Antimicrobial Resistance in the Aconcagua River, Chile: Prevalence and Characterization of Resistant Bacteria in a Watershed Under High Anthropogenic Contamination Pressure
Abstract
1. Introduction
2. Results
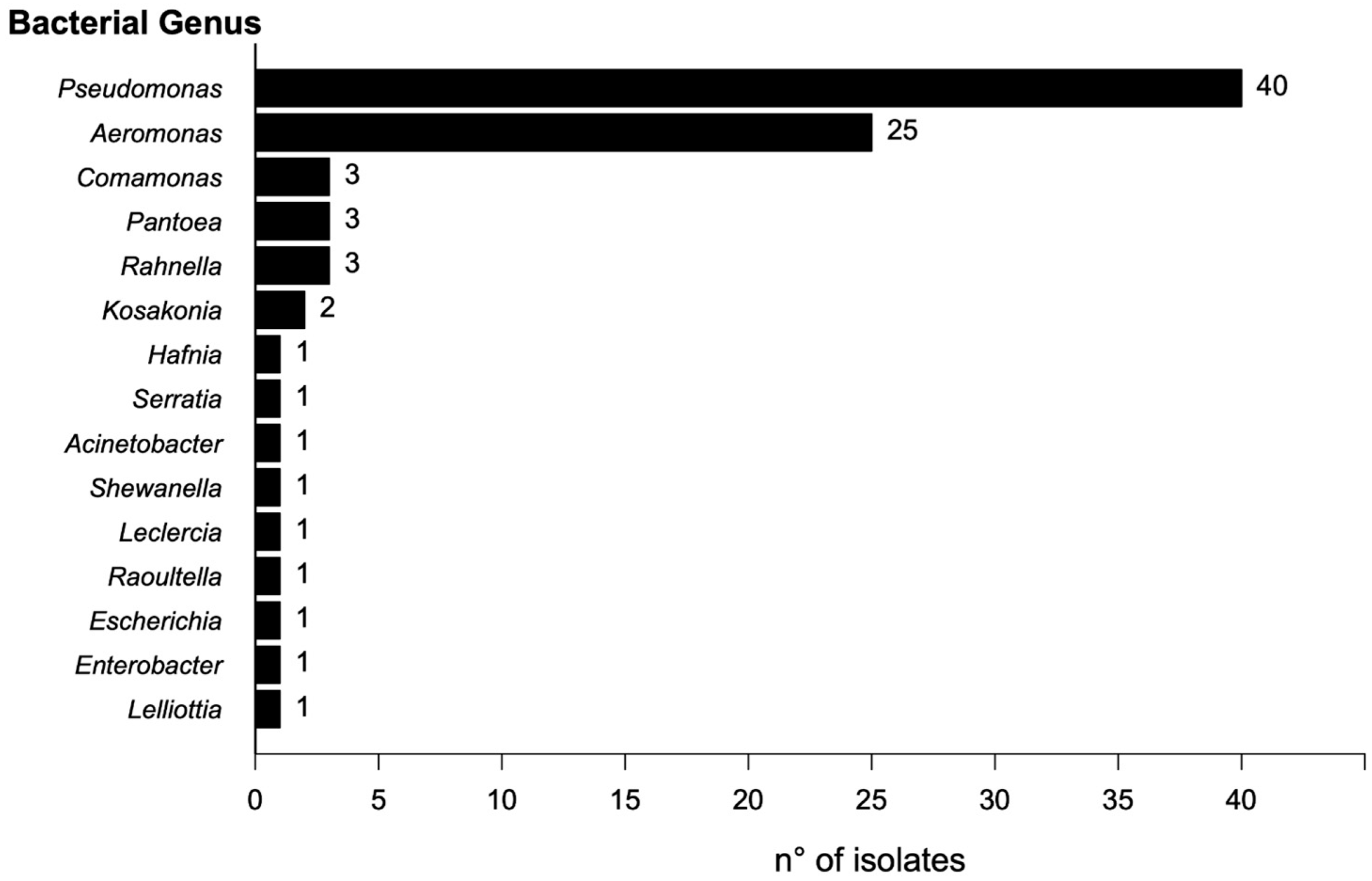
2.1. Isolation and Identification of Bacterial Isolates
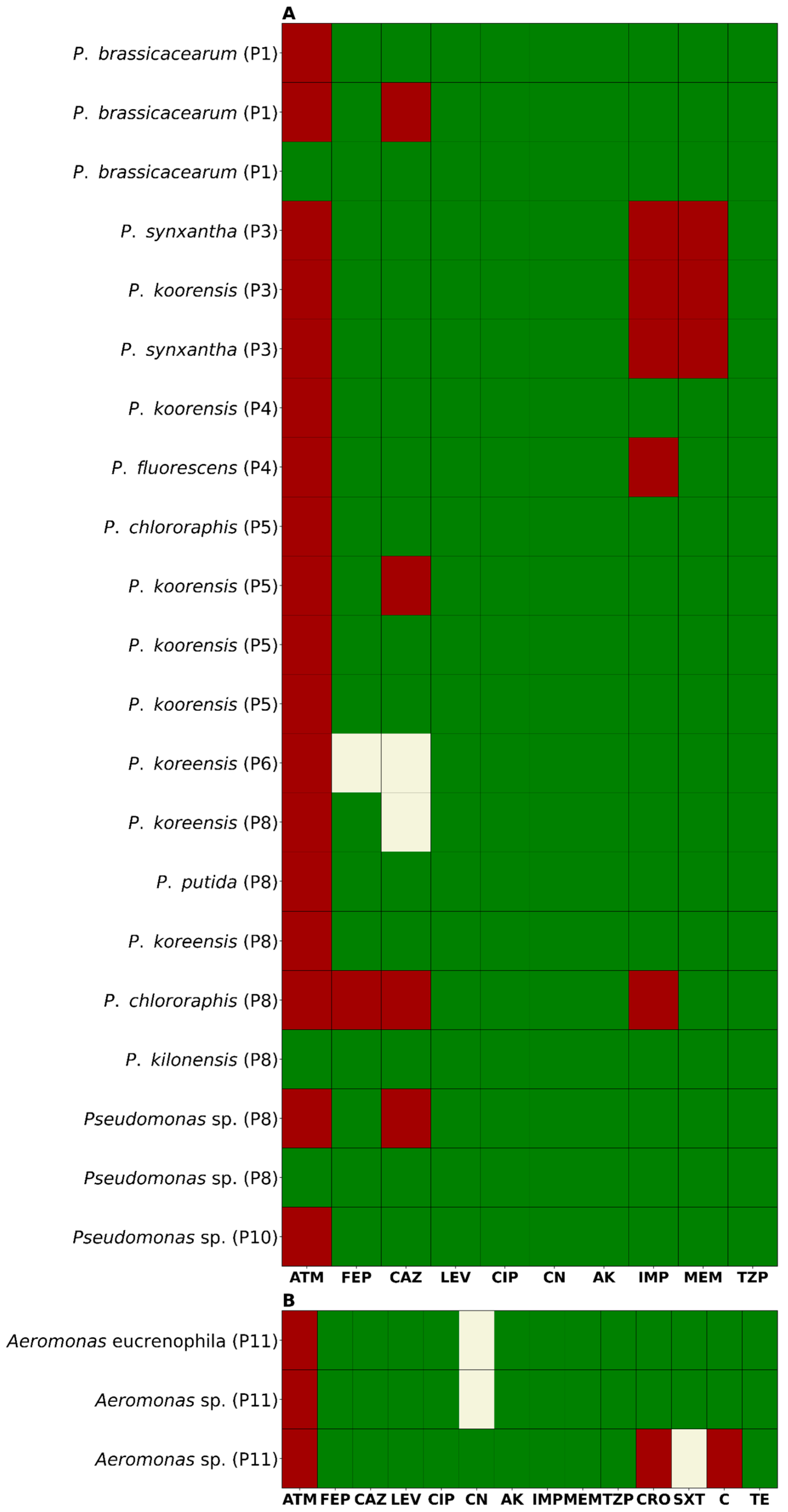
2.2. Antimicrobial Susceptibility Profile
2.3. Detection of Carbapenemase Production
3. Discussion
4. Materials and Methods
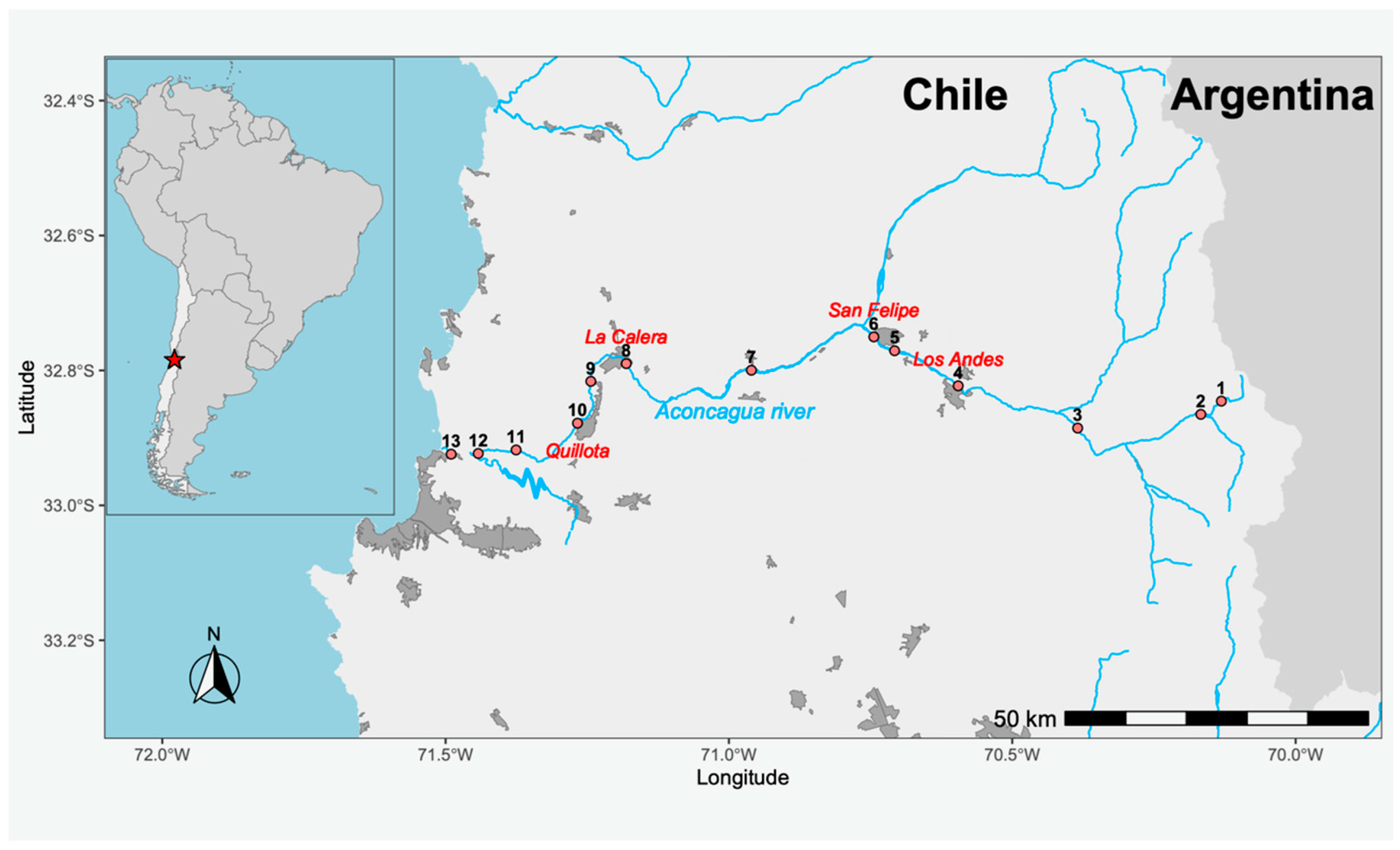
4.1. Study Area and Sampling
4.2. Processing and Isolation of Antibiotic-Resistant Bacteria (ARB)
4.3. Antibiotic Susceptibility Testing
4.4. Detection of Carbapenemase Production Using the Blue-Carba Test
4.5. Genotypic Detection of Clinically Relevant Carbapenemases
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AK | Amikacin |
| AMR | Antimicrobial Resistance |
| AR | Antibiotic Residues |
| ARB | Antimicrobial-Resistant Bacteria |
| ARG | Antimicrobial-Resistant Gene |
| ATM | Aztreonam |
| blaVIM | Betalactamase Verona Integron-encoded Metallo-β-lactamase |
| blaKPC | Betalactamase Klebsiella pneumoniae Carbapenemase |
| blaNDM | Betalactamase New Delhi Metallo-β-lactamase |
| blaIMP | Imipenemase Metallo-β-lactamase |
| C | Chloramphenicol |
| CAZ | Ceftazidime |
| CIP | Ciprofloxacin |
| CLSI | Clinical and Laboratory Standards Institute |
| CN | Gentamicin |
| CRO | Ceftriaxone |
| FEP | Cefepime |
| IMP | Imipenem |
| LEV | Levofloxacin |
| MALDI-TOF | Matrix-Assisted Laser Desorption/Ionization Time-of-Flight |
| MDR | Multidrug-resistant |
| MEM | Meropenem |
| PDR | Pandrug-resistant |
| STX | Trimethoprim-sulfamethoxazole |
| TZP | Piperacillin/tazobactam |
| XDR | Extensively drug-resistant |
References
- Salam, M.A.; Al-Amin, M.Y.; Salam, M.T.; Pawar, J.S.; Akhter, N.; Rabaan, A.A.; Alqumber, M.A.A. Antimicrobial Resistance: A Growing Serious Threat for Global Public Health. Healthcare 2023, 11, 1946. [Google Scholar] [CrossRef]
- Naghavi, M.; Vollset, S.E.; Ikuta, K.S.; Swetschinski, L.R.; Gray, A.P.; Wool, E.E.; Aguilar, G.R.; Mestrovic, T.; Smith, G.; Han, C.; et al. Global Burden of Bacterial Antimicrobial Resistance 1990–2021: A Systematic Analysis with Forecasts to 2050. Lancet 2024, 404, 1199–1226. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, G.; Midiri, A.; Gerace, E.; Biondo, C. Bacterial Antibiotic Resistance: The Most Critical Pathogens. Pathogens 2021, 10, 1310. [Google Scholar] [CrossRef] [PubMed]
- Lesho, E.P.; Laguio-Vila, M. The Slow-Motion Catastrophe of Antimicrobial Resistance and Practical Interventions for All Prescribers. Mayo Clin. Proc. 2019, 94, 1040–1047. [Google Scholar] [CrossRef]
- Davies, S.C.; Oxlade, C. Innovate to Secure the Future: The Future of Modern Medicine. Future Healthc. J. 2021, 8, e251–e256. [Google Scholar] [CrossRef]
- Martínez, J.L. Antibiotics and Antibiotic Resistance Genes in Natural Environments. Science 2008, 321, 365–367. [Google Scholar] [CrossRef]
- Baquero, F.; Martínez, J.L.; Cantón, R.; Martinez, J.L.; Canton, R. Antibiotics and Antibiotic Resistance in Water Environments. Curr. Opin. Biotechnol. 2008, 19, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Fajardo, A.; Martinez, J.L. Antibiotics as Signals That Trigger Specific Bacterial Responses. Curr. Opin. Microbiol. 2008, 11, 161–167. [Google Scholar] [CrossRef]
- Wu, Y.; Gong, Z.; Wang, S.; Song, L. Occurrence and Prevalence of Antibiotic Resistance Genes and Pathogens in an Industrial Park Wastewater Treatment Plant. Sci. Total Environ. 2023, 880, 163278. [Google Scholar] [CrossRef]
- Singh, A.; Pratap, S.G.; Raj, A. Occurrence and Dissemination of Antibiotics and Antibiotic Resistance in Aquatic Environment and Its Ecological Implications: A Review. Environ. Sci. Pollut. Res. Int. 2024, 31, 47505–47529. [Google Scholar] [CrossRef]
- Sousa, M.; Machado, I.; Simões, L.C.; Simões, M. Biocides as Drivers of Antibiotic Resistance: A Critical Review of Environmental Implications and Public Health Risks. Environ. Sci. Ecotechnol. 2025, 25, 100557. [Google Scholar] [CrossRef]
- Checcucci, A.; Buscaroli, E.; Modesto, M.; Luise, D.; Blasioli, S.; Scarafile, D.; Di Vito, M.; Bugli, F.; Trevisi, P.; Braschi, I.; et al. The Swine Waste Resistome: Spreading and Transfer of Antibiotic Resistance Genes in Escherichia coli Strains and the Associated Microbial Communities. Ecotoxicol. Environ. Saf. 2024, 283, 116774. [Google Scholar] [CrossRef] [PubMed]
- Samreen; Ahmad, I.; Malak, H.A.; Abulreesh, H.H. Environmental Antimicrobial Resistance and Its Drivers: A Potential Threat to Public Health. J. Glob. Antimicrob. Resist. 2021, 27, 101–111. [Google Scholar] [CrossRef]
- Skandalis, N.; Maeusli, M.; Papafotis, D.; Miller, S.; Lee, B.; Theologidis, I.; Luna, B. Environmental Spread of Antibiotic Resistance. Antibiotics 2021, 10, 640. [Google Scholar] [CrossRef]
- Ajayi, A.O.; Odeyemi, A.T.; Akinjogunla, O.J.; Adeyeye, A.B.; Ayo-ajayi, I. Review of Antibiotic-Resistant Bacteria and Antibiotic Resistance Genes within the One Health Framework. Infect. Ecol. Epidemiol. 2024, 14, 2312953. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, H.; Yu, S.; Li, D.; Gillings, M.R.; Ren, H.; Mao, D.; Guo, J.; Luo, Y. Inter-Plasmid Transfer of Antibiotic Resistance Genes Accelerates Antibiotic Resistance in Bacterial Pathogens. ISME J. 2024, 18, wrad032. [Google Scholar] [CrossRef] [PubMed]
- Maeusli, M.; Lee, B.; Miller, S.; Reyna, Z.; Lu, P.; Yan, J.; Ulhaq, A.; Skandalis, N.; Spellberg, B.; Luna, B. Horizontal Gene Transfer of Antibiotic Resistance from Acinetobacter baylyi to Escherichia coli on Lettuce and Subsequent Antibiotic Resistance Transmission to the Gut Microbiome. mSphere 2020, 5, 110–1128. [Google Scholar] [CrossRef]
- FRAM Case Study: Aconcagua River in Chile—The Polluted Drinking Water Supplier|University of Gothenburg. Available online: https://www.gu.se/en/research/fram-case-study-aconcagua-river-in-chile-the-polluted-drinking-water-supplier (accessed on 30 April 2025).
- Inostroza, P.A.; Jessen, G.L.; Li, F.; Zhang, X.; Brack, W.; Backhaus, T. Multi-Compartment Impact of Micropollutants and Particularly Antibiotics on Bacterial Communities Using Environmental DNA at River Basin-Level. Environ. Pollut. 2025, 366, 125487. [Google Scholar] [CrossRef]
- Gaete, H.; Aránguiz, F.; Cienfuegos, G.; Tejos, M. Heavy Metals and Toxicity of Waters of the Aconcagua River in Chile. Quim. Nova 2007, 30, 885–891. [Google Scholar] [CrossRef]
- Hernando-Amado, S.; Coque, T.M.; Baquero, F.; Martínez, J.L. Defining and Combating Antibiotic Resistance from One Health and Global Health Perspectives. Nat. Microbiol. 2019, 4, 1432–1442. [Google Scholar] [CrossRef]
- Holmes, A.H.; Moore, L.S.P.; Sundsfjord, A.; Steinbakk, M.; Regmi, S.; Karkey, A.; Guerin, P.J.; Piddock, L.J.V. Understanding the Mechanisms and Drivers of Antimicrobial Resistance. Lancet 2016, 387, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Calero-Cáceres, W.; Marti, E.; Olivares-Pacheco, J.; Rodriguez-Rubio, L. Editorial: Antimicrobial Resistance in Aquatic Environments. Front. Microbiol. 2022, 13, 866268. [Google Scholar] [CrossRef] [PubMed]
- Olivares-Pacheco, J.; Marti, E.; Rodríguez-Rubio, L.; Calero-Cáceres, W. Editorial: Antimicrobial Resistance in Aquatic Environments, Volume II. Front. Microbiol. 2023, 14, 1211464. [Google Scholar] [CrossRef]
- Liu, X.; Lu, S.; Guo, W.; Xi, B.; Wang, W. Antibiotics in the Aquatic Environments: A Review of Lakes, China. Sci. Total Environ. 2018, 627, 1195–1208. [Google Scholar] [CrossRef] [PubMed]
- Kümmerer, K. Antibiotics in the Aquatic Environment—A Review—Part II. Chemosphere 2009, 75, 435–441. [Google Scholar] [CrossRef]
- Manaia, C.M.; Macedo, G.; Fatta-Kassinos, D.; Nunes, O.C. Antibiotic Resistance in Urban Aquatic Environments: Can It Be Controlled? Appl. Microbiol. Biotechnol. 2016, 100, 1543–1557. [Google Scholar] [CrossRef]
- Ashfaq, M.Y.; Da’na, D.A.; Al-Ghouti, M.A. Application of MALDI-TOF MS for Identification of Environmental Bacteria: A Review. J. Environ. Manag. 2022, 305, 114359. [Google Scholar] [CrossRef]
- Chauhan, M.; Kimothi, A.; Sharma, A.; Pandey, A. Cold Adapted Pseudomonas: Ecology to Biotechnology. Front. Microbiol. 2023, 14, 1218708. [Google Scholar] [CrossRef]
- Silby, M.W.; Winstanley, C.; Godfrey, S.A.C.; Levy, S.B.; Jackson, R.W. Pseudomonas Genomes: Diverse and Adaptable. FEMS Microbiol. Rev. 2011, 35, 652–680. [Google Scholar] [CrossRef]
- Horváth, A.; Dobay, O.; Kardos, S.; Ghidán, Á.; Tóth, Á.; Pászti, J.; Ungvári, E.; Horváth, P.; Nagy, K.; Zissman, S.; et al. Varying Fitness Cost Associated with Resistance to Fluoroquinolones Governs Clonal Dynamic of Methicillin-Resistant Staphylococcus aureus. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 2029–2036. [Google Scholar] [CrossRef]
- Tóth, Á.; Kocsis, B.; Damjanova, I.; Kristóf, K.; Jánvári, L.; Pászti, J.; Csercsik, R.; Topf, J.; Szabó, D.; Hamar, P.; et al. Fitness Cost Associated with Resistance to Fluoroquinolones is Diverse across Clones of Klebsiella pneumoniae and May Select for CTX-M-15 Type Extended-Spectrum β-Lactamase. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 837–843. [Google Scholar] [CrossRef]
- Sun, H.; Zeng, J.; Li, S.; Liang, P.; Zheng, C.; Liu, Y.; Luo, T.; Rastogi, N.; Sun, Q. Interaction between rpsL and gyrA Mutations Affects the Fitness and Dual Resistance of Mycobacterium tuberculosis Clinical Isolates against Streptomycin and Fluoroquinolones. Infect. Drug Resist. 2018, 11, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Papp-Wallace, K.M.; Endimiani, A.; Taracila, M.A.; Bonomo, R.A. Carbapenems: Past, Present, and Future. Antimicrob. Agents Chemother. 2011, 55, 4943–4960. [Google Scholar] [CrossRef] [PubMed]
- Halat, D.H.; Moubareck, C.A. The Intriguing Carbapenemases of Pseudomonas aeruginosa: Current Status, Genetic Profile, and Global Epidemiology. Yale J. Biol. Med. 2022, 95, 507–515. [Google Scholar]
- Poirel, L.; Pitout, J.D.; Nordmann, P. Carbapenemases: Molecular Diversity and Clinical Consequences. Future Microbiol. 2007, 2, 501–512. [Google Scholar] [CrossRef]
- Hong, D.J.; Bae, I.K.; Jang, I.H.; Jeong, S.H.; Kang, H.K.; Lee, K. Epidemiology and Characteristics of Metallo-ß-Lactamase-Producing Pseudomonas aeruginosa. Infect. Chemother. 2015, 47, 81–97. [Google Scholar] [CrossRef]
- Catho, G.; Martischang, R.; Boroli, F.; Chraïti, M.N.; Martin, Y.; Koyluk Tomsuk, Z.; Renzi, G.; Schrenzel, J.; Pugin, J.; Nordmann, P.; et al. Outbreak of Pseudomonas aeruginosa Producing VIM Carbapenemase in an Intensive Care Unit and Its Termination by Implementation of Waterless Patient Care. Crit. Care 2021, 25, 301. [Google Scholar] [CrossRef]
- Rodriguez-Mozaz, S.; Chamorro, S.; Marti, E.; Huerta, B.; Gros, M.; Sànchez-Melsió, A.; Borrego, C.M.; Barceló, D.; Balcázar, J.L. Occurrence of Antibiotics and Antibiotic Resistance Genes in Hospital and Urban Wastewaters and Their Impact on the Receiving River. Water Res. 2015, 69, 234–242. [Google Scholar] [CrossRef]
- Basiry, D.; Entezari Heravi, N.; Uluseker, C.; Kaster, K.M.; Kommedal, R.; Pala-Ozkok, I. The Effect of Disinfectants and Antiseptics on Co- and Cross-Selection of Resistance to Antibiotics in Aquatic Environments and Wastewater Treatment Plants. Front. Microbiol. 2022, 13, 1050558. [Google Scholar] [CrossRef]
- Uluseker, C.; Kaster, K.M.; Thorsen, K.; Basiry, D.; Shobana, S.; Jain, M.; Kumar, G.; Kommedal, R.; Pala-Ozkok, I. A Review on Occurrence and Spread of Antibiotic Resistance in Wastewaters and in Wastewater Treatment Plants: Mechanisms and Perspectives. Front. Microbiol. 2021, 12, 717809. [Google Scholar] [CrossRef]
- Díaz-Gavidia, C.; Barría, C.; Rivas, L.; García, P.; Alvarez, F.P.; González-Rocha, G.; Opazo-Capurro, A.; Araos, R.; Munita, J.M.; Cortes, S.; et al. Isolation of Ciprofloxacin and Ceftazidime-Resistant Enterobacterales from Vegetables and River Water is Strongly Associated with the Season and the Sample Type. Front. Microbiol. 2021, 12, 604567. [Google Scholar] [CrossRef] [PubMed]
- Trick, W.E. Decision Making During Healthcare-Associated Infection Surveillance: A Rationale for Automation. Clin. Infect. Dis. 2013, 57, 434–440. [Google Scholar] [CrossRef]
- Bizzini, A.; Greub, G. Matrix-Assisted Laser Desorption Ionization Time-of-Flight Mass Spectrometry, a Revolution in Clinical Microbial Identification. Clin. Microbiol. Infect. 2010, 16, 1614–1619. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing. Manual M100, 33rd ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2023. [Google Scholar]
- Clinical and Laboratory Standards Institute. Methods for Antimicrobial Dilution and Disk Susceptibility Testing of Infrequently Isolated or Fastidious Bacteria. Manual M45, 3rd ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2016. [Google Scholar]
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B. Multidrug-Resistant, Extensively Drug-Resistant and Pandrug-Resistant Bacteria: An International Expert Proposal for Interim Standard Definitions for Acquired Resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Maccari, L.; Ceriana, P.; Granchetti, H.N.; Pezzaniti, A.V.; Lucero, C.; Rapoport, M.; Menocal, A.; Corso, A.; Pasteran, F. Improved Blue Carba Test and Carba NP Test for Detection and Classification of Major Class A and B Carbapenemases, Including Dual Producers, among Gram-Negative Bacilli. J. Clin. Microbiol. 2024, 62, e01255-23. [Google Scholar] [CrossRef]
- Poirel, L.; Walsh, T.R.; Cuvillier, V.; Nordmann, P. Multiplex PCR for Detection of Acquired Carbapenemase Genes. Diagn. Microbiol. Infect. Dis. 2011, 70, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Wolter, D.J.; Khalaf, N.; Robledo, I.E.; Vázquez, J.G.; Santé, I.M.; Aquino, E.E.; Goering, R.V.; Hanson, N.D. Surveillance of Carbapenem-Resistant Pseudomonas aeruginosa Isolates from Puerto Rican Medical Center Hospitals: Dissemination of KPC and IMP-18 β-Lactamases. Antimicrob. Agents Chemother. 2009, 53, 1660–1664. [Google Scholar] [CrossRef]
- Ellington, M.J.; Kistler, J.; Livermore, D.M.; Woodford, N. Multiplex PCR for Rapid Detection of Genes Encoding Acquired Metallo-β-Lactamases. J. Antimicrob. Chemother. 2007, 59, 321–322. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Rojas, N.; Lira-Velásquez, D.; Covarrubia-López, R.; Plaza-Sepúlveda, J.; Munita, J.M.; Carter, M.J.; Olivares-Pacheco, J. Antimicrobial Resistance in the Aconcagua River, Chile: Prevalence and Characterization of Resistant Bacteria in a Watershed Under High Anthropogenic Contamination Pressure. Antibiotics 2025, 14, 669. https://doi.org/10.3390/antibiotics14070669
González-Rojas N, Lira-Velásquez D, Covarrubia-López R, Plaza-Sepúlveda J, Munita JM, Carter MJ, Olivares-Pacheco J. Antimicrobial Resistance in the Aconcagua River, Chile: Prevalence and Characterization of Resistant Bacteria in a Watershed Under High Anthropogenic Contamination Pressure. Antibiotics. 2025; 14(7):669. https://doi.org/10.3390/antibiotics14070669
Chicago/Turabian StyleGonzález-Rojas, Nicolás, Diego Lira-Velásquez, Richard Covarrubia-López, Johan Plaza-Sepúlveda, José M. Munita, Mauricio J. Carter, and Jorge Olivares-Pacheco. 2025. "Antimicrobial Resistance in the Aconcagua River, Chile: Prevalence and Characterization of Resistant Bacteria in a Watershed Under High Anthropogenic Contamination Pressure" Antibiotics 14, no. 7: 669. https://doi.org/10.3390/antibiotics14070669
APA StyleGonzález-Rojas, N., Lira-Velásquez, D., Covarrubia-López, R., Plaza-Sepúlveda, J., Munita, J. M., Carter, M. J., & Olivares-Pacheco, J. (2025). Antimicrobial Resistance in the Aconcagua River, Chile: Prevalence and Characterization of Resistant Bacteria in a Watershed Under High Anthropogenic Contamination Pressure. Antibiotics, 14(7), 669. https://doi.org/10.3390/antibiotics14070669





