | 0.1 | 302.1233 | −0.56 | 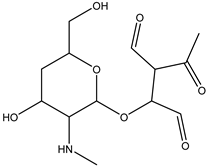 |
| DP-STR-289 C12H20O7N | 1.82 | 0.1 | 290.1232 | −0.79 | 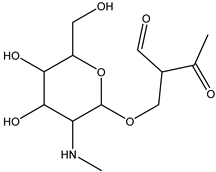 | |
| DP-STR-287 C12H18O7N | 3.07 | 0.1 | 288.1077 | −0.18 |  |
| C4• | C5• | C6• | C9• | C15• | C14• | C19• | |
|---|---|---|---|---|---|---|---|
| EI, kcal/mol | 113.5 | 115.2 | 115.5 | 77.5 | 99.7 | 94.7 | 74.8 |
| GI, kcal/mol | 105.4 | 105.8 | 106.6 | 69.4 | 90.9 | 85.2 | 66.8 |
| Session | Irradiation Time (avg.), s | Charge (avg.), nC | Dose (avg.), Gy |
|---|---|---|---|
| 1–3 | 32.3 | 5375 | 103.4 |
| 4–6 | 122.3 | 52,356.7 | 1007 |
| 7–9 | 73.7 | 157,733.3 | 3033.3 |
| 10–12 | 150 | 365,666.7 | 7032.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oprunenko, A.; Bliznyuk, U.; Ipatova, V.; Nikitchenko, A.; Gloriozov, I.; Braun, A.; Bolotnik, T.; Borshchegovskaya, P.; Kozlova, E.; Ananieva, I.; et al. Electron Beam Irradiation for Efficient Antibiotic Degradation in Aqueous Solutions. Antibiotics 2025, 14, 833. https://doi.org/10.3390/antibiotics14080833
Oprunenko A, Bliznyuk U, Ipatova V, Nikitchenko A, Gloriozov I, Braun A, Bolotnik T, Borshchegovskaya P, Kozlova E, Ananieva I, et al. Electron Beam Irradiation for Efficient Antibiotic Degradation in Aqueous Solutions. Antibiotics. 2025; 14(8):833. https://doi.org/10.3390/antibiotics14080833
Chicago/Turabian StyleOprunenko, Anastasia, Ulyana Bliznyuk, Victoria Ipatova, Alexander Nikitchenko, Igor Gloriozov, Arcady Braun, Timofey Bolotnik, Polina Borshchegovskaya, Elena Kozlova, Irina Ananieva, and et al. 2025. "Electron Beam Irradiation for Efficient Antibiotic Degradation in Aqueous Solutions" Antibiotics 14, no. 8: 833. https://doi.org/10.3390/antibiotics14080833
APA StyleOprunenko, A., Bliznyuk, U., Ipatova, V., Nikitchenko, A., Gloriozov, I., Braun, A., Bolotnik, T., Borshchegovskaya, P., Kozlova, E., Ananieva, I., & Rodin, I. (2025). Electron Beam Irradiation for Efficient Antibiotic Degradation in Aqueous Solutions. Antibiotics, 14(8), 833. https://doi.org/10.3390/antibiotics14080833








