Chronic Heat Stress Can Induce Conjugation of a Novel ermB-Containing ICEFZMF, Increasing Resistance to Erythromycin Among Enterococcus Strains in Diverse Intestinal Segments in the Mouse Model
Abstract
1. Introduction
2. Results and Discussion
2.1. Validation of Heat Stress Model via Molecular Markers of Intestinal Barrier Disruption and Inflammation
2.2. Histopathological Validation of Heat Stress-Induced Intestinal Damage in Mice
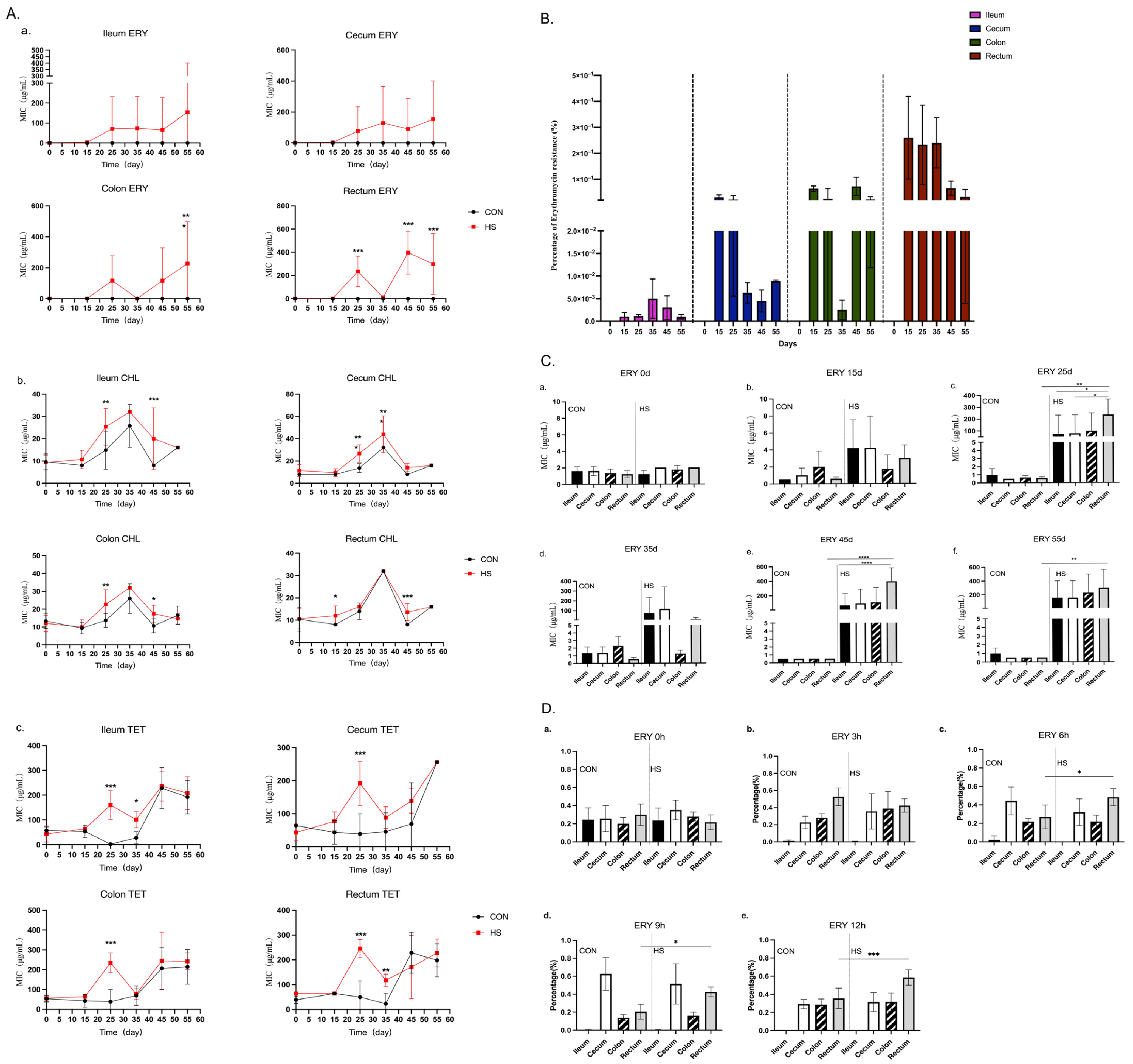
2.3. Determination of MICs for Enterococcus Strains
2.4. Antibiotic Resistance Gene (ARG) Screen and Clonal Relatedness Analysis
2.5. Characterization of a Novel ICEFZMF and Its Transferability
2.6. Heat Stress Facilitated Enhanced Rectal Colonization of Erythromycin-Resistant Enterococcus ER01
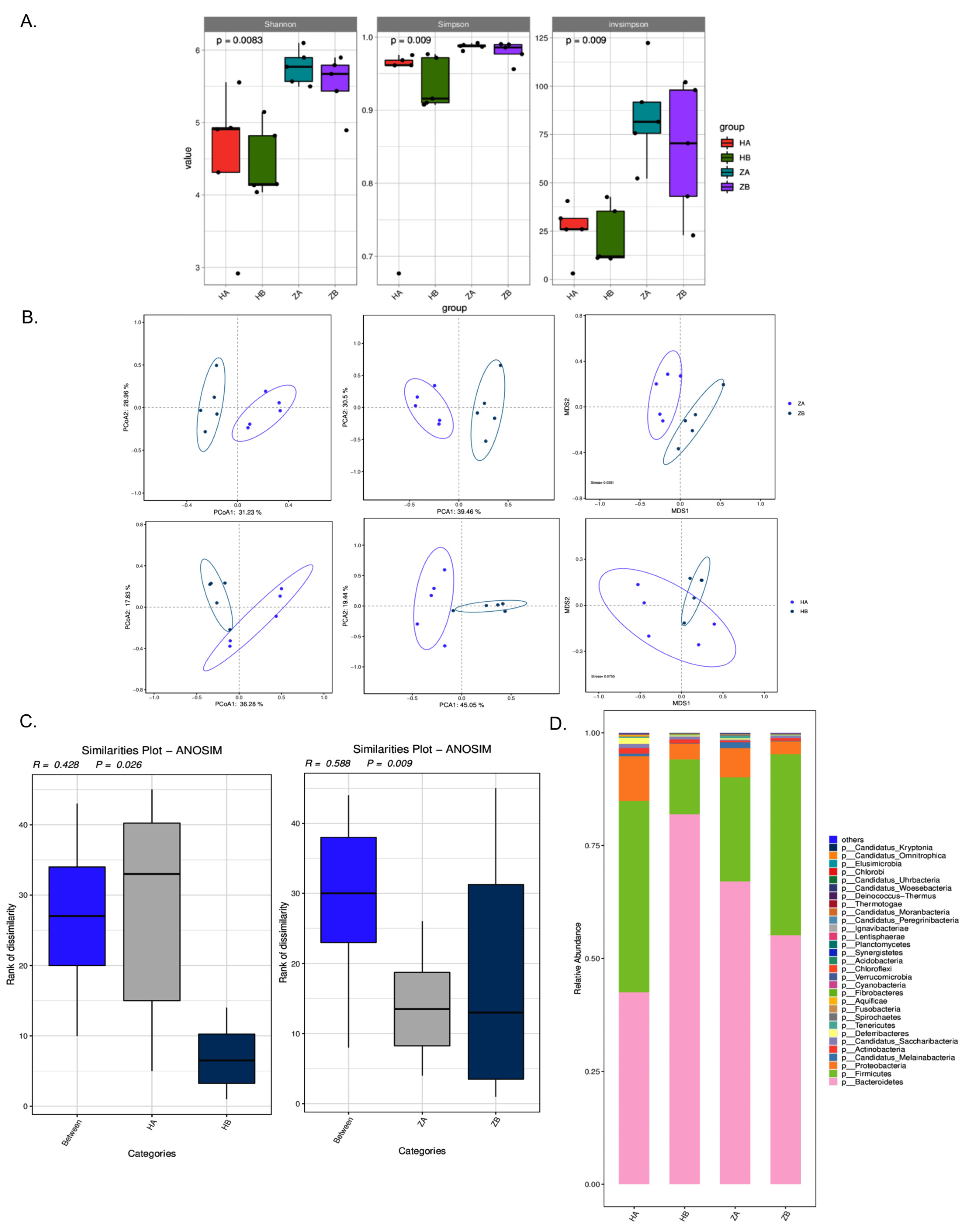
2.7. Effects of Heat Stress on Microbiota Composition in Different Intestinal Segments of Mice
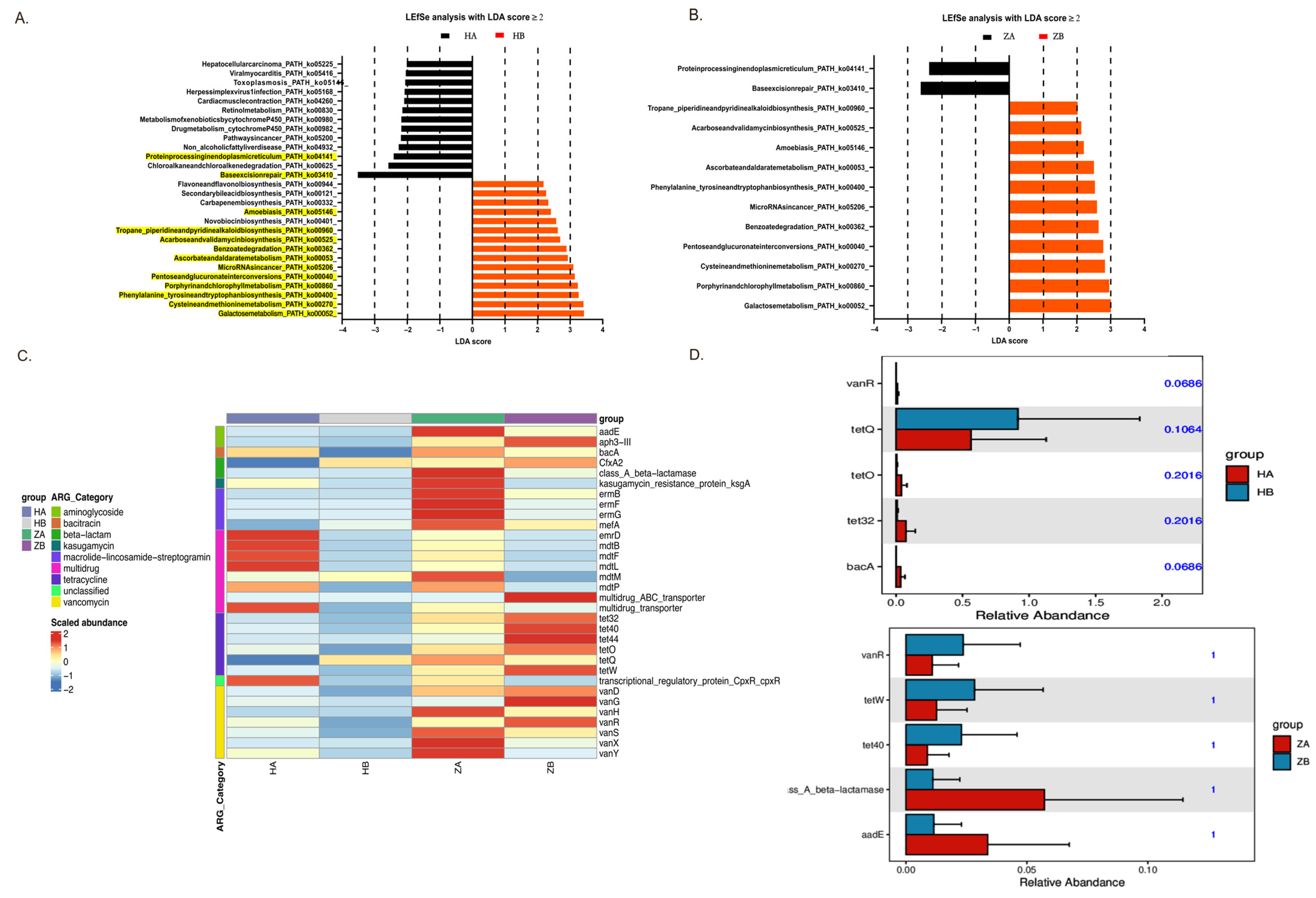
2.8. Metabolic Pathway Alterations in Response to Heat Stress
2.9. Effects of Heat Stress on Antimicrobial Resistance Genes in Gut Microbiota
3. Methods
3.1. Establishment of a Mouse Heat Stress Model
3.2. Histological Analysis
3.3. Detection of mRNA Expression Levels of Heat Shock Proteins, Markers of Intestinal Stress, Integrity and Inflammatory
3.4. Sampling and Isolation of Intestinal Tissues and Bacteria
3.5. Determination of Minimum Inhibitory Concentrations (MICs) of Enterococcus Strains
3.6. The Evaluation of the Genetic Variation of Enterococcus Species Through Enterobacterial Repetitive Intergenic Consensus Sequence PCR (ERIC-PCR)
3.7. Whole Genome Sequencing, Amplification Analysis and Antibiotic Resistance Gene (ARG) Detection
3.8. Transfer Experiments
3.9. Intestinal Colonization with Erythromycin-Resistant Enterococcus and Heat Stress in Mice
3.10. Metagenomic Sequencing and Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hu, H.; Bai, X.; Shah, A.A.; Wen, A.Y.; Hua, J.L.; Che, C.Y.; He, S.J.; Jiang, J.P.; Cai, Z.H.; Dai, S.F. Dietary supplementation with glutamine and gamma-aminobutyric acid improves growth performance and serum parameters in 22- to 35-day-old broilers exposed to hot environment. J. Anim. Physiol. Anim. Nutr. 2016, 100, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Jindal, A.K.; Pandya, K.; Khan, I.D. Antimicrobial resistance: A public health challenge. Med. J. Armed Forces India 2015, 71, 178–181. [Google Scholar] [CrossRef] [PubMed]
- Lane, J.K.; Kelly, T.; Bird, B.; Chenais, E.; Roug, A.; Vidal, G.; Gallardo, R.; Zhou, H.; VanHoy, G.; Smith, W. A One Health Approach to Reducing Livestock Disease Prevalence in Developing Countries: Advances, Challenges, and Prospects. Annu. Rev. Anim. Biosci. 2025, 13, 277–302. [Google Scholar] [CrossRef] [PubMed]
- Bava, R.; Castagna, F.; Lupia, C.; Poerio, G.; Liguori, G.; Lombardi, R.; Naturale, M.D.; Mercuri, C.; Bulotta, R.M.; Britti, D.; et al. Antimicrobial Resistance in Livestock: A Serious Threat to Public Health. Antibiotics 2024, 13, 551. [Google Scholar] [CrossRef]
- Ramos, S.; Silva, V.; Dapkevicius, M.L.E.; Igrejas, G.; Poeta, P. Enterococci, from Harmless Bacteria to a Pathogen. Microorganisms 2020, 8, 1118. [Google Scholar] [CrossRef]
- Torres, C.; Alonso, C.A.; Ruiz-Ripa, L.; Leon-Sampedro, R.; Del Campo, R.; Coque, T.M. Antimicrobial Resistance in Enterococcus spp. of animal origin. Microbiol. Spectr. 2018, 6, 41. [Google Scholar] [CrossRef]
- Guan, L.; Beig, M.; Wang, L.; Navidifar, T.; Moradi, S.; Motallebi Tabaei, F.; Teymouri, Z.; Abedi Moghadam, M.; Sedighi, M. Global status of antimicrobial resistance in clinical Enterococcus faecalis isolates: Systematic review and meta-analysis. Ann. Clin. Microbiol. Antimicrob. 2024, 23, 80. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, S.; Shang, X.; Wang, X.; Yan, Z.; Li, H.; Li, J. Short communication: Antimicrobial resistance and virulence genes of Enterococcus faecalis isolated from subclinical bovine mastitis cases in China. J. Dairy. Sci. 2019, 102, 140–144. [Google Scholar] [CrossRef]
- Yi, L.; Xu, R.; Yuan, X.; Ren, Z.; Song, H.; Lai, H.; Sun, Z.; Deng, H.; Yang, B.; Yu, D. Heat stress enhances the occurrence of erythromycin resistance of Enterococcus isolates in mice feces. J. Therm. Biol. 2024, 120, 103786. [Google Scholar] [CrossRef]
- Wu, S.; Yang, Y.; Wang, T.; Sun, J.; Zhang, Y.; Ji, J.; Sun, X. Effects of acid, alkaline, cold, and heat environmental stresses on the antibiotic resistance of the Salmonella enterica serovar Typhimurium. Food Res. Int. 2021, 144, 110359. [Google Scholar] [CrossRef]
- Al-Nabulsi, A.A.; Osaili, T.M.; Elabedeen, N.A.; Jaradat, Z.W.; Shaker, R.R.; Kheirallah, K.A.; Tarazi, Y.H.; Holley, R.A. Impact of environmental stress desiccation, acidity, alkalinity, heat or cold on antibiotic susceptibility of Cronobacter sakazakii. Int. J. Food Microbiol. 2011, 146, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Dorrian, J.M.; Briggs, D.A.; Ridley, M.L.; Layfield, R.; Kerr, I.D. Induction of a stress response in Lactococcus lactis is associated with a resistance to ribosomally active antibiotics. FEBS J. 2011, 278, 4015–4024. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Verdugo, A.; Gaut, B.S.; Tenaillon, O. Evolution of Escherichia coli rifampicin resistance in an antibiotic-free environment during thermal stress. BMC Evol. Biol. 2013, 13, 50. [Google Scholar] [CrossRef]
- Jacob, P.; Hirt, H.; Bendahmane, A. The heat-shock protein/chaperone network and multiple stress resistance. Plant Biotechnol. J. 2017, 15, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Acunzo, J.; Katsogiannou, M.; Rocchi, P. Small heat shock proteins HSP27 (HspB1), alphaB-crystallin (HspB5) and HSP22 (HspB8) as regulators of cell death. Int. J. Biochem. Cell Biol. 2012, 44, 1622–1631. [Google Scholar] [CrossRef]
- Tamaki, K.; Otaka, M.; Takada, M.; Yamamoto, S.; Odashima, M.; Itoh, H.; Watanabe, S. Evidence for enhanced cytoprotective function of HSP90-overexpressing small intestinal epithelial cells. Dig. Dis. Sci. 2011, 56, 1954–1961. [Google Scholar] [CrossRef]
- Zhang, M.; Lv, Y.; Yue, Z.; Islam, A.; Rehana, B.; Bao, E.; Hartung, J. Effects of transportation on expression of Hsp90, Hsp70, Hsp27 and alphaB-crystallin in the pig stomach. Vet. Rec. 2011, 169, 312. [Google Scholar] [CrossRef]
- Tabler, T.W.; Greene, E.S.; Orlowski, S.K.; Hiltz, J.Z.; Anthony, N.B.; Dridi, S. Intestinal Barrier Integrity in Heat-Stressed Modern Broilers and Their Ancestor Wild Jungle Fowl. Front. Vet. Sci. 2020, 7, 249. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, W.; Wu, E.; Wang, K.; Chen, X.; Cui, Y.; Zhang, G.; Lv, F.; Wang, Y.; Peng, X.; et al. Polysaccharides From Abrus cantoniensis Hance Modulate Intestinal Microflora and Improve Intestinal Mucosal Barrier and Liver Oxidative Damage Induced by Heat Stress. Front. Vet. Sci. 2022, 9, 868433. [Google Scholar] [CrossRef]
- Ouellette, A.J.; Selsted, M.E. Paneth cell defensins: Endogenous peptide components of intestinal host defense. FASEB J. 1996, 10, 1280–1289. [Google Scholar] [CrossRef]
- Ma, T.Y.; Boivin, M.A.; Ye, D.; Pedram, A.; Said, H.M. Mechanism of TNF-alpha modulation of Caco-2 intestinal epithelial tight junction barrier: Role of myosin light-chain kinase protein expression. Am. J. Physiol. Gastrointest. Liver Physiol. 2005, 288, G422–G430. [Google Scholar] [CrossRef]
- Camilleri, M.; Madsen, K.; Spiller, R.; Greenwood-Van Meerveld, B.; Verne, G.N. Intestinal barrier function in health and gastrointestinal disease. Neurogastroenterol. Motil. 2012, 24, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, S.C.; Barbara, G.; Buurman, W.; Ockhuizen, T.; Schulzke, J.D.; Serino, M.; Tilg, H.; Watson, A.; Wells, J.M. Intestinal permeability--a new target for disease prevention and therapy. BMC Gastroenterol. 2014, 14, 189. [Google Scholar] [CrossRef]
- Pastorelli, L.; De Salvo, C.; Mercado, J.R.; Vecchi, M.; Pizarro, T.T. Central role of the gut epithelial barrier in the pathogenesis of chronic intestinal inflammation: Lessons learned from animal models and human genetics. Front. Immunol. 2013, 4, 280. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Yin, J.; Du, M.; Yan, P.; Xu, J.; Zhu, X.; Yu, J. Heat-stress-induced damage to porcine small intestinal epithelium associated with downregulation of epithelial growth factor signaling. J. Anim. Sci. 2009, 87, 1941–1949. [Google Scholar] [CrossRef]
- Nabuurs, M.J.; Van Essen, G.J.; Nabuurs, P.; Niewold, T.A.; Van Der Meulen, J. Thirty minutes transport causes small intestinal acidosis in pigs. Res. Vet. Sci. 2001, 70, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Schmid, T.E.; Multhoff, G. Radiation-induced stress proteins-the role of heat shock proteins (HSP) in anti- tumor responses. Curr. Med. Chem. 2012, 19, 1765–1770. [Google Scholar] [CrossRef]
- Yi, L.; Durand, R.; Grenier, F.; Yang, J.; Yu, K.; Burrus, V.; Liu, J.H. PixR, a Novel Activator of Conjugative Transfer of IncX4 Resistance Plasmids, Mitigates the Fitness Cost of mcr-1 Carriage in Escherichia coli. mBio 2022, 13, e0320921. [Google Scholar] [CrossRef]
- Dobrindt, U.; Hochhut, B.; Hentschel, U.; Hacker, J. Genomic islands in pathogenic and environmental microorganisms. Nat. Rev. Microbiol. 2004, 2, 414–424. [Google Scholar] [CrossRef]
- Siguier, P.; Gourbeyre, E.; Chandler, M. Bacterial insertion sequences: Their genomic impact and diversity. FEMS Microbiol. Rev. 2014, 38, 865–891. [Google Scholar] [CrossRef]
- Cheng, W.; Chen, H.; Su, C.; Yan, S. Abundance and persistence of antibiotic resistance genes in livestock farms: A comprehensive investigation in eastern China. Environ. Int. 2013, 61, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Gomis, J.; Marin, P.; Otal, J.; Galecio, J.S.; Martinez-Conesa, C.; Cubero, M.J. Resistance patterns to C and D antibiotic categories for veterinary use of Campylobacter spp., Escherichia coli and Enterococcus spp. commensal isolates from laying hen farms in Spain during 2018. Prev. Vet. Med. 2021, 186, 105222. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chen, N.; Sun, L.; Zhang, Y.; Wu, Y.; Wang, Y.; Liao, X.; Mi, J. Short-term cold stress can reduce the abundance of antibiotic resistance genes in the cecum and feces in a pig model. J. Hazard. Mater. 2021, 416, 125868. [Google Scholar] [CrossRef] [PubMed]
- Shi, N.; Li, N.; Duan, X.; Niu, H. Interaction between the gut microbiome and mucosal immune system. Mil. Med. Res. 2017, 4, 14. [Google Scholar] [CrossRef]
- Gevers, D.; Kugathasan, S.; Denson, L.A.; Vazquez-Baeza, Y.; Van Treuren, W.; Ren, B.; Schwager, E.; Knights, D.; Song, S.J.; Yassour, M.; et al. The treatment-naive microbiome in new-onset Crohn’s disease. Cell Host Microbe 2014, 15, 382–392. [Google Scholar] [CrossRef]
- Belaich, A.; Belaich, J.P. Microcalorimetric study of the anaerobic growth of Escherichia coli: Measurements of the affinity of whole cells for various energy substrates. J. Bacteriol. 1976, 125, 19–24. [Google Scholar] [CrossRef]
- Renaudeau, D.; Collin, A.; Yahav, S.; de Basilio, V.; Gourdine, J.L.; Collier, R.J. Adaptation to hot climate and strategies to alleviate heat stress in livestock production. Animal 2012, 6, 707–728. [Google Scholar] [CrossRef]
- Gabriele Mack, T.W.; Flury, C. Seasonal alpine grazing trends in Switzerland: Economic importance and impact on biotic communities. Environ. Sci. Policy 2013, 32, 48–57. [Google Scholar] [CrossRef]
- Prochera, A.; Muppirala, A.N.; Kuziel, G.A.; Soualhi, S.; Shepherd, A.; Sun, L.; Issac, B.; Rosenberg, H.J.; Karim, F.; Perez, K.; et al. Enteric glia regulate Paneth cell secretion and intestinal microbial ecology. eLife 2025, 13, RP97144. [Google Scholar] [CrossRef]
- CLSI. CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 32nd ed.; CLSI supplement M100; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2022. [Google Scholar]
- Blanco, A.E.; Barz, M.; Cavero, D.; Icken, W.; Sharifi, A.R.; Voss, M.; Buxade, C.; Preisinger, R. Characterization of Enterococcus faecalis isolates by chicken embryo lethality assay and ERIC-PCR. Avian Pathol. 2018, 47, 23–32. [Google Scholar] [CrossRef]
- Mardis, E.R. Next-generation DNA sequencing methods. Annu. Rev. Genom. Hum. Genet. 2008, 9, 387–402. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Zhu, H.; Ruan, J.; Qian, W.; Fang, X.; Shi, Z.; Li, Y.; Li, S.; Shan, G.; Kristiansen, K.; et al. De novo assembly of human genomes with massively parallel short read sequencing. Genome Res. 2010, 20, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, M.J.; Petty, N.K.; Beatson, S.A. Easyfig: A genome comparison visualizer. Bioinformatics 2011, 27, 1009–1010. [Google Scholar] [CrossRef]
- Chen, L.; Huang, J.; Huang, X.; He, Y.; Sun, J.; Dai, X.; Wang, X.; Shafiq, M.; Wang, L. Horizontal Transfer of Different erm(B)-Carrying Mobile Elements Among Streptococcus suis Strains with Different Serotypes. Front. Microbiol. 2021, 12, 628740. [Google Scholar] [CrossRef]
- Brooks, P.T.; Bell, J.A.; Bejcek, C.E.; Malik, A.; Mansfield, L.S. An antibiotic depleted microbiome drives severe Campylobacter jejuni-mediated Type 1/17 colitis, Type 2 autoimmunity and neurologic sequelae in a mouse model. J. Neuroimmunol. 2019, 337, 577048. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Liu, C.M.; Luo, R.; Sadakane, K.; Lam, T.W. MEGAHIT: An ultra-fast single-node solution for large and complex metagenomics assembly via succinct de Bruijn graph. Bioinformatics 2015, 31, 1674–1676. [Google Scholar] [CrossRef]
- Li, D.; Luo, R.; Liu, C.M.; Leung, C.M.; Ting, H.F.; Sadakane, K.; Yamashita, H.; Lam, T.W. MEGAHIT v1.0: A fast and scalable metagenome assembler driven by advanced methodologies and community practices. Methods 2016, 102, 3–11. [Google Scholar] [CrossRef]
- Zeller, G.; Tap, J.; Voigt, A.Y.; Sunagawa, S.; Kultima, J.R.; Costea, P.I.; Amiot, A.; Bohm, J.; Brunetti, F.; Habermann, N.; et al. Potential of fecal microbiota for early-stage detection of colorectal cancer. Mol. Syst. Biol. 2014, 10, 766. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Goto, S.; Hattori, M.; Aoki-Kinoshita, K.F.; Itoh, M.; Kawashima, S.; Katayama, T.; Araki, M.; Hirakawa, M. From genomics to chemical genomics: New developments in KEGG. Nucleic Acids Res. 2006, 34, D354–D357. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S.; Sato, Y.; Kawashima, M.; Furumichi, M.; Tanabe, M. Data, information, knowledge and principle: Back to metabolism in KEGG. Nucleic Acids Res. 2014, 42, D199–D205. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Pop, M. ARDB--Antibiotic Resistance Genes Database. Nucleic Acids Res. 2009, 37, D443–D447. [Google Scholar] [CrossRef]
- Kesim, B.; Ulger, S.T.; Aslan, G.; Cudal, H.; Ustun, Y.; Kucuk, M.O. Amplicon-based next-generation sequencing for comparative analysis of root canal microbiome of teeth with primary and persistent/secondary endodontic infections. Clin. Oral. Investig. 2023, 27, 995–1004. [Google Scholar] [CrossRef] [PubMed]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- White, J.R.; Nagarajan, N.; Pop, M. Statistical methods for detecting differentially abundant features in clinical metagenomic samples. PLoS Comput. Biol. 2009, 5, e1000352. [Google Scholar] [CrossRef]


| Groups | Segments | ermA | ermB | mefA | fexA | optrA | tetS | tetL |
|---|---|---|---|---|---|---|---|---|
| CON | Ileum | 0.0 (0/32) | 0.0 (0/32) | 0.0 (0/32) | 0.0 (0/32) | 0.0 (0/32) | 0.6 (20/32) | 0.1 (2/32) |
| Cecum | 0.0 (0/48) | 0.0 (0/48) | 0.0 (0/48) | 0.0 (0/48) | 0.0 (0/48) | 0.3 (13/48) | 0.1 (3/48) | |
| Colon | 0.0 (0/34) | 0.0 (0/34) | 0.0 (0/34) | 0.0 (0/34) | 0.0 (0/34) | 0.5 (18/34) | 0.1 (4/34) | |
| Rectum | 0.0 (0/40) | 0.0 (0/40) | 0.0 (0/40) | 0.0 (0/40) | 0.0 (0/40) | 0.5 (20/40) | 0.02 (1/40) | |
| Total | 0.0 (0/154) | 0.0 (0/154) | 0.0 (0/154) | 0.0 (0/154) | 0.0 (0/154) | 0.5 (71/154) | 0.1 (10/154) | |
| HS | Ileum | 2.1 (1/48) | 31.2 (15/48) | 4.2 (0/48) | 2.1 (1/48) | 0.0 (0/48) | 0.3 (12/48) | 0.02 (1/48) |
| Cecum | 0.0 (0/55) | 58.2 (32/55) | 1.8 (0/55) | 10.9 (6/55) | 1.8 (1/55) | 0.4 (20/55) | 0.1 (5/55) | |
| Colon | 0.0 (0/62) | 68.8 (44/62) | 1.6 (0/62) | 1.6 (1/62) | 1.6 (1/62) | 0.5 (30/62) | 0.1 (4/62) | |
| Rectum | 1.7 (1/60) | 76.7 (46/60) | 3.3 (1/60) | 5 (3/60) | 1.7 (1/60) | 0.3 (15/60) | 0.02 (1/60) | |
| Total | 0.9 (2/225) | 60.1 (137/225) | 0.5 (1/225) | 4.9 (11/225) | 1.3 (3/225) | 0.3 (77/225) | 0.05 (11/225) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yi, L.; Ren, Z.; Feng, Y.; Zhang, Y.; Liu, J.; Yuan, X.; Kuang, Q.; Deng, H.; Yang, B.; Yu, D. Chronic Heat Stress Can Induce Conjugation of a Novel ermB-Containing ICEFZMF, Increasing Resistance to Erythromycin Among Enterococcus Strains in Diverse Intestinal Segments in the Mouse Model. Antibiotics 2025, 14, 460. https://doi.org/10.3390/antibiotics14050460
Yi L, Ren Z, Feng Y, Zhang Y, Liu J, Yuan X, Kuang Q, Deng H, Yang B, Yu D. Chronic Heat Stress Can Induce Conjugation of a Novel ermB-Containing ICEFZMF, Increasing Resistance to Erythromycin Among Enterococcus Strains in Diverse Intestinal Segments in the Mouse Model. Antibiotics. 2025; 14(5):460. https://doi.org/10.3390/antibiotics14050460
Chicago/Turabian StyleYi, Lingxian, Zining Ren, Yu Feng, Yechun Zhang, Jianshuo Liu, Xiaowu Yuan, Qihong Kuang, Hui Deng, Bo Yang, and Daojin Yu. 2025. "Chronic Heat Stress Can Induce Conjugation of a Novel ermB-Containing ICEFZMF, Increasing Resistance to Erythromycin Among Enterococcus Strains in Diverse Intestinal Segments in the Mouse Model" Antibiotics 14, no. 5: 460. https://doi.org/10.3390/antibiotics14050460
APA StyleYi, L., Ren, Z., Feng, Y., Zhang, Y., Liu, J., Yuan, X., Kuang, Q., Deng, H., Yang, B., & Yu, D. (2025). Chronic Heat Stress Can Induce Conjugation of a Novel ermB-Containing ICEFZMF, Increasing Resistance to Erythromycin Among Enterococcus Strains in Diverse Intestinal Segments in the Mouse Model. Antibiotics, 14(5), 460. https://doi.org/10.3390/antibiotics14050460






