Evaluation of a Hub-and-Spoke Model to Enhance Healthcare Professionals’ Practice of Antimicrobial Stewardship (AMS) Programmes in the Volta Region of Ghana
Abstract
1. Introduction
2. Results
2.1. Summary of Participant Characteristics
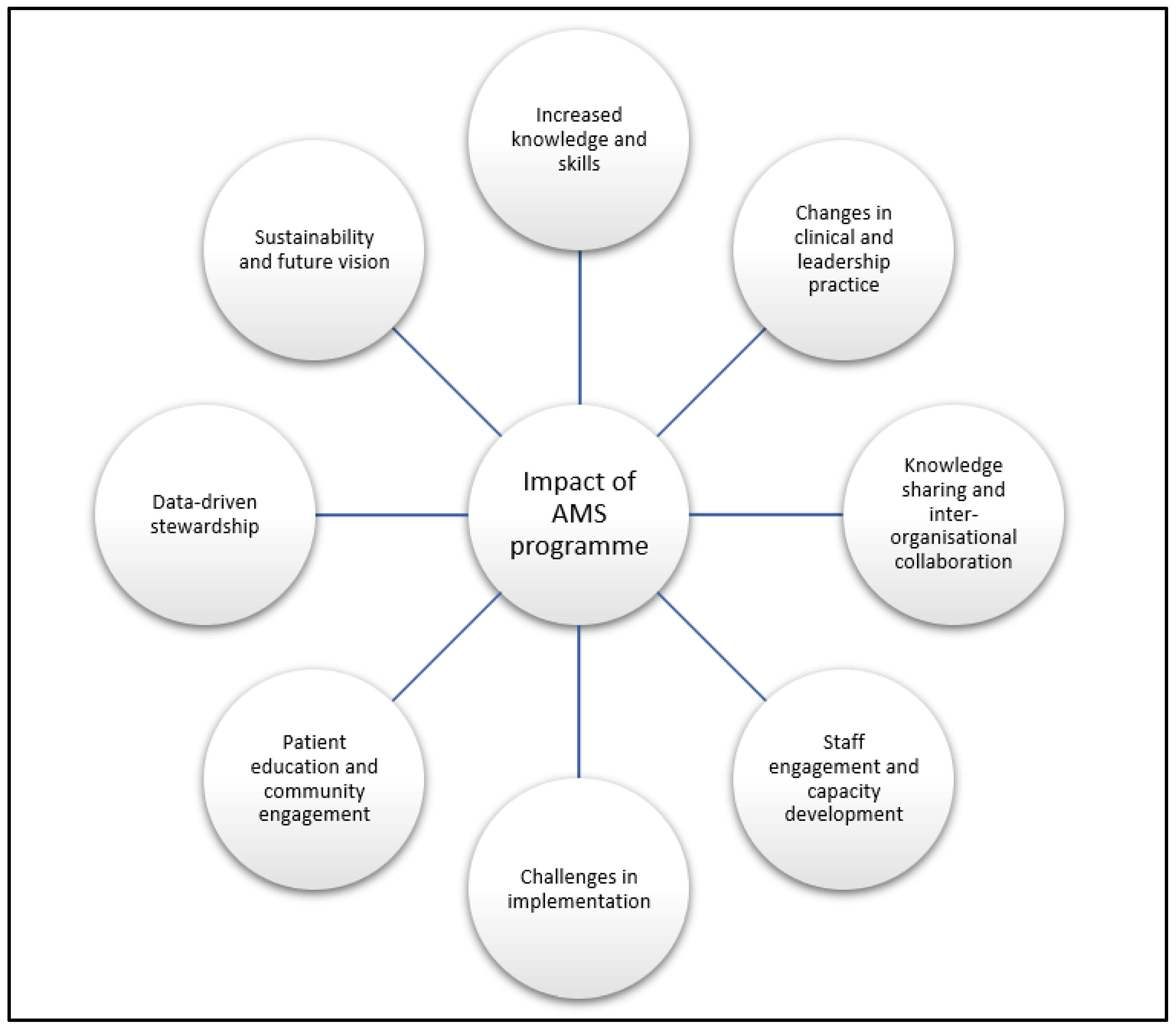
2.2. Emerging Themes from Qualitative Interviews
2.2.1. Increased Knowledge and Skills
“Yes, I knew antimicrobials have resistance; I never knew you had quite a huge number of resistance to antimicrobials prior to this engagement.”(Nurse, Hub site)
“I was a little bit oblivious to the danger we are facing currently with regards to antimicrobial resistance... I actually knew that yes... some bacteria are resistant to some antibiotics, but I always had the impression that we could just switch to a different one.”(Medical Officer, Spoke Site)
“I’m hoping that especially with this current training, that there would be a reduction in hospital acquired infections.”(Administrator, Spoke site)
2.2.2. Changes in Clinical and Leadership Practice
“I make sure the patient really needs an antimicrobial before I prescribe. Meanwhile, at first, I mean, I was a little bit liberal sometimes.”(Medical Officer, Spoke site)
“The need for targeted therapy has increased and the request for culture sensitivity prior to starting some antibiotics or to inform the change of antimicrobial has changed.”(Nurse, Hub site)
“And most of us on the committee are leaders in a way, how are you able to manage your subordinates to be able to send out information for them to grasp, and also how to know the type of leader that you are and what kind of leadership can be used in what circumstance. So yes, it has been very, very, very enlightening.”(Physician Assistant, Hub site)
2.2.3. Knowledge Sharing and Inter-Organisational Collaboration
“We formed an antimicrobial stewardship team within my facility, so we hold meetings, we meet with hospital managers, we meet with our colleagues on all the various topics to present what we learn. We do all the workshops for them to be able to influence their practice and then decision-making and other things, so we are supposed to be something like agents of antimicrobial stewardship.”(Medical Officer, Spoke site)
“We take ideas from each other and after the workshops also we try to link up with other facilities to see what we are doing right and what we aren’t and what they are also doing and what we are also doing …We call ourselves the sister facilities, so we get knowledge from each other.”(Nurse, Spoke site)
2.2.4. Staff Engagement and Capacity Development
“I’ve benefited... a lot from it in terms of project management, publication writing, manuscript writing, public engagement, moderating programmes because I’ve been MC for a number of times and I’m a lead for the medicine disposal, the take back programme.”(Pharmacist, Academic Institute)
“Being involved in the leadership planning has also boosted my abilities and capabilities of leadership.”(Nurse, Hub site)
2.2.5. Challenges in Implementation
“...Most of the time you present certain things [to management] that need to be done, and then the feedback you get is there’s no money; you’ve not budgeted for it.”(Medical Officer, Spoke site)
“Currently my facility doesn’t have a culture and sensitivity test machine, so we do it outside the facility, which is a challenge.”(Nurse, Spoke site)
“In my facility, for instance, we don’t have many doctors. So yes, so when it’s time for a workshop, I often really struggle to get someone to cover for me while I’m away for the three hours of the workshop. But I still try to attend and learn a lot and then come back to impact.”(Medical Officer, Spoke site)
“It’s a challenge; the culture of Ghanaians, most of us, when we are sick, you go to the drugstore first... And you take the medications given to you by the drugstore attendant and you are fine. There’s no need for the hospital. So, most of the time, the community usually dispense antimicrobials anyhow in terms of the pharmacies and because they’re making money, there’s no motivation to stop. So that’s one challenge that I foresee that I’m going to face.”(Medical Officer, Spoke site)
2.2.6. Patient Education and Community Engagement
“We do out-of-hospital public engagements as well. So radio station, TV stations, yes.”(Nurse, Hub site)
2.2.7. Data-Driven Stewardship
“...From the very first GPPS we did, and then the subsequent ones, we’ve realised some change, but we actually reported that in our paper. And we showed the prescribing patterns in a teaching hospital in Ghana. So we did it in, I think, January and then we did in July. So we’re able to compare and realise that there was a positive change in the prescribing patterns.”(Pharmacist, Academic Institute)
“The data from the from the GPPS. Yes, is being presented to management and... the prescribers. And after the GPPS summary, most of the prescribers are also on board. I mean the data is gathered with some of the prescribers. So that also informs on their prescription of antimicrobial, especially with regard to doing targeted treatment I mean, yes.”(Physician Assistant, Hub site)
2.2.8. Sustainability and Future Vision
“…we are putting plans in place... to be able to make sure that it doesn’t end this year or next year like it becomes embedded in... the hospitals, activities. So, we are looking at having an AMS team on the wards or like various representatives on the units so that they are like champions... when new staff come to the facility, there’s training for them. And the push so the prescribers are able to prescribe based on the guidelines.”(Administrator, Spoke site)
“…my expectation is by the end of this project, we’re able to have a solid team; we get more departments and people on board to support, especially if we get the support from management because some of these things will need funding. We’ll get the support from management.”(Nurse, Spoke site)
3. Discussion
3.1. Summary of Main Findings
3.2. Comparison with Literature
3.3. Limitations
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Saleem, Z.; Moore, E.C.; Kalungia, A.C.; Schellack, N.; Ogunleye, O.; Chigome, A.; Chowdhury, K.; Kitutu, F.E.; Massele, A.; Ramdas, N.; et al. Status and implications of the knowledge, attitudes and practices towards AWaRe antibiotic use, resistance and stewardship among low- and middle-income countries. JAC-Antimicrob. Resist. 2025, 7, dlaf033. [Google Scholar] [CrossRef] [PubMed]
- Dodoo, C.C.; Orman, E.; Alalbila, T.; Mensah, A.; Jato, J.; Mfoafo, K.A.; Folitse, I.; Hutton-Nyameaye, A.; Ben, I.O.; Mensah-Kane, P.; et al. Antimicrobial Prescription Pattern in Ho Teaching Hospital, Ghana: Seasonal Determination Using a Point Prevalence Survey. Antibiotics 2021, 10, 199. [Google Scholar] [CrossRef] [PubMed]
- D’arcy, N.; Ashiru-Oredope, D.; Olaoye, O.; Afriyie, D.; Akello, Z.; Ankrah, D.; Asima, D.M.; Banda, D.C.; Barrett, S.; Brandish, C.; et al. Antibiotic Prescribing Patterns in Ghana, Uganda, Zambia and Tanzania Hospitals: Results from the Global Point Prevalence Survey (G-PPS) on Antimicrobial Use and Stewardship Interventions Implemented. Antibiotics 2021, 10, 1122. [Google Scholar] [CrossRef] [PubMed]
- Amponsah, O.K.O.; Buabeng, K.O.; Owusu-Ofori, A.; Ayisi-Boateng, N.K.; Hämeen-Anttila, K.; Enlund, H. Point prevalence survey of antibiotic consumption across three hospitals in Ghana. JAC-Antimicrob. Resist. 2021, 3, dlab008. [Google Scholar] [CrossRef] [PubMed]
- Ankrah, D.; Owusu, H.; Aggor, A.; Osei, A.; Ampomah, A.; Harrison, M.; Nelson, F.; Aboagye, G.O.; Ekpale, P.; Laryea, J.; et al. Point Prevalence Survey of Antimicrobial Utilization in Ghana’s Premier Hospital: Implications for Antimicrobial Stewardship. Antibiotics 2021, 10, 1528. [Google Scholar] [CrossRef] [PubMed]
- Labi, A.-K.; Obeng-Nkrumah, N.; Dayie, N.T.K.D.; Egyir, B.; Sampane-Donkor, E.; Newman, M.J.; Opintan, J.A. Antimicrobial use in hospitalized patients: A multicentre point prevalence survey across seven hospitals in Ghana. JAC-Antimicrob. Resist. 2021, 3, dlab087. [Google Scholar]
- Afriyie, D.K.; Sefah, A.I.; Sneddon, J.; Malcolm, W.; McKinney, R.; Cooper, L.; Kurdi, A.; Godman, B.; Seaton, R.A. Antimicrobial point prevalence surveys in two Ghanaian hospitals: Opportunities for antimicrobial stewardship. JAC-Antimicrob. Resist. 2020, 2, dlaa001. [Google Scholar] [CrossRef] [PubMed]
- Agyare, E.; Acolatse, J.E.E.; Dakorah, M.P.; Akafity, G.; Chalker, V.J.; Spiller, O.B.; Schneider, K.A.; Yevutsey, S.; Aidoo, N.B.; Blankson, S.; et al. Antimicrobial stewardship capacity and antibiotic utilisation practices in the Cape Coast Teaching Hospital, Ghana: A point prevalence survey study. PLoS ONE 2024, 19, e0297626. [Google Scholar] [CrossRef] [PubMed]
- Commonwealth Pharmacists Association. Commonwealth Partnerships for Antimicrobial Stewardship (CwPAMS) Programme Overview [Internet]. Available online: https://commonwealthpharmacy.org/cwpams/ (accessed on 1 July 2025).
- Iqbal, A.; Kumaradev, Y.; Gülpinar, G.; Brandish, C.; Nabiryo, M.; Garraghan, F.; Rosado, H.; Rutter, V. Application of the Hub-and-Spoke Model in Antimicrobial Stewardship Programmes: A Scoping Review. BioMed 2024, 4, 372–394. [Google Scholar] [CrossRef]
- World Health Organization. Antimicrobial Stewardship Programmes in Health-Care Facilities in Low- and Middle-Income Countries: A WHO Practical Toolkit [Internet]; World Health Organization: Geneva, Switzerland, 2019; 71p, Available online: https://iris.who.int/handle/10665/329404 (accessed on 7 May 2025).
- Harun, M.d.G.D.; Sumon, S.A.; Hasan, I.; Akther, F.M.; Islam, M.d.S.; Anwar, M.d.M.U. Barriers, facilitators, perceptions and impact of interventions in implementing antimicrobial stewardship programs in hospitals of low-middle and middle countries: A scoping review. Antimicrob. Resist. Infect. Control. 2024, 13, 8. [Google Scholar] [CrossRef] [PubMed]
- Ayukekbong, J.A.; Ntemgwa, M.; Atabe, A.N. The threat of antimicrobial resistance in developing countries: Causes and control strategies. Antimicrob. Resist. Infect. Control. 2017, 6, 47. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Core Elements of Hospital Antibiotic Stewardship Programs. [Internet]. US Department of Health and Human Services. 2019. Available online: https://www.cdc.gov/antibiotic-use/hcp/core-elements/hospital.html (accessed on 1 July 2025).
- Pauwels, I.; Versporten, A.; Ashiru-Oredope, D.; Costa, S.F.; Maldonado, H.; Porto, A.P.M.; Mehtar, S.; Goossens, H.; Anthierens, S.; Vlieghe, E. Implementation of hospital antimicrobial stewardship programmes in low- and middle-income countries: A qualitative study from a multi-professional perspective in the Global-PPS network. Antimicrob. Resist. Infect. Control. 2025, 14, 26. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Action Plan on Antimicrobial Resistance [Internet]; World Health Organization: Geneva, Switzerland, 2015; 28p, Available online: https://iris.who.int/handle/10665/193736 (accessed on 8 May 2025).
- Auta, A.; Hadi, M.A.; Oga, E.; Adewuyi, E.O.; Abdu-Aguye, S.N.; Adeloye, D.; Strickland-Hodge, B.; Morgan, D.J. Global access to antibiotics without prescription in community pharmacies: A systematic review and meta-analysis. J. Infect. 2019, 78, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Howard, P.; Pulcini, C.; Levy, H.G.; West, R.M.; Gould, I.M.; Harbarth, S.; Nathwani, D.; ESCMID Study Group for Antimicrobial Policies (ESGAP); ISC Group on Antimicrobial Stewardship. An international cross-sectional survey of antimicrobial stewardship programmes in hospitals. J. Antimicrob. Chemother. 2015, 70, 1245–1255. [Google Scholar] [CrossRef] [PubMed]
- Atkins, L.; Francis, J.; Islam, R.; O’cOnnor, D.; Patey, A.; Ivers, N.; Foy, R.; Duncan, E.M.; Colquhoun, H.; Grimshaw, J.M.; et al. A guide to using the Theoretical Domains Framework of behaviour change to investigate implementation problems. Implement. Sci. 2017, 12, 77. [Google Scholar] [CrossRef] [PubMed]
- Tong, A.; Sainsbury, P.; Craig, J. Consolidated criteria for reporting qualitative research (COREQ): A 32-item checklist for interviews and focus groups. Int. J. Qual. Health Care 2007, 19, 349–357. [Google Scholar] [CrossRef] [PubMed]
| Participant Number | Organisation | Profession | Member of AMS Committee | Duration Involved in Project |
|---|---|---|---|---|
| 1 | Ho Teaching Hospital | Nurse | Yes | 5 years |
| 2 | Ho Teaching Hospital | Physician Assistant | Yes | 2 years |
| 3 | Ho Municipal Hospital | Pharmacist | Yes | 6–12 months |
| 4 | Ho Municipal Hospital | Administrator | Yes | 6–12 months |
| 5 | Ketu South Municipal Hospital | Pharmacist | Yes | 6–12 months |
| 6 | Ketu South Municipal Hospital | Nurse | Yes | 6–12 months |
| 7 | Margret Marquart Catholic Hospital | Administrator | Yes | 1–6 months |
| 8 | Margret Marquart Catholic Hospital | Medical Officer | Yes | 6–12 months |
| 9 | Volta Regional Hospital | Administrator | No | <1 month |
| 10 | Volta Regional Hospital | Nurse | No | 6–12 months |
| 11 | University of Health and Allied Sciences | Pharmacist | Yes | 6 years |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McErlean, M.; Kpokiri, E.; Panesar, P.; Cooper, E.E.; Jato, J.; Orman, E.; Odoi, H.; Hutton-Nyameaye, A.; Somuah, S.O.; Folitse, I.; et al. Evaluation of a Hub-and-Spoke Model to Enhance Healthcare Professionals’ Practice of Antimicrobial Stewardship (AMS) Programmes in the Volta Region of Ghana. Antibiotics 2025, 14, 672. https://doi.org/10.3390/antibiotics14070672
McErlean M, Kpokiri E, Panesar P, Cooper EE, Jato J, Orman E, Odoi H, Hutton-Nyameaye A, Somuah SO, Folitse I, et al. Evaluation of a Hub-and-Spoke Model to Enhance Healthcare Professionals’ Practice of Antimicrobial Stewardship (AMS) Programmes in the Volta Region of Ghana. Antibiotics. 2025; 14(7):672. https://doi.org/10.3390/antibiotics14070672
Chicago/Turabian StyleMcErlean, Mairead, Eneyi Kpokiri, Preet Panesar, Emily E. Cooper, Jonathan Jato, Emmanuel Orman, Hayford Odoi, Araba Hutton-Nyameaye, Samuel O. Somuah, Isaac Folitse, and et al. 2025. "Evaluation of a Hub-and-Spoke Model to Enhance Healthcare Professionals’ Practice of Antimicrobial Stewardship (AMS) Programmes in the Volta Region of Ghana" Antibiotics 14, no. 7: 672. https://doi.org/10.3390/antibiotics14070672
APA StyleMcErlean, M., Kpokiri, E., Panesar, P., Cooper, E. E., Jato, J., Orman, E., Odoi, H., Hutton-Nyameaye, A., Somuah, S. O., Folitse, I., Aku, T. A., Ben, I. O., Farragher, M., Hail, L., Dodoo, C. C., & Jani, Y. H. (2025). Evaluation of a Hub-and-Spoke Model to Enhance Healthcare Professionals’ Practice of Antimicrobial Stewardship (AMS) Programmes in the Volta Region of Ghana. Antibiotics, 14(7), 672. https://doi.org/10.3390/antibiotics14070672









