Impact of Timing of Beta-Lactam Therapeutic Drug Monitoring and Therapy Adjustment in Critically Ill Patients
Abstract
1. Introduction
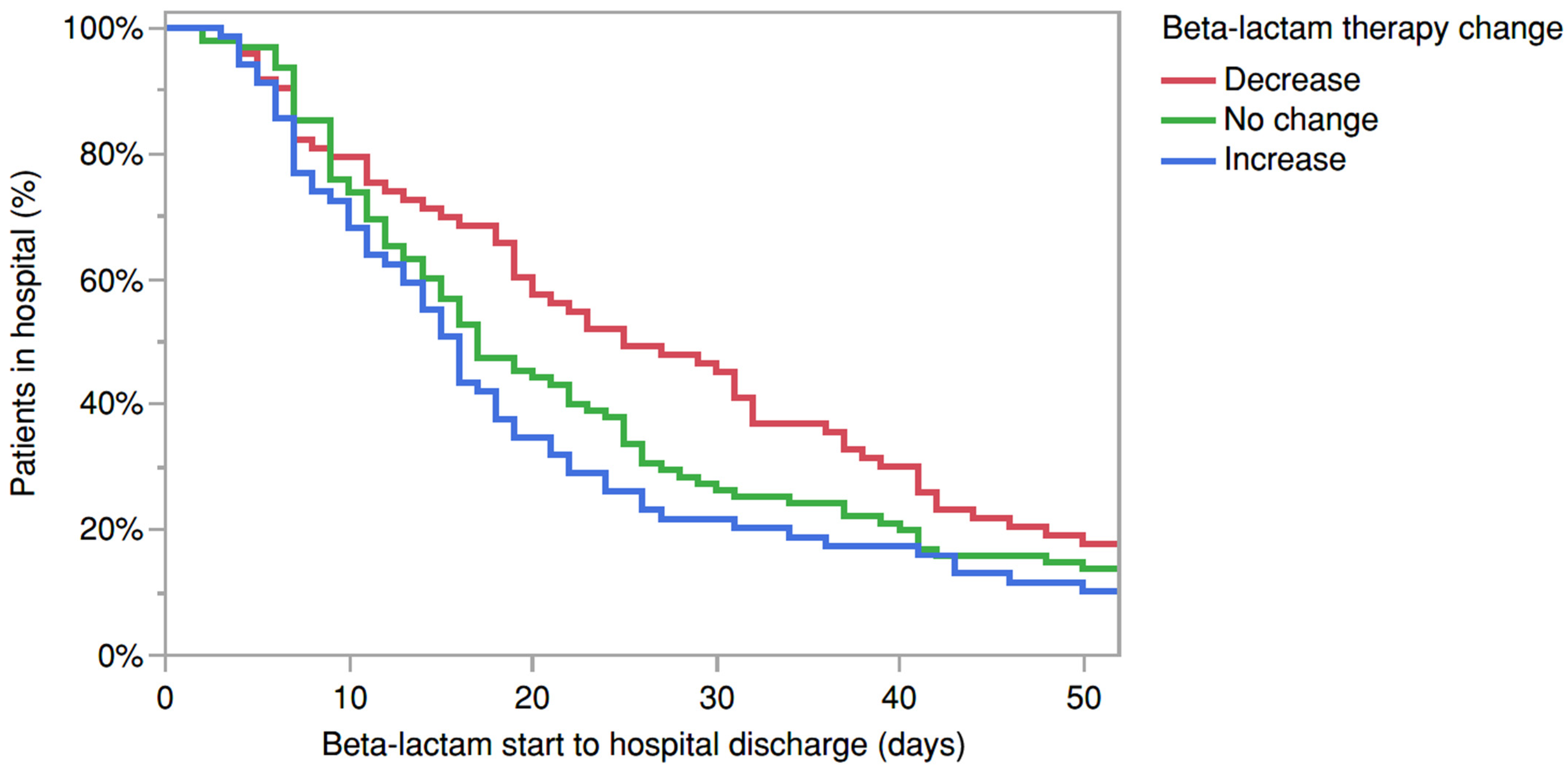
2. Results
3. Discussion
4. Methods
Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Magill, S.S.; O’Leary, E.; Ray, S.M.; Kainer, M.A.; Evans, C.; Bamberg, W.M.; Johnston, H.; Janelle, S.J.; Oyewumi, T.; Lynfield, R.; et al. Antimicrobial Use in US Hospitals: Comparison of Results from Emerging Infections Program Prevalence Surveys, 2015 and 2011. Clin. Infect. Dis. 2021, 72, 1784–1792. [Google Scholar] [CrossRef]
- Craig, W.A. Pharmacokinetic/pharmacodynamic parameters: Rationale for antibacterial dosing of mice and men. Clin. Infect. Dis. 1998, 26, 1–10. [Google Scholar] [CrossRef]
- Mouton, J.W.; den Hollander, J.G. Killing of Pseudomonas aeruginosa during continuous and intermittent infusion of ceftazidime in an in vitro pharmacokinetic model. Antimicrob. Agents Chemother. 1994, 38, 931–936. [Google Scholar] [CrossRef] [PubMed]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; Mcintyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021, 47, 1181–1247. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Roberts, D.; Wood, K.E.; Light, B.; Parrillo, J.E.; Sharma, S.; Suppes, R.; Feinstein, D.; Zanotti, S.; Taiberg, L.; et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit. Care Med. 2006, 34, 1589–1596. [Google Scholar] [CrossRef] [PubMed]
- Harbarth, S.; Garbino, J.; Pugin, J.; Romand, J.A.; Lew, D.; Pittet, D. Inappropriate initial antimicrobial therapy and its effect on survival in a clinical trial of immunomodulating therapy for severe sepsis. Am. J. Med. 2003, 115, 529–535. [Google Scholar] [CrossRef]
- MacArthur, R.D.; Miller, M.; Albertson, T.; Panacek, E.; Johnson, D.; Teoh, L.; Barchuk, W. Adequacy of Early Empiric Antibiotic Treatment and Survival in Severe Sepsis: Experience from the MONARCS Trial. Clin. Infect. Dis. 2004, 38, 284–288. [Google Scholar] [CrossRef]
- Abdul-Aziz, M.H.; Alffenaar, J.-W.C.; Bassetti, M.; Bracht, H.; Dimopoulos, G.; Marriott, D.; Neely, M.N.; Paiva, J.-A.; Pea, F.; Sjovall, F.; et al. Antimicrobial therapeutic drug monitoring in critically ill adult patients: A Position Paper. Intensive Care Med. 2020, 46, 1127–1153. [Google Scholar] [CrossRef]
- Roberts, J.A.; Abdul-Aziz, M.H.; Lipman, J.; Mouton, J.W.; Vinks, A.A.; Felton, T.W.; Hope, W.W.; Farkas, A.; Neely, M.N.; Schentag, J.J.; et al. Individualised antibiotic dosing for patients who are critically ill: Challenges and potential solutions. Lancet Infect. Dis. 2014, 14, 498–509. [Google Scholar] [CrossRef]
- Roberts, J.A.; Paul, S.K.; Akova, M.; Bassetti, M.; De Waele, J.J.; Dimopoulos, G.; Kaukonen, K.-M.; Koulenti, D.; Martin, C.; Montravers, P.; et al. DALI: Defining antibiotic levels in intensive care unit patients: Are current β-lactam antibiotic doses sufficient for critically ill patients? Clin. Infect. Dis. 2014, 58, 1072–1083. [Google Scholar] [CrossRef]
- Al-Shaer, M.H.; Rubido, E.; Cherabuddi, K.; Venugopalan, V.; Klinker, K.; Peloquin, C. Early therapeutic monitoring of β-lactams and associated therapy outcomes in critically ill patients. J. Antimicrob. Chemother. 2020, 75, 3644–3651. [Google Scholar] [CrossRef] [PubMed]
- Huttner, A.; Von Dach, E.; Renzoni, A.; Huttner, B.D.; Affaticati, M.; Pagani, L.; Daali, Y.; Pugin, J.; Karmime, A.; Fathi, M.; et al. Augmented renal clearance, low β-lactam concentrations and clinical outcomes in the critically ill: An observational prospective cohort study. Int. J. Antimicrob. Agents 2015, 45, 385–392. [Google Scholar] [CrossRef]
- Taccone, F.S.; Laterre, P.-F.; Dugernier, T.; Spapen, H.; Delattre, I.; Witebolle, X.; De Backer, D.; Layeux, B.; Wallemacq, P.; Vincent, J.-L.; et al. Insufficient β-lactam concentrations in the early phase of severe sepsis and septic shock. Crit. Care 2010, 14, R126. [Google Scholar] [CrossRef] [PubMed]
- Hagel, S.; Bach, F.; Brenner, T.; Bracht, H.; Brinkmann, A.; Annecke, T.; Hohn, A.; Weigand, M.; Michels, G.; Kluge, S.; et al. Effect of therapeutic drug monitoring-based dose optimization of piperacillin/tazobactam on sepsis-related organ dysfunction in patients with sepsis: A randomized controlled trial. Intensive Care Med. 2022, 48, 311–321. [Google Scholar] [CrossRef]
- Alshaer, M.H.; Maranchick, N.; Bai, C.; Maguigan, K.L.; Shoulders, B.; Felton, T.W.; Mathew, S.K.; Mardini, M.T.; Peloquin, C.A. Using Machine Learning to Define the Impact of Beta-Lactam Early and Cumulative Target Attainment on Outcomes in Intensive Care Unit Patients with Hospital-Acquired and Ventilator-Associated Pneumonia. Antimicrob. Agents Chemother. 2022, 66, e0056322. [Google Scholar] [CrossRef]
- Alshaer, M.H.; Maranchick, N.; Alexander, K.M.; Manigaba, K.; Shoulders, B.R.; Felton, T.W.; Mathew, S.K.; Peloquin, C.A. Beta-lactam target attainment and associated outcomes in patients with bloodstream infections. Int. J. Antimicrob. Agents 2023, 61, 106727. [Google Scholar] [CrossRef] [PubMed]
- Venugopalan, V.; Hamza, M.; Santevecchi, B.; DeSear, K.; Cherabuddi, K.; Peloquin, C.A.; Alshaer, M.H. Implementation of a β-lactam therapeutic drug monitoring program: Experience from a large academic medical center. Am. J. Health-Syst. Pharm. 2022, 79, 1586–1591. [Google Scholar] [CrossRef]
- Wong, G.; Briscoe, S.; Adnan, S.; McWhinney, B.; Ungerer, J.; Lipman, J.; Roberts, J.A. Protein Binding of β-Lactam Antibiotics in Critically Ill Patients: Can We Successfully Predict Unbound Concentrations? Antimicrob. Agents Chemother. 2013, 57, 6165–6170. [Google Scholar] [CrossRef]
- Kessler, R.E.; Bies, M.; Buck, R.E.; Chisholm, D.R.; Pursiano, T.A.; Tsai, Y.H.; Misiek, M.; Price, K.E.; Leitner, F. Comparison of a new cephalosporin, BMY 28142, with other broad-spectrum beta-lactam antibiotics. Antimicrob. Agents Chemother. 1985, 27, 207–216. [Google Scholar] [CrossRef]
- Craig, W.A. The pharmacology of meropenem, a new carbapenem antibiotic. Clin. Infect. Dis. 1997, 24 (Suppl. 2), S266–S275. [Google Scholar] [CrossRef]
- Swabb, E.A. Review of the clinical pharmacology of the monobactam antibiotic aztreonam. Am. J. Med. 1985, 78, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Baxter Healthcare. Oxacillin Injection [Prescribing Information]; Baxter Healthcare: Deerfield, IL, USA, 2017. [Google Scholar]
| Characteristic | Total (n = 268) | Increase (n = 71) | Decrease (n = 79) | No Change (n = 118) | p-Value |
|---|---|---|---|---|---|
| Age, years | 56 (17) | 55 (18) | 58 (15) | 56 (18) | 0.32 |
| Weight, kg | 82 (32) | 84 (35) | 86 (38) | 77 (24) | 0.07 |
| Male | 152 (57) | 48 (68) | 36 (46) | 68 (58) | 0.10 |
| Baseline serum creatinine, mg/dL | 1.26 (1.14) | 0.95 (0.82) | 1.45 (0.93) | 1.27 (1.31) | 0.05 |
| Baseline SOFA score | 6 (4) | 5 (3) | 6 (4) | 6 (4) | 0.13 |
| ICU length of stay, days | 15 (7–30) | 24 (38) | 24 (23) | 23 (31) | 0.78 |
| Hospital length of stay, days | 22 (13–41) | 29 (39) | 32 (31) | 31 (34) | 0.61 |
| Renal replacement therapy, yes | 38 (14) | 7 (10) | 8 (10) | 23 (19) | 0.08 |
| Most common infection source | N = 297 | N = 75 | N = 93 | N = 129 | |
| Lung | 131 (44) | 28 (37) | 38 (41) | 65 (50) | 0.15 |
| Bacteremia | 51 (17) | 14 (19) | 18 (19) | 19 (15) | 0.62 |
| Skin/soft tissue | 26 (9) | 10 (13) | 10 (11) | 6 (5) | 0.08 |
| Intra-abdominal | 20 (7) | 1 (1) | 9 (10) | 10 (8) | 0.08 |
| Urinary tract | 18 (6) | 3 (4) | 5 (5) | 10 (8) | 0.53 |
| Bone/joint | 14 (5) | 8 (11) | 3 (3) | 3 (2) | 0.02 |
| Endocarditis | 12 (4) | 3 (4) | 4 (4) | 5 (4) | 0.99 |
| Concomitant antimicrobials | |||||
| Aminoglycoside | 9 (3) | 1 (1) | 3 (3) | 5 (4) | 0.31 |
| Daptomycin | 6 (2) | 0 (0) | 2 (2) | 4 (3) | 0.37 |
| Fluoroquinolone | 2 (<1) | 0 (0) | 0 (0) | 2 (20) | 0.99 |
| Linezolid | 24 (8) | 1 (1) | 10 (11) | 13 (10) | 0.05 |
| TMP/SMZ | 9 (3) | 0 (0) | 3 (3) | 6 (5) | 0.88 |
| Vancomycin | 68 (23) | 19 (25) | 18 (19) | 31 (24) | 0.57 |
| Most common isolated bacteria † | |||||
| Pseudomonas aeruginosa | 115 (2) | 37 (4) | 34 (1) | 44 (3) | 0.84 |
| Escherichia coli | 46 (1) | 12 (2) | 19 (0.25) | 15 (1) | 0.14 |
| Klebsiella pneumoniae | 39 (1) | 4 (0.25) | 17 (1) | 18 (1) | 0.04 |
| Staphylococcus aureus | 30 (2) | 21 (0.5) | 2 (1.25) | 7 (2) | <0.0001 |
| Enterobacter cloacae | 26 (1) | 3 (1) | 6 (1) | 17 (1) | 0.03 |
| Proteus mirabilis | 16 (1) | 3 (1) | 10 (1) | 3 (1) | 0.03 |
| Serratia marcescens | 14 (1) | 2 (1.5) | 9 (1) | 3 (1) | 0.04 |
| Empiric Breakpoints used, n | 79 (26) | 22 | 20 | 37 | 0.17 |
| Predictors | Univariate Analysis—Clinical Cure, ORs (95% CI) | Multiple Regression—Clinical Cure, aORs (95% CI) |
|---|---|---|
| SOFA score | 0.94 (0.88, 1.003) | - |
| RRT, yes | 0.57 (0.30, 1.11) | - |
| Age | 0.99 (0.97, 1.002) | - |
| Days to TDM | 0.92 (0.88–0.98) * | 0.92 (0.88, 0.98) * |
| Baseline SCr, mg/dL | 0.91 (0.73–1.13) | - |
| Regimen change | ||
| Increase vs. no change | - | 1.17 (0.60, 2.30) |
| Decrease vs. no change | - | 1.25 (0.67, 2.33) |
| Increase vs. decrease | - | 0.94 (0.45, 1.95) |
| Predictors | Univariate Analysis, ORs (95% CI) | Multivariate Analysis, aORs (95% CI) |
|---|---|---|
| SOFA score | 1.17 (1.09, 1.27) * | 1.14 (1.04, 1.25)* |
| RRT, yes | 3.59 (1.85, 6.96) * | 2.07 (0.91, 4.67) |
| Age | 1.04 (1.02, 1.06) * | 1.05 (1.02–1.07) * |
| Days to TDM | 0.93 (0.86, 1.02) | - |
| Baseline SCr, mg/dL | 1.17 (0.94, 1.46) | - |
| Infection source, intra-abdominal | 4.54 (1.79, 11.49) * | 4.82 (1.53, 15.21) * |
| Regimen change | ||
| Increase vs. no change | - | 0.36 (0.13, 0.97) * |
| Decrease vs. no change | - | 0.67 (0.33, 1.35) |
| Increase vs. decrease | - | 0.45 (0.16, 1.24) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alshaer, M.H.; Maranchick, N.F.; Maguigan, K.L.; Shoulders, B.R.; Mousa, M.J.; Murray, M.; Ashton, J.; Alexander, K.; Santevecchi, B.A.; DeSear, K.; et al. Impact of Timing of Beta-Lactam Therapeutic Drug Monitoring and Therapy Adjustment in Critically Ill Patients. Antibiotics 2025, 14, 463. https://doi.org/10.3390/antibiotics14050463
Alshaer MH, Maranchick NF, Maguigan KL, Shoulders BR, Mousa MJ, Murray M, Ashton J, Alexander K, Santevecchi BA, DeSear K, et al. Impact of Timing of Beta-Lactam Therapeutic Drug Monitoring and Therapy Adjustment in Critically Ill Patients. Antibiotics. 2025; 14(5):463. https://doi.org/10.3390/antibiotics14050463
Chicago/Turabian StyleAlshaer, Mohammad H., Nicole F. Maranchick, Kelly L. Maguigan, Bethany R. Shoulders, Mays J. Mousa, Melissa Murray, Jennifer Ashton, Kaitlin Alexander, Barbara A. Santevecchi, Kathryn DeSear, and et al. 2025. "Impact of Timing of Beta-Lactam Therapeutic Drug Monitoring and Therapy Adjustment in Critically Ill Patients" Antibiotics 14, no. 5: 463. https://doi.org/10.3390/antibiotics14050463
APA StyleAlshaer, M. H., Maranchick, N. F., Maguigan, K. L., Shoulders, B. R., Mousa, M. J., Murray, M., Ashton, J., Alexander, K., Santevecchi, B. A., DeSear, K., Venugopalan, V., Cherabuddi, K., & Peloquin, C. A. (2025). Impact of Timing of Beta-Lactam Therapeutic Drug Monitoring and Therapy Adjustment in Critically Ill Patients. Antibiotics, 14(5), 463. https://doi.org/10.3390/antibiotics14050463





