The Influence of the Seasonal Variability of Candida spp. Bloodstream Infections and Antifungal Treatment: A Mediterranean Pilot Study
Abstract
:1. Introduction
2. Results
3. Discussion
4. Limitations
5. Materials and Methods
5.1. Microbiological Methods
5.2. Statistical Analysis
Seasonal Variable
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. WHO Fungal Priority Pathogens List to Guide Research, Development and Public Health Action 2022. Available online: https://iris.who.int/bitstream/handle/10665/363682/9789240060241-eng.pdf?sequence=1> (accessed on 8 March 2025).
- Pappas, P.G.; Lionakis, M.S.; Arendrup, M.C.; Kullberg, B.J. Invasive candidiasis. Nat. Rev. Dis. Primers 2018, 4, 18026. [Google Scholar] [CrossRef] [PubMed]
- Koehler, P.; Stecher, M.; Cornely, O.A.; Koehler, D.; Vehreschild, M.J.G.T.; Bohlius, J.; Wisplinghoff, H.; Vehreschild, J.J. Morbidity and mortality of candidaemia in Europe: An epidemiologic meta-analysis. Clin. Microbiol. Infect. 2019, 25, 1200–1212. [Google Scholar] [CrossRef] [PubMed]
- Rodolico, V.; Di Carlo, P.; Gulotta, G.; D’Arpa, F.; Salamone, G.; Cocorullo, G.; Agrusa, A.; Giammanco, A.; Sergi, C. Intra-abdominal Candida spp. infection in acute abdomen in a quality assurance (QA)-certified academic setting. J. Clin. Pathol. 2017, 70, 579–583. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Arendrup, M.C.; Arikan-Akdagli, S.; Jørgensen, K.M.; Barac, A.; Steinmann, J.; Toscano, C.; Arsenijevic, V.A.; Sartor, A.; Lass-Flörl, C.; Hamprecht, A.; et al. European candidaemia is characterised by notable differential epidemiology and susceptibility pattern: Results from the ECMM Candida III study. J. Infect. 2023, 87, 428–437. [Google Scholar] [CrossRef]
- Meneghello, S.; Bernabè, G.; Di Pietra, G.; Di Sopra, S.; Del Vecchio, C.; Cattelan, A.M.; Castagliuolo, I.; Brun, P. Prevalence, Species Distribution and Resistance of Candidemia in Pediatric and Adult Patients in a Northeast Italy University Hospital. J. Fungi 2024, 10, 707. [Google Scholar] [CrossRef]
- Bassetti, M.; Peghin, M.; Carnelutti, A.; Righi, E.; Merelli, M.; Ansaldi, F.; Trucchi, C.; Alicino, C.; Sartor, A.; Toniutto, P.; et al. Clinical characteristics and predictors of mortality in cirrhotic patients with candidemia and intra-abdominal candidiasis: A multicenter study. Intensive Care Med. 2017, 43, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Kojic, E.M.; Darouiche, R.O. Candida infections of medical devices. Clin. Microbiol. Rev. 2004, 17, 255–267. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ranđelović, M.; Ignjatović, A.; Đorđević, M.; Golubović, M.; Stalević, M.; Rančić, N.; Otašević, S. Superficial Candidiasis: Cluster Analysis of Species Distribution and Their Antifungal Susceptibility In Vitro. J. Fungi 2025, 11, 338. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Diekema, D.J.; Rinaldi, M.G.; Barnes, R.; Hu, B.; Veselov, A.V.; Tiraboschi, N.; Nagy, E.; Gibbs, D.L. Results from the ARTEMIS DISK Global Antifungal Surveillance Study: A 6.5-year analysis of susceptibilities of Candida and other yeast species to fluconazole and voriconazole by standardized disk diffusion testing. J. Clin. Microbiol. 2005, 43, 5848–5859. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- De Gaetano, S.; Midiri, A.; Mancuso, G.; Avola, M.G.; Biondo, C. Candida auris Outbreaks: Current Status and Future Perspectives. Microorganisms 2024, 12, 927. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, H.; Xu, P.; Nie, C.; Geng, X.; Chen, J.; Liu, S.; Wei, M. Inhalable Fungi and Opportunistic Pathogens During Haze and Haze-Dust Events from Winter to Springtime in One Typical Inland City of Northern China. J. Geophys. Res. Atmos. 2024, 129, e2024JD040792. [Google Scholar] [CrossRef]
- Case, N.T.; Gurr, S.J.; Fisher, M.C.; Blehert, D.S.; Boone, C.; Casadevall, A.; Chowdhary, A.; Cuomo, C.A.; Currie, C.R.; Denning, D.W.; et al. Fungal impacts on Earth’s ecosystems. Nature 2025, 638, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, A.; Khadija, B.; Saqib, M.A.; Tara, T.; Umar, M. Unveiling the Impact of Atmospheric Temperature on Antifungal Resistance and Virulence Factors in Candida spp. Isolated from Forest Ecosystem. SSRN 2025, 20. [Google Scholar] [CrossRef]
- High time to tackle drug-resistant fungal infections. Nature 2025, 640, 569. [CrossRef] [PubMed]
- Gadre, A.; Enbiale, W.; Andersen, L.K.; Coates, S.J. The effects of climate change on fungal diseases with cutaneous manifestations: A report from the International Society of Dermatology Climate Change Committee. J. Clim. Change Health 2022, 6, 100156. [Google Scholar] [CrossRef]
- Lemos-Carolino, M.; Madeira-Lopes, A. The temperature profile of the pathogenic yeast Candida albicans. Z. Für Allg. Mikrobiol. 1981, 22, 705–709. [Google Scholar] [CrossRef]
- Al-Fattani, M.A.; Douglas, L.J. Biofilm matrix of Candida albicans and Candida tropicalis: Chemical composition and role in drug resistance. J. Med. Microbiol. 2006, 55 Pt 8, 999–1008. [Google Scholar] [CrossRef] [PubMed]
- Ahamad, I.; Bano, F.; Anwer, R.; Srivastava, P.; Kumar, R.; Fatma, T. Antibiofilm Activities of Biogenic Silver Nanoparticles Against Candida albicans. Front. Microbiol. 2022, 12, 741493. [Google Scholar] [CrossRef]
- Alim, D.; Sircaik, S.; Panwar, S.L. The Significance of Lipids to Biofilm Formation in Candida albicans: An Emerging Perspective. J. Fungi 2018, 4, 140. [Google Scholar] [CrossRef]
- Pierantoni, D.C.; Corte, L.; Casadevall, A.; Robert, V.; Cardinali, G.; Tascini, C. How does temperature trigger biofilm adhesion and growth in Candida albicans and two non-Candida albicans Candida species? Mycoses 2021, 64, 1412–1421. [Google Scholar] [CrossRef]
- Vineis, P.; Romanello, M.; Michelozzi, P.; Martuzzi, M. Health co-benefits of climate change action in Italy. Lancet Planet. Health 2022, 6, e293–e294. [Google Scholar] [CrossRef] [PubMed]
- Seidel, D.; Wurster, S.; Jenks, J.D.; Sati, H.; Gangneux, J.P.; Egger, M.; Alastruey-Izquierdo, A.; Ford, N.P.; Chowdhary, A.; Sprute, R.; et al. Impact of climate change and natural disasters on fungal infections. Lancet Microbe. 2024, 5, e594–e605. [Google Scholar] [CrossRef] [PubMed]
- Edi-Osagie, N.E.; BEmmerson, A.J. Seasonality of invasive Candida infection in neonates. Acta PæDiatrica 2004, 94, 72–74. [Google Scholar] [CrossRef] [PubMed]
- Donders, G.G.; Ruban, K.; Donders, F.; Reybrouck, R. Lab-Based Retrospective 10-Year Analysis Shows Seasonal Variation of Vaginal Candida Infection Rates in Belgium. J. Clin. Med. 2022, 11, 574. [Google Scholar] [CrossRef]
- Actor, J.K. Mycology. In Elsevier’s Integrated Review Immunology and Microbiology, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 139–146. [Google Scholar] [CrossRef]
- Robert, V.; Cardinali, G.; Casadevall, A. Distribution and impact of yeast thermal tolerance permissive for mammalian infection. BMC Biol. 2015, 13, 18. [Google Scholar] [CrossRef]
- George, M.E.; Gaitor, T.T.; Cluck, D.B.; Henao-Martínez, A.F.; Sells, N.R.; Chastain, D.B. The impact of climate change on the epidemiology of fungal infections: Implications for diagnosis, treatment, and public health strategies. Ther. Adv. Infect. Dis. 2025, 12, 20499361251313841. [Google Scholar] [CrossRef]
- Kashyap, B.; Das, S.; Gupta, K.; Sagar, T. Current Scenario of Geriatric Fungal Infections: A Prevalence Study from East Delhi. Aging Med. Healthc. 2019, 10, 46–50. [Google Scholar] [CrossRef]
- Wolfgruber, S.; Sedik, S.; Klingspor, L.; Tortorano, A.; Gow, N.A.R.; Lagrou, K.; Gangneux, P.; Maertens, J.; Meis, J.F.; Lass-Flörl, C.; et al. Insights from Three Pan-European Multicentre Studies on Invasive Candida Infections and Outlook to ECMM Candida IV. Mycopathologia 2024, 189, 70. [Google Scholar] [CrossRef]
- Barchiesi, F.; Orsetti, E.; Mazzanti, S.; Trave, F.; Salvi, A.; Nitti, C.; Manso, E. Candidemia in the elderly: What does it change? PLoS ONE 2017, 12, e0176576. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Asogan, M.; Kim, H.Y.; Kidd, S.; Alastruey-Izquierdo, A.; Govender, N.P.; Dao, A.; Shin, H.; Heim, J.; Ford, N.P.; Gigante, V.; et al. Candida parapsilosis: A systematic review to inform the World Health Organization fungal priority pathogens list. Med. Mycol. 2024, 62, myad131. [Google Scholar] [CrossRef]
- Viale, P.L.; Mirandola, S.; Natalini, C.; Esposti, L.D.; Dovizio, M.; Veronesi, C.; Forcina, G.; Navalesi, P.; Boscolo, A. A retrospective Italian analysis on the characteristics of invasive fungal infections in the intensive care unit setting: CHARTER-IFI study. Mycoses 2024, 67, e13779. [Google Scholar] [CrossRef] [PubMed]
- Vena, A.; Tiseo, G.; Falcone, M.; Bartalucci, C.; Marelli, C.; Cesaretti, M.; Di Pilato, V.; Escribano, P.; Forniti, A.; Giacobbe, D.R.; et al. Impact of Fluconazole Resistance on the Outcomes of Patients with Candida parapsilosis Bloodstream Infections: A Retrospective Multicenter Study. Clin. Infect. Dis. 2025, 80, 540–550. [Google Scholar] [CrossRef] [PubMed]
- Caggiano, G.; Fioriti, S.; Morroni, G.; Apollonio, F.; Triggiano, F.; D’Achille, G.; Stefanizzi, P.; Dalfino, L.; Ronga, L.; Mosca, A.; et al. Genotypic and phenotypic characteristics of Candida parapsilosis bloodstream isolates: Health Care Associated Infections in a teaching Hospital in Italy. J. Infect. Public Health 2024, 17, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Klingspor, L.; Tortorano, A.; Peman, J.; Willinger, B.; Hamal, P.; Sendid, B.; Velegraki, A.; Kibbler, C.; Meis, J.; Sabino, R.; et al. Invasive Candida infections in surgical patients in intensive care units: A prospective, multicentre survey initiated by the European Confederation of Medical Mycology (ECMM) (2006–2008). Clin. Microbiol. Infect. 2014, 21, e1–e87. [Google Scholar] [CrossRef] [PubMed]
- Arastehfar, A.; Daneshnia, F.; Salehi, M.; Yaşar, M.; Hoşbul, T.; Ilkit, M.; Pan, W.; Hagen, F.; Arslan, N.; Perlin, D.S. Low level of antifungal resistance of Candida glabrata blood isolates in Turkey: Fluconazole minimum inhibitory concentration and FKS mutations can predict therapeutic failure. Mycoses 2020, 63, 911. [Google Scholar] [CrossRef]
- De Francesco, M.A.; Piccinelli, G.; Gelmi, M.; Gargiulo, F.; Ravizzola, G.; Pinsi, G.; Peroni, L.; Bonfanti, C.; Caruso, A. Invasive Candidiasis in Brescia, Italy: Analysis of Species Distribution and Antifungal Susceptibilities During Seven Years. Mycopathologia 2017, 182, 897–905. [Google Scholar] [CrossRef]
- Dekkers, B.G.J.; Veringa, A.; EMarriott, D.J.; Boonstra, J.M.; Doukas, F.F.; McLachlan, A.J.; Alffenaar, W.C. Invasive Candidiasis in the Elderly: Considerations for Drug Therapy. Drugs Aging 2018, 35, 781. [Google Scholar] [CrossRef]
- Salmanton-García, J.; Cornely, O.A.; Stemler, J.; Barać, A.; Steinmann, J.; Siváková, A.; Akalin, E.H.; Arikan-Akdagli, S.; Loughlin, L.; Toscano, C.; et al. Attributable mortality of candidemia—Results from the ECMM Candida III multinational European Observational Cohort Study. J. Infect. 2024, 89, 106229. [Google Scholar] [CrossRef]
- Sharifi, M.; Badiee, P.; Abastabar, M.; Morovati, H.; Haghani, I.; Noorbakhsh, M.; Mohammadi, R. A 3-year study of Candida infections among patients with malignancy: Etiologic agents and antifungal susceptibility profile. Front. Cell. Infect. Microbiol. 2023, 13, 1152552. [Google Scholar] [CrossRef]
- Corsello, S.; Spinillo, A.; Osnengo, G.; Penna, C.; Guaschino, S.; Beltrame, A.; Blasi, N.; Festa, A. An epidemiological survey of vulvovaginal candidiasis in Italy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2003, 110, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.Y.; Lim, Y.K.; Choe, K.W.; ho Choi, Y.; Lee, M.K. Seasonality and epidemiological trends in species distribution and antifungal susceptibility of Candida species isolated from various clinical specimens conducted during 2011–2022, Korea: A retrospective surveillance study. Ann. Clin. Microbiol. 2024, 27, 185–196. [Google Scholar] [CrossRef]
- Hossain, M.M.; Sultana, F.; Mostafa, M.; Ferdus, H.; Kundu, M.; Adhikary, S.; Sabbir, M.A.A. Stress Responses and Mechanisms of Phytopathogens Infecting Humans: Threats, Drivers, and Recommendations. Stresses 2025, 5, 28. [Google Scholar] [CrossRef]
- Khan, S.; Cai, L.; Bilal, H.; Khan, M.N.; Fang, W.; Zhang, D.; Yao, F.; Wang, X.; Wang, Q.; Hou, B.; et al. An 11-Year retrospective analysis of candidiasis epidemiology, risk factors, and antifungal susceptibility in a tertiary care hospital in China. Sci. Rep. 2025, 15, 7240. [Google Scholar] [CrossRef] [PubMed]
- Lima, R.; Ribeiro, F.C.; Colombo, A.L.; De Almeida, J.N. The emerging threat antifungal-resistant Candida tropicalis in humans, animals, and environment. Front. Fungal Biol. 2022, 3, 957021. [Google Scholar] [CrossRef]
- Cornely, O.A.; Sprute, R.; Bassetti, M.; Chen, S.C.; Groll, A.H.; Kurzai, O.; Lass-Flörl, C.; Ostrosky-Zeichner, L.; Rautemaa-Richardson, R.; Revathi, G.; et al. Global guideline for the diagnosis and management of candidiasis: An initiative of the ECMM in cooperation with ISHAM and ASM. Lancet Infect. Dis. 2025, 25, E280–E293. [Google Scholar] [CrossRef]
- Russo Fiorino, G.; Maniglia, M.; Marchese, V.; Aprea, L.; Torregrossa, M.V.; Campisi, F.; Favaro, D.; Calamusa, G.; Amodio, E. Healthcare-associated infections over an eight year period in a large university hospital in Sicily (Italy, 2011–2018). J. Infect. Prev. 2021, 22, 220–230. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- De Pauw, B.; Walsh, T.J.; Donnelly, J.P.; Stevens, D.A.; Edwards, J.E.; Calandra, T.; Pappas, P.G.; Maertens, J.; Lortholary, O.; Kauffman, C.A.; et al. Revised Definitions of Invasive Fungal Disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin. Infect. Dis. 2008, 46, 1813–1821. [Google Scholar]
- Sherertz, R.J.; Gledhill, K.S.; Hampton, K.D.; Pfaller, M.A.; Givner, L.B.; Abramson, J.S.; Dillard, R.G. Outbreak of Candida bloodstream infections associated with retrograde medication administration in a neonatal intensive care unit. J. Pediatr. 1992, 120, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Serra, N.; Di Carlo, P.; Andriolo, M.; Mazzola, G.; Diprima, E.; Rea, T.; Anastasia, A.; Fasciana, T.M.; Pipitò, L.; Capra, G.; et al. Staphylococcus aureus and Coagulase-Negative Staphylococci from Bloodstream Infections: Frequency of Occurrence and Antimicrobial Resistance, 2018–2021. Life 2023, 13, 1356. [Google Scholar] [CrossRef]
- Di Carlo, P.D.; Serra, N.; Assunta Fasciana, T.M.; Giammanco, A.; Rea, T.; Napolitano, M.S.; Lucchesi, A.; Cascio, A.; Sergi, C.M. Microbial profile in bile from pancreatic and extra-pancreatic biliary tract cancer. PLoS ONE 2024, 19, e0294049. [Google Scholar] [CrossRef]
- Lacroix, C.; Gicquel, A.; Sendid, B.; Meyer, J.; Accoceberry, I.; François, N.; Morio, F.; Desoubeaux, G.; Chandenier, J.; Kauffmann-Lacroix, C.; et al. Evaluation of two matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS) systems for the identification of Candida species. Clin. Microbiol. Infect. 2014, 20, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.A.; Young, S.; Timm, K.; Novak-Weekley, S.; Marlowe, E.M.; Madisen, N.; Lillie, J.L.; Ledeboer, N.A.; Smith, R.; Hyke, J.; et al. Multicenter Evaluation of the Bruker MALDI Biotyper CA System for the Identification of Clinically Important Bacteria and Yeasts. Am. J. Clin. Pathol. 2017, 147, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Astvad, K.M.T.; Arikan-Akdagli, S.; Arendrup, M.C. A Pragmatic Approach to Susceptibility Classification of Yeasts without EUCAST Clinical Breakpoints. J. Fungi 2022, 8, 141. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- European Committee on Antimicrobial Susceptibility Testing Breakpoint Tables for Interpretation of MICs for Antifungal Agents. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/AFST/Clinical_breakpoints/AFST_BP_v11.0.pdf (accessed on 3 April 2024).
- García-Gutiérrez, L.; Baena Rojas, B.; Ruiz, M.; Hernández Egido, S.; Ruiz-Gaitán, A.C.; Laiz, L.; Pemán, J.; Cuétara-García, M.S.; Mellado, E.; Martin-Sanchez, P.M. Fungal burden assessment in hospital zones with different protection degrees. Build. Environ. 2025, 269, 112454. [Google Scholar] [CrossRef]
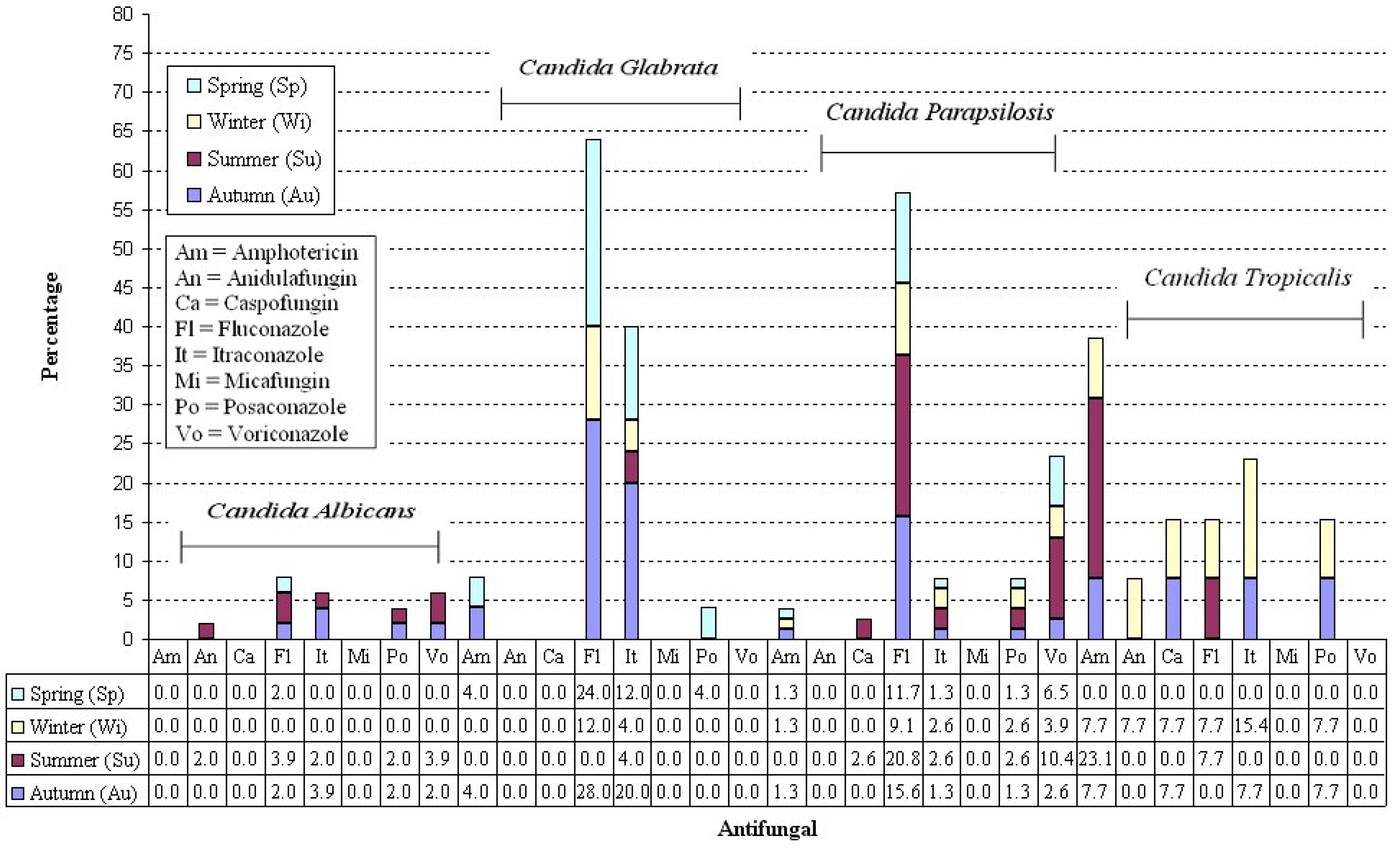
| Parameters | Sample |
|---|---|
| Patients | 175 |
| Age at hospitalization | |
| Mean ± SD | 68.3 (14.2) |
| Median (IQR) | 70.0 (61.3, 77.8) |
| Gender | |
| Male | 57.1% (100) |
| Female | 42.9% (75) |
| Deaths | 41.7% (73) |
| Hospital setting | |
| ICU | 37.7% (66) |
| Medical setting | 51.4% (90) |
| Surgical setting | 10.9% (19) |
| Comorbidities † | |
| Acute coronary syndrome (ACS) | 6.9% (12) |
| Autoimmune diseases (AD) | 2.3% (4) |
| Chronic heart failure (CHF) | 34.3% (60) |
| Chronic obstructive pulmonary disease (COPD) | 22.3% (39) |
| Chronic renal failure (CRF) | 20.0% (35) |
| Dementia (DM) | 9.1% (16) |
| Diabetes | 20.6% (36) |
| Organ solid tumors | 20.6% (36) |
| Hematological tumors | 4.6% (8) |
| Cerebrovascular (brain) insult | 13.1% (23) |
| Haemodialysis (HD) | 10.9% (19) |
| Liver cirrhosis (LC) | 4.0% (7) |
| Candida spp. by Season | |
| Autumn | 28.6% (50) |
| Summer | 32.0% (56) |
| Winter | 18.3% (32) |
| Spring | 21.1% (37) |
| Hospital Setting/Seasons | Autumn | Summer | Winter | Spring |
|---|---|---|---|---|
| ICU | 10.9% (19) | 11.4% (20) | 8.0% (14) | 7.4% (13) |
| Medical | 15.4% (27) | 17.7% (31) | 9.7% (17) | 8.6% (15) |
| Surgical | 2.3% (4) | 2.9% (5) | 0.6% (1) | 5.1% (9) |
| Antifungal | Total | Autumn (Au) | Summer (Su) | Winter (Wi) | Spring (Sp) | Analysis Among Seasons p-Value (Test) |
|---|---|---|---|---|---|---|
| Total | 175 | 28.6% (50) | 32.0% (56) | 18.3% (32) | 21.1% (37) | 0.0364 * (Cg) Su **, p = 0.0263(Z) Effect size: phi = 0.644, large effect |
| (1) Amphotericin | 7.4% (13) | 2.9% (5) | 2.3% (4) | 1.1% (2) | 1.1% (2) | 0.56 (Cg) |
| (2) Anidulafungin | 1.1% (2) | 0.0% (0) | 0.6% (1) | 0.6% (1) | 0.0% (0) | N/A |
| (3) Caspofungin | 2.3% (4) | 0.6% (1) | 1.1% (2) | 0.6% (1) | 0.0% (0) | N/A |
| (4) Fluconazole | 39.4% (69) | 12.6% (22) | 10.9% (19) | 6.3% (11) | 9.7% (17) | 0.29 (Cg) |
| (5) Itraconazole | 13.7% (24) | 5.7% (10) | 2.3% (4) | 2.9% (5) | 2.9% (5) | 0.30 (Cg) |
| (6) Micafungin | 0.0% (0) | 0.0% (0) | 0.0% (0) | 0.0% (0) | 0.0% (0) | N/A |
| (7) Posaconazole | 6.9% (12) | 2.3% (4) | 1.7% (3) | 1.7% (3) | 1.1% (2) | 0.88 (Cg) |
| (8) Voriconazole | 12.0% (21) | 1.7% (3) | 5.7% (10) | 1.7% (3) | 2.9% (5) | 0.10 (Cg) |
| Analysis in season, p-value (test) | p < 0.001 * (Q) (4)>(1),(2),(3),(5),(6),(7),(8), p < 0.05 * (B) Effect size: η2 = 0.17, large effect | p < 0.001 * (Q) (4)>(1),(2),(3),(5),(6),(7),(8), p < 0.05 * (B) Effect size: η2 = 0.20, large effect | p < 0.001 * (Q) (4)>(1),(2),(3),(5),(6),(7), p < 0.05 * (B) Effect size: η2 = 0.15, large effect | p < 0.001 * (Q) (4)>(1),(2),(3),(6),(7),(8), p < 0.05 * (B) Effect size: η2 = 0.15, large effect | p < 0.001 * (Q) (4)>(1),(2),(3),(5),(6),(7),(8), p < 0.05 * (B) Effect size: η2 = 0.22, large effect |
| Isolated Strains | Age % (n) Mean (SD) Median (IQR) | Gender Males/Females % (n) | Status (††) Dead (Yes) % (n) | Hospital Setting ICU; Medical; Surgical |
|---|---|---|---|---|
| Candida albicans (C.A.) (n = 51) | n = 51 69.6 (13.9) 71 (62, 78.5) | 56.9% (29) 43.1% (22) | 30.5% (18) | 17.6% (9); 72.5% (37); 9.8% (5) |
| Candida glabrata (C.G.) (n = 25) | n = 25 70.3 (11.5) 73 (62.8, 77.5) | 60% (15) 40% (10) | 28.0% (7) | 28.0% (7); 48.0% (12); 24.0% (7) |
| Candida krusei (C.K.) (n = 4) | n = 4 69.8 (16.0) 66.5 (60, 79.5) | 25% (1) 75% (3) | 0.0% (0) | 25.0% (1); 75.0% (3); 0.0% (0) |
| Candida lusitaniae (C.L.) (n = 3) | n = 3 73.7 (10.1) 79 (66.3, 79.8) | 66.7% (2) 33.3% (1) | 66.7% (2) | 66.7% (2); 0.0% (0); 33.3% (1) |
| Candida norvegensis (C.N.) † (n = 1) | n = 1 65 | 100% (1) 0.0% (0) | 100% (1) | 0.0% (0); 100% (1); 0.0% (0) |
| Candida parapsilosis (C.P.) (n = 77) | n = 77 66.1 (15.7) 68 (58.5, 77) | 57.1% (44) 42.9% (33) | 53.2% (41) | 54.5% (42); 39.0% (30); 6.5% (5) |
| Candida pulcherrima (C.Pu.) † (n = 1) | n = 1 71 | 100% (1) 0.0% (0) | 0.0% (0) | 0.0% (0); 0.0% (0); 100% (1) |
| Candida tropicalis (C.T.) (n = 13) | n = 13 70.8 ± 12.9 71 (67.5, 79) | 53.8% (7) 46.2% (6) | 30.8% (4) | 38.5% (5); 53.8% (7); 7.7% (1) |
| p-value (test) | p = 0.86 (KW) | p = 0.88 (F) | p < 0.0402 * (F) C.P. **, 53.2%, p = 0.005 (Z) Effect size: phi = 0.86, large effect | p = 0.0001 * (F) C.P./ICU **, 54.5%, p = 0.0001(Z) C.A./Medical **, 72.5%, p = 0.0003(Z) C.G./Surgical **, 24%, p = 0.0161 (Z) Effect size: phi = 2.37, large effect |
| Isolated Strains | Total | Autumn (Au) | Summer (Su) | Winter (Wi) | Spring (Sp) | p-Value Among Seasons (Test) |
|---|---|---|---|---|---|---|
| Total | 175 | 28.6% (50) | 32.0% (56) | 18.3% (32) | 21.1% (37) | 0.053 (Cg) |
| Candida albicans (C.A.) | 29.1% (51) | 8.0% (14) | 11.4% (20) | 5.1% (9) | 4.6% (8) | 0.07 (Cg) |
| Candida glabrata (C.G.) | 14.3% (25) | 5.7% (10) | 0.6% (1) | 2.9% (5) | 5.1% (9) | 0.0436 * (Cg) Au **, p = 0.0481 (Z) Effect size: phi = 1.62, large effect |
| Candida krusei (C.K.) | 2.3% (4) | 1.7% (3) | 0.6% (1) | 0.0% (0) | 0.0% (0) | N/A |
| Candida lusitaniae (C.L.) | 1.7% (3) | 0.6% (1) | 0.0% (0) | 0.0% (0) | 1.1% (2) | N/A |
| Candida norvegensis (C.N.) | 0.6% (1) | 0.0% (0) | 0.6% (1) | 0.0% (0) | 0.0% (0) | N/A |
| Candida parapsilosis (C.P.) | 44.0% (77) | 10.3% (18) | 15.4% (27) | 8.6% (15) | 9.7% (17) | 0.22 (Cg) |
| Candida pulcherrima (C.Pu.) | 0.6% (1) | 0.0% (0) | 0.6% (1) | 0.0% (0) | 0.0% (0) | N/A |
| Candida tropicalis (C.T.) | 7.4% (13) | 2.3% (4) | 2.9% (5) | 1.7% (3) | 0.6% (1) | 0.44 (Cg) |
| Analysis into groups p-value (test) | p < 0.0001* (Cg) C.A. **, p < 0.0001(Z) C.P. **, p < 0.0001(Z) Effect size: phi = 11.5, large effect | p < 0.0001 * (Cg) C. A. **, p = 0.0002 (Z) C.P. **, p < 0.0001 (Z) Effect size: phi = 2.37, large effect | p < 0.0001 * (Cg) C. A. **, p < 0.0001 (Z) C.P. **, p < 0.0001 (Z) Effect size: phi = 3.89, large effect | p < 0.0001 * (Cg) C. A. **, p = 0.0027 (Z) C.P. **, p < 0.0001 (Z) Effect size: phi = 5.61, large effect | p < 0.0001 * (Cg) C.G. **, p = 0.0237 (Z) C.P. **, p < 0.0001 (Z) Effect size: phi = 5.62, large effect |
| Parameters | Sample |
|---|---|
| Central venous catheters | 28.6% (50) |
| Hospital setting | |
| ICU | 38.0% (19) |
| Medical | 44.0% (22) |
| Surgical | 18.0% (9) |
| Comorbidities † | |
| Chronic heart failure | 24.0% (12) |
| Chronic obstructive pulmonary disease | 18.0% (9) |
| Chronic renal failure | 28.0% (14) |
| Diabetes | 16.0% (8) |
| Oncologic patients ††† | 30.0% (15) |
| Candida spp. †† | |
| Candida albicans | 30.0% (15) |
| Candida glabrata | 10.0% (5) |
| Candida parapsilosis | 50.0% (25) * |
| Candida tropicalis | 10.0% (5) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Carlo, P.; Serra, N.; Collotta, O.; Colomba, C.; Firenze, A.; Aprea, L.; Distefano, S.A.; Cortegiani, A.; Giammanco, G.; Fasciana, T.M.A.; et al. The Influence of the Seasonal Variability of Candida spp. Bloodstream Infections and Antifungal Treatment: A Mediterranean Pilot Study. Antibiotics 2025, 14, 452. https://doi.org/10.3390/antibiotics14050452
Di Carlo P, Serra N, Collotta O, Colomba C, Firenze A, Aprea L, Distefano SA, Cortegiani A, Giammanco G, Fasciana TMA, et al. The Influence of the Seasonal Variability of Candida spp. Bloodstream Infections and Antifungal Treatment: A Mediterranean Pilot Study. Antibiotics. 2025; 14(5):452. https://doi.org/10.3390/antibiotics14050452
Chicago/Turabian StyleDi Carlo, Paola, Nicola Serra, Ornella Collotta, Claudia Colomba, Alberto Firenze, Luigi Aprea, Salvatore Antonino Distefano, Andrea Cortegiani, Giovanni Giammanco, Teresa Maria Assunta Fasciana, and et al. 2025. "The Influence of the Seasonal Variability of Candida spp. Bloodstream Infections and Antifungal Treatment: A Mediterranean Pilot Study" Antibiotics 14, no. 5: 452. https://doi.org/10.3390/antibiotics14050452
APA StyleDi Carlo, P., Serra, N., Collotta, O., Colomba, C., Firenze, A., Aprea, L., Distefano, S. A., Cortegiani, A., Giammanco, G., Fasciana, T. M. A., Virruso, R., Capuano, A., Sergi, C. M., & Cascio, A. (2025). The Influence of the Seasonal Variability of Candida spp. Bloodstream Infections and Antifungal Treatment: A Mediterranean Pilot Study. Antibiotics, 14(5), 452. https://doi.org/10.3390/antibiotics14050452












