Misconceptions and Behavioral Risks in Parental Antibiotic Use on Romanian Children: A Cross-Sectional Study on Knowledge, Attitudes, and Practices
Abstract
1. Introduction
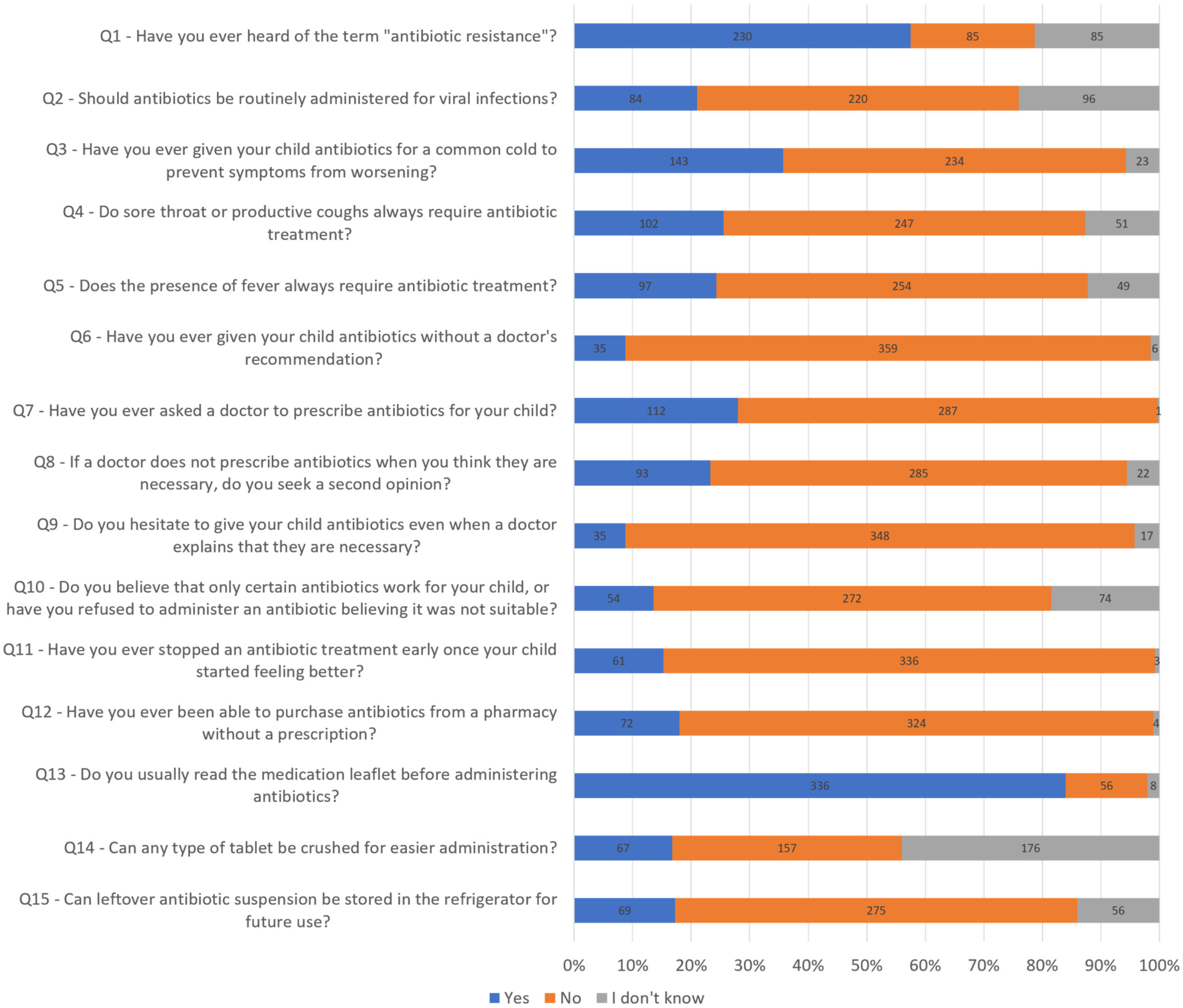
2. Results
3. Discussion
3.1. Knowledge and Misconceptions About Antibiotics
3.2. Self-Medication and Unregulated Antibiotic Use
3.3. Compliance with Antibiotic Treatment
3.4. Implications and Future Directions
4. Materials and Methods
4.1. Study Design and Participants
4.2. Survey Structure
4.3. Survey Questions
- Have you ever heard of the term “antibiotic resistance”?
- Should antibiotics be administered for viral infections?
- Have you ever given your child antibiotics for a common cold to prevent symptoms from worsening?
- Do sore throat or productive coughs always require antibiotic treatment?
- Does the presence of fever always require antibiotic treatment?
- Have you ever given your child antibiotics without a doctor’s recommendation?
- Have you ever asked a doctor to prescribe antibiotics for your child?
- If a doctor does not prescribe antibiotics when you think they are necessary, do you seek a second opinion?
- Do you hesitate to give your child antibiotics even when a doctor explains that they are necessary?
- Do you believe that only certain antibiotics work for your child, or have you refused to administer an antibiotic believing it was not suitable?
- Have you ever stopped an antibiotic treatment early once your child started feeling better?
- Have you ever been able to purchase antibiotics from a pharmacy without a prescription?
- Do you usually read the medication leaflet before administering antibiotics?
- Can any type of tablet be crushed for easier administration?
- Can leftover antibiotic suspension be stored in the refrigerator for future use?
4.4. Data Analysis
4.5. Ethical Considerations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMR | antimicrobial resistance |
| IPC | infection prevention and control |
| ASP | antimicrobial stewardship program(s) |
| AMC | antimicrobial consumption |
| EU/EEA | European Union/European Economic Area |
| WHO | World Health Organization |
References
- World Health Organization (WHO). Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report: 2022; WHO: Geneva, Switzerland, 2022; Available online: https://www.who.int/publications/i/item/9789240062702 (accessed on 1 February 2025).
- European Centre for Disease Prevention and Control (ECDC). Surveillance of Antimicrobial Resistance in Europe, 2023 Data; ECDC: Stockholm, Sweden, 2024; Available online: https://www.ecdc.europa.eu/en/publications-data/surveillance-antimicrobial-resistance-europe-2023-data-executive-summary (accessed on 1 February 2025).
- Centers for Disease Control and Prevention (CDC). Antibiotic Resistance Threats in the United States, 2019; U.S. Department of Health and Human Services, CDC: Atlanta, GA, USA, 2019. Available online: https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf (accessed on 1 February 2025).
- Aslam, B.; Asghar, R.; Muzammil, S.; Shafique, M.; Siddique, A.B.; Khurshid, M.; Ijaz, M.; Rasool, M.H.; Chaudhry, T.H.; Aamir, A.; et al. AMR and Sustainable Development Goals: At a crossroads. Glob. Health 2024, 20, 73. [Google Scholar] [CrossRef] [PubMed]
- Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Balea, L.B.; Glasdam, S. Practices, strategies, and challenges in antibiotic treatment and prevention of antimicrobial resistance from the perspectives of Romanian community pharmacists and general practitioners: A Goffman-inspired qualitative interview study. Front. Antibiot. 2024, 3, 1439688. [Google Scholar] [CrossRef]
- Bernatchez, S.F. Reducing antimicrobial resistance by practicing better infection prevention and control. Am. J. Infect. Control 2023, 51, 1063–1066. [Google Scholar] [CrossRef]
- Abejew, A.A.; Wubetu, G.Y.; Fenta, T.G. Relationship between Antibiotic Consumption and Resistance: A Systematic Review. Can. J. Infect. Dis. Med. Microbiol. 2024, 2024, 9958678. [Google Scholar] [CrossRef] [PubMed]
- Barbu, I.C.; Gheorghe-Barbu, I.; Grigore, G.A.; Vrancianu, C.O.; Chifiriuc, M.C. Antimicrobial Resistance in Romania: Updates on Gram-Negative ESCAPE Pathogens in the Clinical, Veterinary, and Aquatic Sectors. Int. J. Mol. Sci. 2023, 24, 7892. [Google Scholar] [CrossRef]
- Gheorghe, I.; Czobor, I.; Chifiriuc, M.C.; Borcan, E.; Ghiţă, C.; Banu, O.; Lazăr, V.; Mihăescu, G.; Mihăilescu, D.F.; Zhiyong, Z. Molecular screening of carbapenemase-producing Gram-negative strains in Romanian intensive care units during a one year survey. J. Med. Microbiol. 2014, 63 Pt 10, 1303–1310. [Google Scholar] [CrossRef]
- Golli, A.L.; Cristea, O.M.; Zlatian, O.; Glodeanu, A.D.; Balasoiu, A.T.; Ionescu, M.; Popa, S. Prevalence of Multidrug-Resistant Pathogens Causing Bloodstream Infections in an Intensive Care Unit. Infect. Drug Resist. 2022, 15, 5981–5992. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control (ECDC). Antimicrobial Consumption in the EU/EEA (ESAC-Net)—Annual Epidemiological Report for 2023; ECDC: Stockholm, Sweden, 2024; Available online: https://www.ecdc.europa.eu/en/publications-data/antimicrobial-consumption-eueea-esac-net-annual-epidemiological-report-2023 (accessed on 28 February 2025).
- European Commission. Special Eurobarometer 2190: Attitudes of Europeans Towards Antibiotics; European Commission, Updated November 2018. Available online: https://europa.eu/eurobarometer/surveys/detail/2190 (accessed on 25 February 2025).
- Donà, D.; Barbieri, E.; Brigadoi, G.; Liberati, C.; Bosis, S.; Castagnola, E.; Colomba, C.; Galli, L.; Lancella, L.; Lo Vecchio, A.; et al. State of the Art of Antimicrobial and Diagnostic Stewardship in Pediatric Setting. Antibiotics 2025, 14, 132. [Google Scholar] [CrossRef]
- Chai, G.; Governale, L.; McMahon, A.W.; Trinidad, J.P.; Staffa, J.; Murphy, D. Trends of outpatient prescription drug utilization in US children, 2002–2010. Pediatrics 2012, 130, 23–31. [Google Scholar] [CrossRef]
- Butler, A.M.; Brown, D.S.; Durkin, M.J.; Sahrmann, J.M.; Nickel, K.B.; O’Neil, C.A.; Olsen, M.A.; Hyun, D.Y.; Zetts, R.M.; Newland, J.G. Association of Inappropriate Outpatient Pediatric Antibiotic Prescriptions with Adverse Drug Events and Health Care Expenditures. JAMA Netw. Open 2022, 5, e2214153. [Google Scholar] [CrossRef] [PubMed]
- Wattles, B.A.; Smith, M.J.; Feygin, Y.; Jawad, K.; Flinchum, A.; Corley, B.; Spicer, K.B. Inappropriate Prescribing of Antibiotics to Pediatric Patients Receiving Medicaid: Comparison of High-Volume and Non-High-Volume Antibiotic Prescribers-Kentucky, 2019. Healthcare 2023, 11, 2307. [Google Scholar] [CrossRef] [PubMed]
- Nasso, C.; Scarfone, A.; Pirrotta, I.; Rottura, M.; Giorgi, D.A.; Pallio, G.; Irrera, N.; Squadrito, V.; Squadrito, F.; Irrera, P.; et al. Appropriateness of Antibiotic Prescribing in Hospitalized Children: A Focus on the Real-World Scenario of the Different Paediatric Subspecialties. Front. Pharmacol. 2022, 13, 890398. [Google Scholar] [CrossRef]
- Laxminarayan, R.; Duse, A.; Wattal, C.; Zaidi, A.K.M.; Wertheim, H.F.L.; Sumpradit, N.; Vlieghe, E.; Hara, G.L.; Gould, I.M.; Goossens, H.; et al. Antibiotic Resistance—The Need for Global Solutions. Lancet Infect. Dis. 2013, 13, 1057–1098. [Google Scholar] [CrossRef]
- Kiggundu, R.; Lusaya, E.; Seni, J.; Waswa, J.P.; Kakooza, F.; Tjipura, D.; Kikule, K.; Muiva, C.; Joshi, M.P.; Stergachis, A.; et al. Identifying and addressing challenges to antimicrobial use surveillance in the human health sector in low- and middle-income countries: Experiences and lessons learned from Tanzania and Uganda. Antimicrob. Resist. Infect. Control 2023, 12, 9. [Google Scholar] [CrossRef]
- Horwood, J.; Cabral, C.; Hay, A.D.; Ingram, J. Primary care clinician antibiotic prescribing decisions in consultations for children with RTIs: A qualitative interview study. Br. J. Gen. Pract. 2016, 66, e207–e213. [Google Scholar] [CrossRef] [PubMed]
- Marsh, S.A.; Byrne, M.K.; Parsafar, S. What influences parental decisions about antibiotic use with their children: A qualitative study in rural Australia. PLoS ONE 2023, 18, e0288480. [Google Scholar] [CrossRef]
- Chalkidou, A.; Lambert, M.; Cordoba, G.; Taxis, K.; Hansen, M.P.; Bjerrum, L. Misconceptions and Knowledge Gaps on Antibiotic Use and Resistance in Four Healthcare Settings and Five European Countries-A Modified Delphi Study. Antibiotics 2023, 12, 1435. [Google Scholar] [CrossRef]
- Pogurschi, E.N.; Petcu, C.D.; Mizeranschi, A.E.; Zugravu, C.A.; Cirnatu, D.; Pet, I.; Ghimpețeanu, O.-M. Knowledge, Attitudes and Practices Regarding Antibiotic Use and Antibiotic Resistance: A Latent Class Analysis of a Romanian Population. Int. J. Environ. Res. Public Health 2022, 19, 7263. [Google Scholar] [CrossRef]
- Ghiga, I.; Pitchforth, E.; Stålsby Lundborg, C.; Machowska, A. Family doctors’ roles and perceptions on antibiotic consumption and antibiotic resistance in Romania: A qualitative study. BMC Prim. Care 2023, 24, 93. [Google Scholar] [CrossRef]
- Tangcharoensathien, V.; Chanvatik, S.; Kosiyaporn, H.; Kirivan, S.; Kaewkhankhaeng, W.; Thunyahan, A.; Lekagul, A. Population knowledge and awareness of antibiotic use and antimicrobial resistance: Results from national household survey 2019 and changes from 2017. BMC Public Health 2021, 21, 2188. [Google Scholar] [CrossRef] [PubMed]
- Auta, A.; Hadi, M.A.; Oga, E.; Adewuyi, E.O.; Abdu-Aguye, S.N.; Adeloye, D.; Strickland-Hodge, B.; Morgan, D.J. Global access to antibiotics without prescription in community pharmacies: A systematic review and meta-analysis. J. Infect. 2019, 78, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, G.V.; Roberts, R.M.; Albert, A.P.; Johnson, D.D.; Hicks, L.A. Effects of knowledge, attitudes, and practices of primary care providers on antibiotic selection, United States. Emerg. Infect. Dis. 2014, 20, 2041–2047. [Google Scholar] [CrossRef] [PubMed]
- Al-Makramani, A.A.; Shawish, A.M.; Sabi, A.Y.; Ghareeb, H.I.; Faqih, F.A.; Daghas, H.A.; Bahri, N.A.; Muyidi, A.Y.; Bajawi, R.H.; Maghrabi, E.A.; et al. Parental Awareness of Antibiotic Use for Upper Respiratory Tract Infection in Children in Jazan Region, Saudi Arabia. Cureus 2024, 16, e69438. [Google Scholar] [CrossRef]
- Oikonomou, M.E.; Gkentzi, D.; Karatza, A.; Fouzas, S.; Vervenioti, A.; Dimitriou, G. Parental Knowledge, Attitude, and Practices on Antibiotic Use for Childhood Upper Respiratory Tract Infections during COVID-19 Pandemic in Greece. Antibiotics 2021, 10, 802. [Google Scholar] [CrossRef]
- Masadeh, M.; Harun, S.N.; Mukattash, T.; Alrabadi, N. Parental Knowledge and Attitudes Towards Antibiotic Resistance in Children: A Review Article. Curr. Pediatr. Rev. 2025, in press. [Google Scholar] [CrossRef]
- Pierantoni, L.; Vecchio, A.L.; Lenzi, J.; Corsi, V.; Campana, L.; Trobia, G.L.; Amendolea, A.; Di Felice, B.; Alighieri, G.; Fabrizio, G.C.; et al. Parents’ Perspective of Antibiotic Usage in Children: A Nationwide Survey in Italy. Pediatr. Infect. Dis. J. 2021, 40, 906–911. [Google Scholar] [CrossRef]
- Chanvatik, S.; Kosiyaporn, H.; Lekagul, A.; Kaewkhankhaeng, W.; Vongmongkol, V.; Thunyahan, A.; Tangcharoensathien, V. Knowledge and use of antibiotics in Thailand: A 2017 national household survey. PLoS ONE 2019, 14, e0220990. [Google Scholar] [CrossRef]
- Tsuzuki, S.; Fujitsuka, N.; Horiuchi, K.; Ijichi, S.; Gu, Y.; Fujitomo, Y.; Takahashi, R.; Ohmagari, N. Factors associated with sufficient knowledge of antibiotics and antimicrobial resistance in the Japanese general population. Sci. Rep. 2020, 10, 3502. [Google Scholar] [CrossRef]
- Waaseth, M.; Adan, A.; Røen, I.L.; Eriksen, K.; Stanojevic, T.; Halvorsen, K.H.; Garcia, B.H.; Holst, L.; Ulshagen, K.M.; Blix, H.S.; et al. Knowledge of antibiotics and antibiotic resistance among Norwegian pharmacy customers—A cross-sectional study. BMC Public Health 2019, 19, 66. [Google Scholar] [CrossRef]
- Ranji, S.R.; Steinman, M.A.; Shojania, K.G.; Gonzales, R. Interventions to reduce unnecessary antibiotic prescribing: A systematic review and quantitative analysis. Med. Care 2008, 46, 847–862. [Google Scholar] [CrossRef] [PubMed]
- Mallah, N.; Orsini, N.; Figueiras, A.; Takkouche, B. Education level and misuse of antibiotics in the general population: A systematic review and dose–response meta-analysis. Antimicrob. Resist. Infect. Control 2022, 11, 24. [Google Scholar] [CrossRef] [PubMed]
- Glibić, M.; Bedeković, L.; Maglica, M.; Marijanović, I.; Vukoja, D. Behavioral and Knowledge Patterns Regarding the Use of Antibiotics Among Urban and Rural Population in Bosnia and Herzegovina-a Cross-sectional Study. Mater. Sociomed. 2023, 35, 33–41. [Google Scholar] [CrossRef]
- Kamata, K.; Tokuda, Y.; Gu, Y.; Ohmagari, N.; Yanagihara, K. Public knowledge and perception about antimicrobials and antimicrobial resistance in Japan: A national questionnaire survey in 2017. PLoS ONE 2018, 13, e0207017. [Google Scholar] [CrossRef] [PubMed]
- Tsuzuki, S.; Koizumi, R.; Matsunaga, N.; Ohmagari, N. Decline in Antimicrobial Consumption and Stagnation in Reducing Disease Burden due to Antimicrobial Resistance in Japan. Infect. Dis. Ther. 2023, 12, 1823–1834. [Google Scholar] [CrossRef]
- Machowska, A.; Stålsby Lundborg, C. Drivers of Irrational Use of Antibiotics in Europe. Int. J. Environ. Res. Public Health 2018, 16, 27. [Google Scholar] [CrossRef]
- Iuhas, A.; Marinău, C.; Niulaș, L.; Futaki, Z.; Balmoș, A.; Kozma, K.; Indrieș, M.; Sava, C. Familial Mediterranean fever in Romania: A case report and literature review. Front. Pediatr. 2025, 12, 1546387. [Google Scholar] [CrossRef]
- Marinău, C.; Csep, A.; Sava, C.; Iuhas, A.; Niulaș, L.; Szilagyi, A.; Ritli, L.; Balmoș, A.; Jurca, C. Difficulties in the management of an Askin tumor in a pediatric patient with cystic fibrosis: Case report and literature review. Front. Pediatr. 2023, 11, 1289256. [Google Scholar] [CrossRef]
- European Commission. Antimicrobial Resistance and Causes of Non-Prudent Use of Antibiotics in Human Medicine in the EU; European Commission: Brussels, Belgium, 2016. [Google Scholar]
- Ministry of Health of Romania. Ministerial Order No. 63/2024 on the Regulation and Monitoring of the Prescription and Dispensing of Antibiotics and Antifungals. 2024. Available online: https://legislatie.just.ro/public/DetaliiDocument/278215 (accessed on 16 March 2025).
- Ghiga, I.; Stålsby Lundborg, C. ‘Struggling to be a defender of health’—A qualitative study on the pharmacists’ perceptions of their role in antibiotic consumption and antibiotic resistance in Romania. J. Pharm. Policy Pract. 2016, 9, 10. [Google Scholar] [CrossRef]
- Sava, C.N.; Bodog, T.-M.; Niulas, L.R.; Iuhas, A.R.; Marinau, C.P.; Negrut, N.; Balmos, A.B.; Pasca, B.; Roman, N.A.; Nistor-Cseppento, C.D. Biomarker Changes in Pediatric Patients With COVID-19: A Retrospective Study from a Single Center Database. In Vivo 2022, 36, 2813–2822. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Overuse of Antibiotics in COVID-19 Patients Worsens Antimicrobial Resistance Crisis; WHO: Geneva, Switzerland, 2024; Available online: https://www.who.int/news/item/26-04-2024-who-reports-widespread-overuse-of-antibiotics-in-patients--hospitalized-with-covid-19 (accessed on 25 February 2025).
- Konstantinidis, T.; Tsigalou, C.; Karvelas, A.; Stavropoulou, E.; Voidarou, C.; Bezirtzoglou, E. Effects of Antibiotics upon the Gut Microbiome: A Review of the Literature. Biomedicines 2020, 8, 502. [Google Scholar] [CrossRef] [PubMed]
- Stivers, T. Participating in decisions about treatment: Overt parent pressure for antibiotic medication in pediatric encounters. Soc. Sci. Med. 2002, 54, 1111–1130. [Google Scholar] [CrossRef] [PubMed]
- Stivers, T.; Mangione-Smith, R.; Elliott, M.N.; McDonald, L.; Heritage, J. Why do physicians think parents expect antibiotics? What parents report vs what physicians believe. J. Fam. Pract. 2003, 52, 140–148. [Google Scholar] [PubMed]
- Stivers, T. Managing Patient Pressure to Prescribe Antibiotics in the Clinic. Paediatr. Drugs 2021, 23, 437–443. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Antimicrobial Resistance: Does Stopping a Course of Antibiotics Early Lead to Antibiotic Resistance? WHO: Geneva, Switzerland, 2024; Available online: https://www.who.int/news-room/questions-and-answers/item/antimicrobial-resistance-does-stopping-a-course-of-antibiotics-early-lead-to-antibiotic-resistance (accessed on 21 March 2025).
- Souvatzi, E.; Katsikidou, M.; Arvaniti, A.; Plakias, S.; Tsiakiri, A.; Samakouri, M. Trust in Healthcare, Medical Mistrust, and Health Outcomes in Times of Health Crisis: A Narrative Review. Societies 2024, 14, 269. [Google Scholar] [CrossRef]
- Iuhas, A.; Jurca, C.; Kozma, K.; Riza, A.-L.; Streață, I.; Petcheși, C.; Dan, A.; Sava, C.; Balmoș, A.; Marinău, C.; et al. PAH Pathogenic Variants and Clinical Correlations in a Group of Hyperphenylalaninemia Patients from North-Western Romania. Diagnostics 2023, 13, 1483. [Google Scholar] [CrossRef]
- Sava, C.; Sava, M.; Drăgan, A.-M.; Iuhas, A.; Niulaș, L.; Marinău, C.P.; Balmoș, A.B. The Use of Xpert MTB/RIF Ultra Testing for Early Diagnosis of Tuberculosis: A Retrospective Study from a Single-Center Database. Genes 2023, 14, 1231. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iuhas, A.; Galiș, R.; Rus, M.; Balmoș, A.; Marinău, C.; Niulaș, L.; Futaki, Z.; Matioc, D.; Sava, C. Misconceptions and Behavioral Risks in Parental Antibiotic Use on Romanian Children: A Cross-Sectional Study on Knowledge, Attitudes, and Practices. Antibiotics 2025, 14, 479. https://doi.org/10.3390/antibiotics14050479
Iuhas A, Galiș R, Rus M, Balmoș A, Marinău C, Niulaș L, Futaki Z, Matioc D, Sava C. Misconceptions and Behavioral Risks in Parental Antibiotic Use on Romanian Children: A Cross-Sectional Study on Knowledge, Attitudes, and Practices. Antibiotics. 2025; 14(5):479. https://doi.org/10.3390/antibiotics14050479
Chicago/Turabian StyleIuhas, Alin, Radu Galiș, Marius Rus, Andreea Balmoș, Cristian Marinău, Larisa Niulaș, Zsolt Futaki, Dorina Matioc, and Cristian Sava. 2025. "Misconceptions and Behavioral Risks in Parental Antibiotic Use on Romanian Children: A Cross-Sectional Study on Knowledge, Attitudes, and Practices" Antibiotics 14, no. 5: 479. https://doi.org/10.3390/antibiotics14050479
APA StyleIuhas, A., Galiș, R., Rus, M., Balmoș, A., Marinău, C., Niulaș, L., Futaki, Z., Matioc, D., & Sava, C. (2025). Misconceptions and Behavioral Risks in Parental Antibiotic Use on Romanian Children: A Cross-Sectional Study on Knowledge, Attitudes, and Practices. Antibiotics, 14(5), 479. https://doi.org/10.3390/antibiotics14050479





