Use of Daptomycin to Manage Severe MRSA Infections in Humans
Abstract
1. Introduction
2. MRSA—Clinical Impact
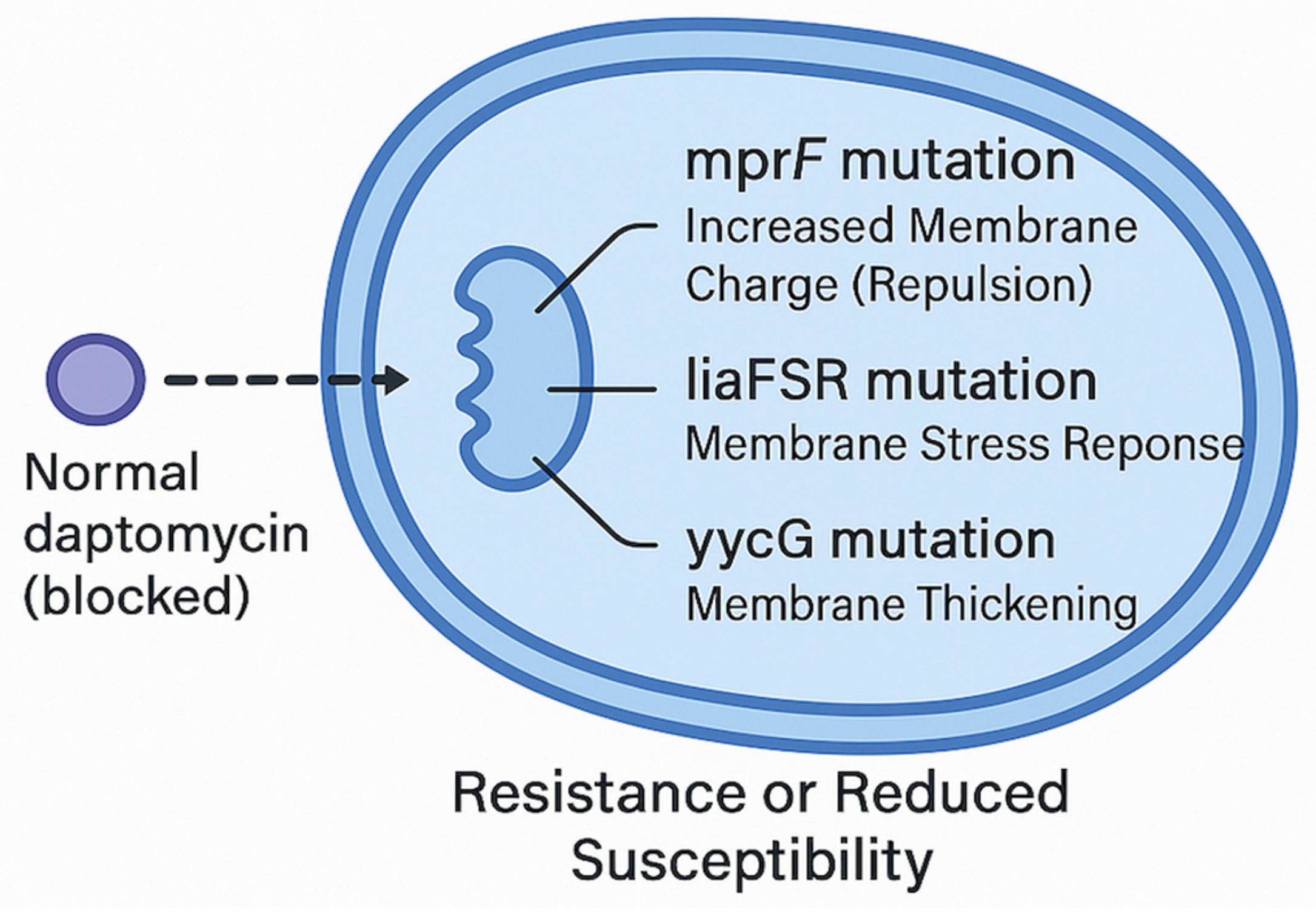
3. Daptomycin—Microbiology and Resistance
4. Clinical Use—Daptomycin Monotherapy
4.1. Critical Appraisal of Recent Evidence and Guideline Updates
| Study | Year | Study Design | Population | Main Findings |
|---|---|---|---|---|
| Fowler et al. [36] | 2006 | Randomized controlled trial | Adults with MRSA bacteraemia and right-sided endocarditis | DAP non-inferior to standard therapy, fewer nephrotoxic events |
| Murray et al. [39] | 2013 | Observational study | Adults with MRSA bacteraemia | Faster clearance of bacteraemia with DAP compared to VAN |
| Kullar et al. [40] | 2013 | Observational cohort | Critically ill patients with MRSA bacteraemia | DAP associated with higher rates of microbiologic success |
4.2. Pharmacokinetic Advances and TDM
5. Clinical Use—Daptomycin Plus Fosfomycin
6. Clinical Use—Daptomycin Plus Β-Lactams
7. Clinical Use in Paediatric Patients
8. Discussion
8.1. Monotherapy
8.2. Combination Regimens
8.3. Special Settings and Pharmacokinetics
8.4. Limitations of Current Evidence and Future Directions
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MRSA | Methicillin-resistant Staphylococcus aureus |
| DAP | Daptomycin |
| VAN | Vancomycin |
| VISA | Vancomycin-intermediate Staphylococcus aureus |
| hVISA | Heterogeneous vancomycin-intermediate Staphylococcus aureus |
| MIC | Minimum inhibitory concentration |
| cSSSIs | Complicated skin and skin structure infections |
| CPT | Ceftaroline |
| BPR | Ceftobiprole |
| FOS | Fosfomycin |
| TDM | Therapeutic drug monitoring |
| MSSA | Methicillin-susceptible Staphylococcus aureus |
| OR | odds ratio |
| CI | confidence interval |
References
- Ippolito, G.; Leone, S.; Lauria, F.N.; Nicastri, E.; Wenzel, R.P. Methicillin-resistant Staphylococcus aureus: The superbug. Int. J. Infect. Dis. 2010, 14 (Suppl. S4), S7–S11. [Google Scholar] [CrossRef] [PubMed]
- Chambers, H.F.; Deleo, F.R. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat. Rev. Microbiol. 2009, 7, 629–641. [Google Scholar] [CrossRef] [PubMed]
- Leone, S.; Pezone, I.; Pisaturo, M.; McCaffery, E.; Alfieri, A.; Fiore, M. Pharmacotherapies for multidrug-resistant gram-positive infections: Current options and beyond. Expert. Opin. Pharmacother. 2024, 25, 1027–1037. [Google Scholar] [CrossRef] [PubMed]
- Rybak, M.; Lomaestro, B.; Rotschafer, J.C.; Moellering, R., Jr.; Craig, W.; Billeter, M.; Dalovisio, J.R.; Levine, D.P. Therapeutic monitoring of vancomycin in adult patients: A consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am. J. Health Syst. Pharm. 2009, 66, 82–98. [Google Scholar] [CrossRef]
- Hidayat, L.K.; Hsu, D.I.; Quist, R.; Shriner, K.A.; Wong-Beringer, A. High-dose vancomycin therapy for methicillin-resistant Staphylococcus aureus infections: Efficacy and toxicity. Arch. Intern. Med. 2006, 166, 2138–2144. [Google Scholar] [CrossRef]
- Howden, B.P.; Davies, J.K.; Johnson, P.D.; Stinear, T.P.; Grayson, M.L. Reduced vancomycin susceptibility in Staphylococcus aureus, including vancomycin-intermediate and heterogeneous vancomycin-intermediate strains: Resistance mechanisms, laboratory detection, and clinical implications. Clin. Microbiol. Rev. 2010, 23, 99–139. [Google Scholar] [CrossRef]
- Charles, P.G.; Ward, P.B.; Johnson, P.D.; Howden, B.P.; Grayson, M.L. Clinical features associated with bacteremia due to heterogeneous vancomycin-intermediate Staphylococcus aureus. Clin. Infect. Dis. 2004, 38, 448–451. [Google Scholar] [CrossRef]
- Arbeit, R.D.; Maki, D.; Tally, F.P.; Campanaro, E.; Eisenstein, B.I. The safety and efficacy of daptomycin for the treatment of complicated skin and skin-structure infections. Clin. Infect. Dis. 2004, 38, 1673–1681. [Google Scholar] [CrossRef]
- Silverman, J.A.; Perlmutter, N.G.; Shapiro, H.M. Correlation of daptomycin bactericidal activity and membrane depolarization in Staphylococcus aureus. Antimicrob. Agents Chemother. 2003, 47, 2538–2544. [Google Scholar] [CrossRef]
- Dvorchik, B.H.; Brazier, D.; DeBruin, M.F.; Arbeit, R.D. Daptomycin pharmacokinetics and safety following administration of escalating doses once daily to healthy subjects. Antimicrob. Agents Chemother. 2003, 47, 1318–1323. [Google Scholar] [CrossRef]
- Cosgrove, S.E. The relationship between antimicrobial resistance and patient outcomes: Mortality, length of hospital stay, and health care costs. Clin. Infect. Dis. 2006, 42 (Suppl. S2), S82–S89. [Google Scholar] [CrossRef] [PubMed]
- Engemann, J.J.; Carmeli, Y.; Cosgrove, S.E.; Fowler, V.G.; Bronstein, M.Z.; Trivette, S.L.; Briggs, J.P.; Sexton, D.J.; Kaye, K.S. Adverse clinical and economic outcomes attributable to methicillin resistance among patients with Staphylococcus aureus surgical site infection. Clin. Infect. Dis. 2003, 36, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Hirabayashi, A.; Yahara, K.; Oka, K.; Kajihara, T.; Ohkura, T.; Hosaka, Y.; Shibayama, K.; Sugai, M.; Yagi, T. Comparison of disease and economic burden between MRSA infection and MRSA colonization in a university hospital: A retrospective data integration study. Antimicrob. Resist. Infect. Control 2024, 13, 27. [Google Scholar] [CrossRef] [PubMed]
- Hamdy, R.F.; Hsu, A.J.; Stockmann, C.; Olson, J.A.; Bryan, M.; Hersh, A.L.; Tamma, P.D.; Gerber, J.S. Epidemiology of Methicillin-Resistant Staphylococcus aureus Bacteremia in Children. Pediatrics 2017, 139, e20170183. [Google Scholar] [CrossRef]
- Park, D.A.; Lee, S.M.; Peck, K.R.; Joo, E.J.; Oh, E.G. Impact of Methicillin-Resistance on Mortality in Children and Neonates with Staphylococcus aureus Bacteremia: A Meta-analysis. Infect. Chemother. 2013, 45, 202–210. [Google Scholar] [CrossRef]
- Song, X.; Perencevich, E.; Campos, J.; Short, B.L.; Singh, N. Clinical and economic impact of methicillin-resistant Staphylococcus aureus colonization or infection on neonates in intensive care units. Infect. Control Hosp. Epidemiol. 2010, 31, 177–182. [Google Scholar] [CrossRef]
- Whitby, M.; McLaws, M.L.; Berry, G. Risk of death from methicillin-resistant Staphylococcus aureus bacteraemia: A meta-analysis. Med. J. Aust. 2001, 175, 264–267. [Google Scholar] [CrossRef]
- Cosgrove, S.E.; Sakoulas, G.; Perencevich, E.N.; Schwaber, M.J.; Karchmer, A.W.; Carmeli, Y. Comparison of mortality associated with methicillin-resistant and methicillin-susceptible Staphylococcus aureus bacteremia: A meta-analysis. Clin. Infect. Dis. 2003, 36, 53–59. [Google Scholar] [CrossRef]
- Bai, A.D.; Lo, C.K.L.; Komorowski, A.S.; Suresh, M.; Guo, K.; Garg, A.; Tandon, P.; Senecal, J.; Del Corpo, O.; Stefanova, I.; et al. Staphylococcus aureus bacteraemia mortality: A systematic review and meta-analysis. Clin. Microbiol. Infect. 2022, 28, 1076–1084. [Google Scholar] [CrossRef]
- Gonzalez-Ruiz, A.; Seaton, R.A.; Hamed, K. Daptomycin: An evidence-based review of its role in the treatment of Gram-positive infections. Infect. Drug Resist. 2016, 9, 47–58. [Google Scholar] [CrossRef]
- Steenbergen, J.N.; Alder, J.; Thorne, G.M.; Tally, F.P. Daptomycin: A lipopeptide antibiotic for the treatment of serious Gram-positive infections. J. Antimicrob. Chemother. 2005, 55, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Algammal, A.M.; Hetta, H.F.; Elkelish, A.; Alkhalifah, D.H.H.; Hozzein, W.N.; Batiha, G.E.; El Nahhas, N.; Mabrok, M.A. Methicillin-Resistant Staphylococcus aureus (MRSA): One Health Perspective Approach to the Bacterium Epidemiology, Virulence Factors, Antibiotic-Resistance, and Zoonotic Impact. Infect. Drug Resist. 2020, 13, 3255–3265. [Google Scholar] [CrossRef] [PubMed]
- Raad, I.; Hanna, H.; Jiang, Y.; Dvorak, T.; Reitzel, R.; Chaiban, G.; Sherertz, R.; Hachem, R. Comparative activities of daptomycin, linezolid, and tigecycline against catheter-related methicillin-resistant Staphylococcus bacteremic isolates embedded in biofilm. Antimicrob. Agents Chemother. 2007, 51, 1656–1660. [Google Scholar] [CrossRef] [PubMed]
- Boutet-Dubois, A.; Magnan, C.; Lienard, A.; Pouget, C.; Bouchet, F.; Marchandin, H.; Larcher, R.; Lavigne, J.P.; Pantel, A. In Vivo-Acquired Resistance to Daptomycin during Methicillin-Resistant Staphylococcus aureus Bacteremia. Antibiotics 2023, 12, 1647. [Google Scholar] [CrossRef]
- Cui, L.; Ma, X.; Sato, K.; Okuma, K.; Tenover, F.C.; Mamizuka, E.M.; Gemmell, C.G.; Kim, M.N.; Ploy, M.C.; El-Solh, N.; et al. Cell wall thickening is a common feature of vancomycin resistance in Staphylococcus aureus. J. Clin. Microbiol. 2003, 41, 5–14. [Google Scholar] [CrossRef]
- Yang, S.J.; Mishra, N.N.; Rubio, A.; Bayer, A.S. Causal role of single nucleotide polymorphisms within the mprF gene of Staphylococcus aureus in daptomycin resistance. Antimicrob. Agents Chemother. 2013, 57, 5658–5664. [Google Scholar] [CrossRef]
- Bayer, A.S.; Schneider, T.; Sahl, H.G. Mechanisms of daptomycin resistance in Staphylococcus aureus: Role of the cell membrane and cell wall. Ann. N. Y. Acad. Sci. 2013, 1277, 139–158. [Google Scholar] [CrossRef]
- Bayer, A.S.; Mishra, N.N.; Chen, L.; Kreiswirth, B.N.; Rubio, A.; Yang, S.J. Frequency and Distribution of Single-Nucleotide Polymorphisms within mprF in Methicillin-Resistant Staphylococcus aureus Clinical Isolates and Their Role in Cross-Resistance to Daptomycin and Host Defense Antimicrobial Peptides. Antimicrob. Agents Chemother. 2015, 59, 4930–4937. [Google Scholar] [CrossRef]
- Rose, W.E.; Fallon, M.; Moran, J.J.; Vanderloo, J.P. Vancomycin tolerance in methicillin-resistant Staphylococcus aureus: Influence of vancomycin, daptomycin, and telavancin on differential resistance gene expression. Antimicrob. Agents Chemother. 2012, 56, 4422–4427. [Google Scholar] [CrossRef]
- Stefani, S.; Campanile, F.; Santagati, M.; Mezzatesta, M.L.; Cafiso, V.; Pacini, G. Insights and clinical perspectives of daptomycin resistance in Staphylococcus aureus: A review of the available evidence. Int. J. Antimicrob. Agents 2015, 46, 278–289. [Google Scholar] [CrossRef]
- Pujol, M.; Miró, J.M.; Shaw, E.; Aguado, J.M.; San-Juan, R.; Puig-Asensio, M.; Pigrau, C.; Calbo, E.; Montejo, M.; Rodriguez-Álvarez, R.; et al. Daptomycin Plus Fosfomycin Versus Daptomycin Alone for Methicillin-resistant Staphylococcus aureus Bacteremia and Endocarditis: A Randomized Clinical Trial. Clin. Infect. Dis. 2021, 72, 1517–1525. [Google Scholar] [CrossRef] [PubMed]
- Rose, W.E.; Schulz, L.T.; Andes, D.; Striker, R.; Berti, A.D.; Hutson, P.R.; Shukla, S.K. Addition of ceftaroline to daptomycin after emergence of daptomycin-nonsusceptible Staphylococcus aureus during therapy improves antibacterial activity. Antimicrob. Agents Chemother. 2012, 56, 5296–5302. [Google Scholar] [CrossRef] [PubMed]
- U.S Food and Drug Administration. Daptomycin for Injection—Full Prescribing Information. 2021. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/210282s000lbl.pdf (accessed on 12 June 2025).
- Seaton, R.A.; Menichetti, F.; Dalekos, G.; Beiras-Fernandez, A.; Nacinovich, F.; Pathan, R.; Hamed, K. Evaluation of Effectiveness and Safety of High-Dose Daptomycin: Results from Patients Included in the European Cubicin(®) Outcomes Registry and Experience. Adv. Ther. 2015, 32, 1192–1205. [Google Scholar] [CrossRef] [PubMed]
- Kullar, R.; Casapao, A.M.; Davis, S.L.; Levine, D.P.; Zhao, J.J.; Crank, C.W.; Segreti, J.; Sakoulas, G.; Cosgrove, S.E.; Rybak, M.J. A multicentre evaluation of the effectiveness and safety of high-dose daptomycin for the treatment of infective endocarditis. J. Antimicrob. Chemother. 2013, 68, 2921–2926. [Google Scholar] [CrossRef]
- Fowler, V.G., Jr.; Boucher, H.W.; Corey, G.R.; Abrutyn, E.; Karchmer, A.W.; Rupp, M.E.; Levine, D.P.; Chambers, H.F.; Tally, F.P.; Vigliani, G.A.; et al. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N. Engl. J. Med. 2006, 355, 653–665. [Google Scholar] [CrossRef]
- Konychev, A.; Heep, M.; Moritz, R.K.; Kreuter, A.; Shulutko, A.; Fierlbeck, G.; Bouylout, K.; Pathan, R.; Trostmann, U.; Chaves, R.L. Safety and efficacy of daptomycin as first-line treatment for complicated skin and soft tissue infections in elderly patients: An open-label, multicentre, randomized phase IIIb trial. Drugs Aging 2013, 30, 829–836. [Google Scholar] [CrossRef]
- Bookstaver, P.B.; Bland, C.M.; Qureshi, Z.P.; Faulkner-Fennell, C.M.; Sheldon, M.A.; Caulder, C.R.; Hartis, C. Safety and effectiveness of daptomycin across a hospitalized obese population: Results of a multicenter investigation in the southeastern United States. Pharmacotherapy 2013, 33, 1322–1330. [Google Scholar] [CrossRef]
- Murray, K.P.; Zhao, J.J.; Davis, S.L.; Kullar, R.; Kaye, K.S.; Lephart, P.; Rybak, M.J. Early use of daptomycin versus vancomycin for methicillin-resistant Staphylococcus aureus bacteremia with vancomycin minimum inhibitory concentration >1 mg/L: A matched cohort study. Clin. Infect. Dis. 2013, 56, 1562–1569. [Google Scholar] [CrossRef]
- Kullar, R.; Davis, S.L.; Kaye, K.S.; Levine, D.P.; Pogue, J.M.; Rybak, M.J. Implementation of an antimicrobial stewardship pathway with daptomycin for optimal treatment of methicillin-resistant Staphylococcus aureus bacteremia. Pharmacotherapy 2013, 33, 3–10. [Google Scholar] [CrossRef]
- Moise, P.A.; Culshaw, D.L.; Wong-Beringer, A.; Bensman, J.; Lamp, K.C.; Smith, W.J.; Bauer, K.; Goff, D.A.; Adamson, R.; Leuthner, K.; et al. Comparative Effectiveness of Vancomycin Versus Daptomycin for MRSA Bacteremia with Vancomycin MIC >1 mg/L: A Multicenter Evaluation. Clin. Ther. 2016, 38, 16–30. [Google Scholar] [CrossRef]
- Bassetti, M.; Villa, G.; Ansaldi, F.; De Florentiis, D.; Tascini, C.; Cojutti, P.; Righi, E.; Sartor, A.; Crapis, M.; De Rosa, F.G.; et al. Risk factors associated with the onset of daptomycin non-susceptibility in Staphylococcus aureus infections in critically ill patients. Intensive Care Med. 2015, 41, 366–368. [Google Scholar] [CrossRef]
- Barber, K.E.; Werth, B.J.; Rybak, M.J. The combination of ceftaroline plus daptomycin allows for therapeutic de-escalation and daptomycin sparing against MRSA. J. Antimicrob. Chemother. 2015, 70, 505–509. [Google Scholar] [CrossRef]
- Liu, C.; Bayer, A.; Cosgrove, S.E.; Daum, R.S.; Fridkin, S.K.; Gorwitz, R.J.; Kaplan, S.L.; Karchmer, A.W.; Levine, D.P.; Murray, B.E.; et al. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin. Infect. Dis. 2011, 52, e18–e55. [Google Scholar] [CrossRef] [PubMed]
- Schweizer, M.L.; Richardson, K.; Vaughan Sarrazin, M.S.; Goto, M.; Livorsi, D.J.; Nair, R.; Alexander, B.; Beck, B.F.; Jones, M.P.; Puig-Asensio, M.; et al. Comparative Effectiveness of Switching to Daptomycin Versus Remaining on Vancomycin Among Patients with Methicillin-resistant Staphylococcus aureus (MRSA) Bloodstream Infections. Clin. Infect. Dis. 2021, 72, S68–S73. [Google Scholar] [CrossRef]
- Adamu, Y.; Puig-Asensio, M.; Dabo, B.; Schweizer, M.L. Comparative effectiveness of daptomycin versus vancomycin among patients with methicillin-resistant Staphylococcus aureus (MRSA) bloodstream infections: A systematic literature review and meta-analysis. PLoS ONE 2024, 19, e0293423. [Google Scholar] [CrossRef]
- D’Avolio, A.; Pensi, D.; Baietto, L.; Pacini, G.; Di Perri, G.; De Rosa, F.G. Daptomycin Pharmacokinetics and Pharmacodynamics in Septic and Critically Ill Patients. Drugs 2016, 76, 1161–1174. [Google Scholar] [CrossRef]
- Soraluce, A.; Asin-Prieto, E.; Rodriguez-Gascon, A.; Barrasa, H.; Maynar, J.; Carcelero, E.; Soy, D.; Isla, A. Population pharmacokinetics of daptomycin in critically ill patients. Int. J. Antimicrob. Agents 2018, 52, 158–165. [Google Scholar] [CrossRef]
- Gregoire, N.; Chauzy, A.; Buyck, J.; Rammaert, B.; Couet, W.; Marchand, S. Clinical Pharmacokinetics of Daptomycin. Clin. Pharmacokinet. 2021, 60, 271–281. [Google Scholar] [CrossRef]
- Heitzmann, J.; Thoma, Y.; Bricca, R.; Gagnieu, M.C.; Leclerc, V.; Roux, S.; Conrad, A.; Ferry, T.; Goutelle, S. Implementation and Comparison of Two Pharmacometric Tools for Model-Based Therapeutic Drug Monitoring and Precision Dosing of Daptomycin. Pharmaceutics 2022, 14, 114. [Google Scholar] [CrossRef] [PubMed]
- Tuloup, V.; Millet, A.; Taricco, A.; Parant, F.; Ferry, T.; Goutelle, S. Evaluation of Limited Sampling Strategies for Bayesian Estimation of Daptomycin Area Under the Concentration-Time Curve: A Short Communication. Ther. Drug Monit. 2023, 45, 562–565. [Google Scholar] [CrossRef] [PubMed]
- Cairns, K.A.; Abbott, I.J.; Dooley, M.J.; Peleg, A.Y.; Peel, T.N.; Udy, A.A. The impact of daptomycin therapeutic drug monitoring on clinical outcomes: A systematic review. Int. J. Antimicrob. Agents 2023, 61, 106712. [Google Scholar] [CrossRef] [PubMed]
- Bhavnani, S.M.; Rubino, C.M.; Ambrose, P.G.; Drusano, G.L. Daptomycin exposure and the probability of elevations in the creatine phosphokinase level: Data from a randomized trial of patients with bacteremia and endocarditis. Clin. Infect. Dis. 2010, 50, 1568–1574. [Google Scholar] [CrossRef] [PubMed]
- Klinker, K.P.; Borgert, S.J. Beyond Vancomycin: The Tail of the Lipoglycopeptides. Clin. Ther. 2015, 37, 2619–2636. [Google Scholar] [CrossRef] [PubMed]
- Dhand, A.; Sakoulas, G. Daptomycin in Combination with Other Antibiotics for the Treatment of Complicated Methicillin-Resistant Staphylococcus aureus Bacteremia. Clin. Ther. 2014, 36, 1303–1316. [Google Scholar] [CrossRef]
- Miró, J.M.; Entenza, J.M.; Del Río, A.; Velasco, M.; Castañeda, X.; Garcia de la Mària, C.; Giddey, M.; Armero, Y.; Pericàs, J.M.; Cervera, C.; et al. High-dose daptomycin plus fosfomycin is safe and effective in treating methicillin-susceptible and methicillin-resistant Staphylococcus aureus endocarditis. Antimicrob. Agents Chemother. 2012, 56, 4511–4515. [Google Scholar] [CrossRef]
- Parker, S.L.; Frantzeskaki, F.; Wallis, S.C.; Diakaki, C.; Giamarellou, H.; Koulenti, D.; Karaiskos, I.; Lipman, J.; Dimopoulos, G.; Roberts, J.A. Population Pharmacokinetics of Fosfomycin in Critically Ill Patients. Antimicrob. Agents Chemother. 2015, 59, 6471–6476. [Google Scholar] [CrossRef]
- Aktas, G.; Derbentli, S. In vitro activity of daptomycin combinations with rifampicin, gentamicin, fosfomycin and fusidic acid against MRSA strains. J. Glob. Antimicrob. Resist. 2017, 10, 223–227. [Google Scholar] [CrossRef]
- García-de-la-Mària, C.; Gasch, O.; García-Gonzalez, J.; Soy, D.; Shaw, E.; Ambrosioni, J.; Almela, M.; Pericàs, J.M.; Tellez, A.; Falces, C.; et al. The Combination of Daptomycin and Fosfomycin Has Synergistic, Potent, and Rapid Bactericidal Activity against Methicillin-Resistant Staphylococcus aureus in a Rabbit Model of Experimental Endocarditis. Antimicrob. Agents Chemother. 2018, 62, 10–1128. [Google Scholar] [CrossRef]
- Mishra, N.N.; Lew, C.; Abdelhady, W.; Lapitan, C.K.; Proctor, R.A.; Rose, W.E.; Bayer, A.S. Synergy Mechanisms of Daptomycin-Fosfomycin Combinations in Daptomycin-Susceptible and -Resistant Methicillin-Resistant Staphylococcus aureus: In Vitro, Ex Vivo, and In Vivo Metrics. Antimicrob. Agents Chemother. 2022, 66, e0164921. [Google Scholar] [CrossRef]
- Omori, K.; Kitagawa, H.; Takada, M.; Maeda, R.; Nomura, T.; Kubo, Y.; Shigemoto, N.; Ohge, H. Fosfomycin as salvage therapy for persistent methicillin-resistant Staphylococcus aureus bacteremia: A case series and review of the literature. J. Infect. Chemother. 2024, 30, 352–356. [Google Scholar] [CrossRef]
- Coronado-Álvarez, N.M.; Parra, D.; Parra-Ruiz, J. Clinical efficacy of fosfomycin combinations against a variety of gram-positive cocci. Enferm. Infecc. Microbiol. Clin. 2019, 37, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Barber, K.E.; Werth, B.J.; Ireland, C.E.; Stone, N.E.; Nonejuie, P.; Sakoulas, G.; Pogliano, J.; Rybak, M.J. Potent synergy of ceftobiprole plus daptomycin against multiple strains of Staphylococcus aureus with various resistance phenotypes. J. Antimicrob. Chemother. 2014, 69, 3006–3010. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Jiang, S.; Sun, L.; Wang, H.; Di, L.; Liu, Y.; Zhang, Y.; Zhuang, H.; Hong, Y.; Wang, Z.; et al. “Seesaw effect” between daptomycin and ceftobiprole in daptomycin-resistant methicillin-resistant Staphylococcus aureus isolates. Int. J. Antimicrob. Agents 2025, 65, 107469. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.J.; Xiong, Y.Q.; Boyle-Vavra, S.; Daum, R.; Jones, T.; Bayer, A.S. Daptomycin-oxacillin combinations in treatment of experimental endocarditis caused by daptomycin-nonsusceptible strains of methicillin-resistant Staphylococcus aureus with evolving oxacillin susceptibility (the “seesaw effect”). Antimicrob. Agents Chemother. 2010, 54, 3161–3169. [Google Scholar] [CrossRef]
- Shafiq, I.; Bulman, Z.P.; Spitznogle, S.L.; Osorio, J.E.; Reilly, I.S.; Lesse, A.J.; Parameswaran, G.I.; Mergenhagen, K.A.; Tsuji, B.T. A combination of ceftaroline and daptomycin has synergistic and bactericidal activity in vitro against daptomycin nonsusceptible methicillin-resistant Staphylococcus aureus (MRSA). Infect. Dis. 2017, 49, 410–416. [Google Scholar] [CrossRef]
- Werth, B.J.; Sakoulas, G.; Rose, W.E.; Pogliano, J.; Tewhey, R.; Rybak, M.J. Ceftaroline increases membrane binding and enhances the activity of daptomycin against daptomycin-nonsusceptible vancomycin-intermediate Staphylococcus aureus in a pharmacokinetic/pharmacodynamic model. Antimicrob. Agents Chemother. 2013, 57, 66–73. [Google Scholar] [CrossRef]
- Barber, K.E.; Smith, J.R.; Ireland, C.E.; Boles, B.R.; Rose, W.E.; Rybak, M.J. Evaluation of Ceftaroline Alone and in Combination against Biofilm-Producing Methicillin-Resistant Staphylococcus aureus with Reduced Susceptibility to Daptomycin and Vancomycin in an In Vitro Pharmacokinetic/Pharmacodynamic Model. Antimicrob. Agents Chemother. 2015, 59, 4497–4503. [Google Scholar] [CrossRef]
- Sakoulas, G.; Moise, P.A.; Casapao, A.M.; Nonejuie, P.; Olson, J.; Okumura, C.Y.; Rybak, M.J.; Kullar, R.; Dhand, A.; Rose, W.E.; et al. Antimicrobial salvage therapy for persistent staphylococcal bacteremia using daptomycin plus ceftaroline. Clin. Ther. 2014, 36, 1317–1333. [Google Scholar] [CrossRef]
- Geriak, M.; Haddad, F.; Rizvi, K.; Rose, W.; Kullar, R.; LaPlante, K.; Yu, M.; Vasina, L.; Ouellette, K.; Zervos, M.; et al. Clinical Data on Daptomycin plus Ceftaroline versus Standard of Care Monotherapy in the Treatment of Methicillin-Resistant Staphylococcus aureus Bacteremia. Antimicrob. Agents Chemother. 2019, 63, 10–1128. [Google Scholar] [CrossRef]
- McCreary, E.K.; Kullar, R.; Geriak, M.; Zasowski, E.J.; Rizvi, K.; Schulz, L.T.; Ouellette, K.; Vasina, L.; Haddad, F.; Rybak, M.J.; et al. Multicenter Cohort of Patients With Methicillin-Resistant Staphylococcus aureus Bacteremia Receiving Daptomycin Plus Ceftaroline Compared With Other MRSA Treatments. Open Forum Infect. Dis. 2020, 7, ofz538. [Google Scholar] [CrossRef]
- Johnson, T.M.; Molina, K.C.; Miller, M.A.; Kiser, T.H.; Huang, M.; Mueller, S.W. Combination ceftaroline and daptomycin salvage therapy for complicated methicillin-resistant Staphylococcus aureus bacteraemia compared with standard of care. Int. J. Antimicrob. Agents 2021, 57, 106310. [Google Scholar] [CrossRef] [PubMed]
- Hicks, A.S.; Dolan, M.A.; Shah, M.D.; Elwood, S.E.; Platts-Mills, J.A.; Madden, G.R.; Elliott, Z.S.; Eby, J.C. Early initiation of ceftaroline-based combination therapy for methicillin-resistant Staphylococcus aureus bacteremia. Ann. Clin. Microbiol. Antimicrob. 2025, 24, 3. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Chen, I.; Lin, L. Comparing the Outcomes of Ceftaroline plus Vancomycin or Daptomycin Combination Therapy versus Vancomycin or Daptomycin Monotherapy in Adults with Methicillin-Resistant Staphylococcus aureus Bacteremia—A Meta-Analysis. Antibiotics 2022, 11, 1104. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.H.; Wang, J.L.; Yin, W.J.; Xu, W.H. Vancomycin or Daptomycin Plus a β-Lactam Versus Vancomycin or Daptomycin Alone for Methicillin-Resistant Staphylococcus aureus Bloodstream Infections: A Systematic Review and Meta-Analysis. Microb. Drug Resist. 2021, 27, 1044–1056. [Google Scholar] [CrossRef]
- Jorgensen, S.C.J.; Zasowski, E.J.; Trinh, T.D.; Lagnf, A.M.; Bhatia, S.; Sabagha, N.; Abdul-Mutakabbir, J.C.; Alosaimy, S.; Mynatt, R.P.; Davis, S.L.; et al. Daptomycin Plus β-Lactam Combination Therapy for Methicillin-resistant Staphylococcus aureus Bloodstream Infections: A Retrospective, Comparative Cohort Study. Clin. Infect. Dis. 2020, 71, 1–10. [Google Scholar] [CrossRef]
- Oltolini, C.; Castiglioni, B.; Tassan Din, C.; Castiglioni, A.; Ossi, C.; La Canna, G.; Pajoro, U.; Scarpellini, P. Meticillin-resistant Staphylococcus aureus endocarditis: First report of daptomycin plus ceftobiprole combination as salvage therapy. Int. J. Antimicrob. Agents 2016, 47, 502–504. [Google Scholar] [CrossRef]
- Tascini, C.; Attanasio, V.; Ripa, M.; Carozza, A.; Pallotto, C.; Bernardo, M.; Francisci, D.; Oltolini, C.; Palmiero, G.; Scarpellini, P. Ceftobiprole for the treatment of infective endocarditis: A case series. J. Glob. Antimicrob. Resist. 2020, 20, 56–59. [Google Scholar] [CrossRef]
- Werth, B.J.; Barber, K.E.; Ireland, C.E.; Rybak, M.J. Evaluation of ceftaroline, vancomycin, daptomycin, or ceftaroline plus daptomycin against daptomycin-nonsusceptible methicillin-resistant Staphylococcus aureus in an in vitro pharmacokinetic/pharmacodynamic model of simulated endocardial vegetations. Antimicrob. Agents Chemother. 2014, 58, 3177–3181. [Google Scholar] [CrossRef]
- Bradley, J.; Glasser, C.; Patino, H.; Arnold, S.R.; Arrieta, A.; Congeni, B.; Daum, R.S.; Kojaoghlanian, T.; Yoon, M.; Anastasiou, D.; et al. Daptomycin for Complicated Skin Infections: A Randomized Trial. Pediatrics 2017, 139, e20162477. [Google Scholar] [CrossRef]
- Arrieta, A.C.; Bradley, J.S.; Popejoy, M.W.; Bensaci, M.; Grandhi, A.; Bokesch, P.; Glasser, C.; Du, L.; Patino, H.; Kartsonis, N.A. Randomized Multicenter Study Comparing Safety and Efficacy of Daptomycin Versus Standard-of-care in Pediatric Patients With Staphylococcal Bacteremia. Pediatr. Infect. Dis. J. 2018, 37, 893–900. [Google Scholar] [CrossRef]
- Bradley, J.S.; Arrieta, A.C.; Digtyar, V.A.; Popejoy, M.W.; Grandhi, A.; Bokesch, P.; Hershberger, E.; Dorr, M.B.; Tan, C.M.; Murata, Y.; et al. Daptomycin for Pediatric Gram-Positive Acute Hematogenous Osteomyelitis. Pediatr. Infect. Dis. J. 2020, 39, 814–823. [Google Scholar] [CrossRef] [PubMed]
- Persha, H.; Thacker, S.A.; Hornback, K.M.; Alvira-Arill, G.R.; Lueking, R.; Morrisette, T. Real-World Clinical Characteristics and Outcomes with Daptomycin Use in Pediatric Patients: A Retrospective Case Series. Antibiotics 2024, 13, 833. [Google Scholar] [CrossRef] [PubMed]
- Olney, K.B.; Howard, J.I.; Burgess, D.S. Daptomycin Dose Optimization in Pediatric Staphylococcus aureus Bacteremia: A Pharmacokinetic/Pharmacodynamic Investigation. J. Clin. Pharmacol. 2024, 64, 860–865. [Google Scholar] [CrossRef] [PubMed]
| Study | Year | Study Design | Population | Main Findings |
|---|---|---|---|---|
| García-de-la-Mària et al. [59] | 2018 | Rabbit endocarditis model | Experimental MRSA infection | DAP+FOS combination improved bacterial clearance |
| Mishra et al. [60] | 2022 | In vitro, ex vivo, in vivo models | DAP-susceptible and DAP-resistant MRSA | Synergy, resensitisation of resistant strains |
| Coronado-Álvarez et al. [62] | 2019 | Retrospective cohort | Patients with persistent bacteraemia | DAP+FOS associated with improved microbiologic outcomes |
| BACSARM trial [31] | 2021 | Randomized controlled trial | MRSA bacteraemia patients | DAP+FOS lowered microbiological failure rates |
| Study | Year | Study Design | Population | Main Findings |
|---|---|---|---|---|
| Werth et al. [79] | 2013 | Pharmacokinetic/pharmacodynamic model | Simulated endocardial vegetations | DAP+CPT enhanced bactericidal activity |
| Sakoulas et al. [69] | 2014 | Case series | Persistent MRSA bacteraemia | Successful salvage therapy with DAP+CPT |
| Geriak et al. [70] | 2019 | Pilot randomized trial | MRSA bacteraemia | Trend towards lower mortality with early use of DAP+CPT |
| Barber et al. [79] | 2014 | In vitro study | MRSA strains | DAP+BPR combination reduced MIC and enhanced killing |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiore, M.; Alfieri, A.; Fiore, D.; Iuliano, P.; Spatola, F.G.; Limone, A.; Pezone, I.; Leone, S. Use of Daptomycin to Manage Severe MRSA Infections in Humans. Antibiotics 2025, 14, 617. https://doi.org/10.3390/antibiotics14060617
Fiore M, Alfieri A, Fiore D, Iuliano P, Spatola FG, Limone A, Pezone I, Leone S. Use of Daptomycin to Manage Severe MRSA Infections in Humans. Antibiotics. 2025; 14(6):617. https://doi.org/10.3390/antibiotics14060617
Chicago/Turabian StyleFiore, Marco, Aniello Alfieri, Daniela Fiore, Pasquale Iuliano, Francesco Giuseppe Spatola, Andrea Limone, Ilaria Pezone, and Sebastiano Leone. 2025. "Use of Daptomycin to Manage Severe MRSA Infections in Humans" Antibiotics 14, no. 6: 617. https://doi.org/10.3390/antibiotics14060617
APA StyleFiore, M., Alfieri, A., Fiore, D., Iuliano, P., Spatola, F. G., Limone, A., Pezone, I., & Leone, S. (2025). Use of Daptomycin to Manage Severe MRSA Infections in Humans. Antibiotics, 14(6), 617. https://doi.org/10.3390/antibiotics14060617








