Delivery of Outpatient Parenteral Antimicrobial Therapy (OPAT) in an Ever-Changing National Health Service (UK): Benefits, Barriers, and Opportunities
Abstract
1. Introduction
2. OPAT in the UK
2.1. Clinical Practice
2.2. National Initiatives
2.3. The Changing NHS Landscape
3. Benefits of OPAT Programmes
3.1. Clinical Effectiveness and Safety
3.2. Cost-Effectiveness
3.3. Patient Experience and Acceptability
3.4. Other Patient Benefits
3.5. Organisational Benefits
4. Risks of OPAT
4.1. Overuse of OPAT
4.2. OPAT-Related Complications
4.2.1. Adverse Drug Reactions
4.2.2. Vascular Access-Related Complications
4.2.3. Infusion and Elastomeric Device Complications
4.2.4. OPAT Failure
4.2.5. Patient Non-Compliance
4.2.6. Unplanned Hospital Readmission
5. Barriers and Challenges
5.1. Commissioning and Funding of Services
5.2. Variation in Service Delivery
5.3. Inequitable Access to OPAT Care
5.4. Antimicrobial Stewardship (AMS)
5.5. Sustainability in OPAT
6. Opportunities and Prospects
6.1. Future of OPAT in the UK
6.2. Opportunities for OPAT
6.2.1. Changes in OPAT Structure and Modes of Delivery
6.2.2. Evolving Clinical Responsibilities
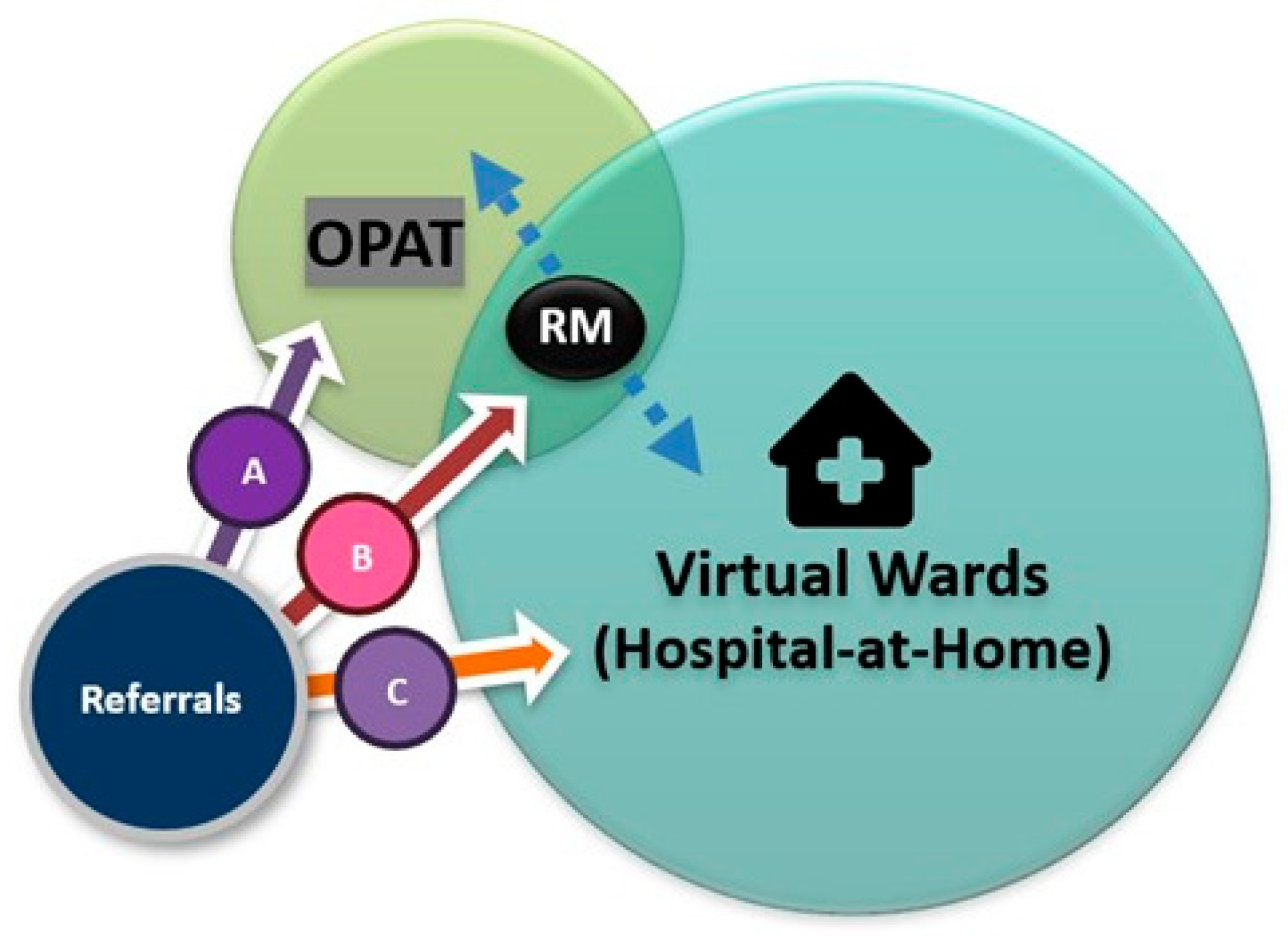
6.2.3. OPAT and Virtual Wards
6.2.4. Complex Outpatient Antimicrobial Therapy (COpAT)
6.2.5. Long-Acting Parenteral Agents
6.2.6. OPAT in Hard-to-Reach Groups
6.2.7. Continuous Antimicrobial Infusions and Infusion Devices
6.2.8. Telemedicine in OPAT (Tele-OPAT)
6.2.9. Palliative OPAT
6.2.10. Outpatient Subcutaneous Antimicrobial Therapy (OSCAT)
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADRs | Adverse drug reactions |
| AMR | Antimicrobial resistance |
| AMS | Antimicrobial stewardship |
| BJIs | Bone and joint infections |
| BSAC | British Society for Antimicrobial Chemotherapy |
| CCI | Charlson comorbidity index |
| CDI | Clostridioides difficile infection |
| COpAT | Complex outpatient antimicrobial therapy |
| GPRs | Good practice recommendations |
| HIV | Human immunodeficiency virus |
| ICB | Integrated care board |
| ID | Infectious diseases |
| IV | Intravenous |
| IVOST | Intravenous to oral therapy |
| MDR | Multi-drug resistant |
| MDT | Multidisciplinary team |
| MHRA | Medicines and Healthcare Products Regulatory Agency |
| NHS | National Health Service |
| NORS | National outcomes registry system |
| OPAT | Outpatient parenteral antimicrobial therapy |
| OSCAT | Outpatient subcutaneous antimicrobial therapy |
| PICCs | Peripheral inserted central catheters |
| PK/PD | Pharmacokinetic/pharmacodynamic |
| PWID | People who inject drugs |
| RM | Remote monitoring |
| SSTIs | Skin and soft tissue infections |
| QoL | Quality of life |
| RCTs | Randomised controlled trials |
| TDM | Therapeutic drug monitoring |
| Tele-OPAT | Telemedicine in OPAT |
| UK | United Kingdom |
| US | United States |
| UTIs | Urinary tract infections |
References
- Rucker, R.W.; Harrison, G.M. Outpatient intravenous medications in the management of cystic fibrosis. Pediatrics 1974, 54, 358–360. [Google Scholar] [CrossRef]
- Subedi, S.; Looke, D.; McDougall, D.; Sehu, M.; Playford, E. Supervised self-administration of outpatient parenteral antibiotic therapy: A report from a large tertiary hospital in Australia. Int. J. Infect. Dis. 2015, 30, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Shah, B.; Norris, A.H. (Eds.) Handbook of Outpatient Parenteral Antimicrobial Therapy for Infectious Diseases, 3rd ed.; CRG Publishing and Infectious Disease Society of America: New York, NY, USA, 2016. [Google Scholar]
- Chapman, A.L.N.; Patel, S.; Horner, C.; Green, H.; Guleri, A.; Hedderwick, S.; Snape, S.; Statham, J.; Wilson, E.; Gilchrist, M.; et al. Updated good practice recommendations for outpatient parenteral antimicrobial therapy (OPAT) in adults and children in the UK. JAC Antimicrob. Resist. 2019, 1, dlz026. [Google Scholar] [CrossRef] [PubMed]
- Durojaiye, O.C.; Cartwright, K.; Ntziora, F. Outpatient parenteral antimicrobial therapy (OPAT) in the UK: A cross-sectional survey of acute hospital trusts and health boards. Diagn. Microbiol. Infect. Dis. 2019, 93, 58–62. [Google Scholar] [CrossRef]
- Chapman, A.L. Outpatient parenteral antimicrobial therapy in a changing NHS: Challenges and opportunities. Clin. Med. 2013, 13, 35–36. [Google Scholar] [CrossRef]
- Acuram, G.; Desales, M.V.J. Hospital admission avoidance in a London NHS trust: A retrospective review of King’s OPAT service. Br. J. Nurs. 2025, 34, S4–S12. [Google Scholar] [CrossRef]
- Department of Health and Social Care (DHSC). Change NHS [Internet]; DHSC: London, UK, 2024. Available online: https://change.nhs.uk/en-GB (accessed on 20 December 2024).
- Minton, J.; Murray, C.C.; Meads, D.; Hess, S.; Vargas-Palacios, A.; Mitchell, E.; Wright, J.; Hulme, C.; Raynor, D.K.; Gregson, A.; et al. The Community IntraVenous Antibiotic Study (CIVAS): A mixed-methods evaluation of patient preferences for and cost-effectiveness of different service models for delivering outpatient parenteral antimicrobial therapy. Health Soc. Care Deliv. Res. 2017, 5. Available online: https://pubmed.ncbi.nlm.nih.gov/28211658/ (accessed on 22 December 2024). [CrossRef]
- Matthews, P.C.; Conlon, C.P.; Berendt, A.R.; Kayley, J.; Jefferies, L.; Atkins, B.L.; Byren, I. Outpatient parenteral antimicrobial therapy (OPAT): Is it safe for selected patients to self-administer at home? A retrospective analysis of a large cohort over 13 years. J. Antimicrob. Chemother. 2007, 60, 356–362. [Google Scholar] [CrossRef]
- Chapman, A.L. Outpatient parenteral antimicrobial therapy. BMJ 2013, 346, f1585. [Google Scholar] [CrossRef]
- Ashiru-Oredope, D.; Sharland, M.; Charani, E.; McNulty, C.; Cooke, J.; on behalf of ARHAI Antimicrobial Stewardship Group. Improving the quality of antibiotic prescribing in the NHS by developing a new Antimicrobial Stewardship Programme: Start Smart—Then Focus. J. Antimicrob. Chemother. 2012, 67 (Suppl. S1), i51–i63. [Google Scholar] [CrossRef]
- UK Government. Confronting Antimicrobial Resistance 2024 to 2029. Available online: https://assets.publishing.service.gov.uk/media/664394d9993111924d9d3465/confronting-antimicrobial-resistance-2024-to-2029.pdf (accessed on 25 February 2025).
- Harbour, J.; Dimitrova, M.; Herbert, P.; Miller, C.; Mac Gann, T.; Steward, J.; SHTG Recommendations No 01-21 Outpatient parenteral antimicrobial therapy (OPAT). Scottish Health Technologies Group. 2021. Available online: https://shtg.scot/our-advice/outpatient-parenteral-antimicrobial-therapy-opat (accessed on 5 January 2025).
- Norris, A.H.; Shrestha, N.K.; Allison, G.M.; Keller, S.C.; Bhavan, K.P.; Zurlo, J.J.; Hersh, A.L.; Gorski, L.A.; Bosso, J.A.; Rathore, M.H.; et al. 2018 Infectious Diseases Society of America clinical practice guideline for the management of outpatient parenteral antimicrobial therapy. Clin. Infect. Dis. 2019, 68, e1–e35. [Google Scholar] [CrossRef] [PubMed]
- Hanumunthadu, B.; Harrison, T.; Mathew, D.; Cotter, M. Multidrug-resistant (MDR) and extensively drug-resistant (XDR) Tuberculosis: Successes and complications on outpatient parenteral antimicrobial therapy at a London Teaching Hospital between 2009 and 2016. Open Forum Infect. Dis. 2016, 3, 558. [Google Scholar] [CrossRef]
- Otu, A.A.; Bongomin, F.; Bazaz, R.; Harris, C.; Denning, D.W.; Kosmidis, C. Micafungin may be safely administered as outpatient parenteral antimicrobial therapy for chronic pulmonary aspergillosis. Mycoses 2019, 62, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Seaton, R.; Morrison, J.; Man, I.; Watson, J.; Nathwani, D. Out-patient parenteral antimicrobial therapy—A viable option for the management of cutaneous leishmaniasis. QJM 1999, 92, 659–667. [Google Scholar] [CrossRef]
- Parkash, V.; Laundy, N.; Durojaiye, O.C. Outpatient parenteral antimicrobial therapy for leishmaniasis: 13 years’ experience at a large UK infectious diseases centre. Trans. R. Soc. Trop. Med. Hyg. 2023, 117, 473–475. [Google Scholar] [CrossRef]
- Gilchrist, M.; Barr, D.; Drummond, F.; Muir, A.; Williams, J.; Scriven, J.; Snape, S.; Hemsley, C.; Durojaiye, C.O.; Patel, S.; et al. Outpatient parenteral antimicrobial therapy (OPAT) in the UK: Findings from the BSAC National Outcomes Registry (2015–19). J. Antimicrob. Chemother. 2022, 77, 1481–1490. [Google Scholar] [CrossRef]
- UK Health Security Agency. UK Access, Watch, Reserve, and Other Classification for Antibiotics (UK-AWaRe Antibiotic Classification); UKHSA: London, UK, 2025. Available online: https://www.gov.uk/government/publications/uk-aware-antibiotic-classification/uk-access-watch-reserve-and-other-classification-for-antibiotics-uk-aware-antibiotic-classification (accessed on 20 April 2025).
- Durojaiye, O.C.; Cole, J.; Kritsotakis, E.I. Effectiveness and safety of a disposable elastomeric continuous infusion pump for outpatient parenteral antimicrobial therapy (OPAT) in a UK setting. J. Chemother. 2024, 36, 119–126. [Google Scholar] [CrossRef]
- McSorley, J.C.; Reyes, D.; Tonna, I.; Bateman, V. Experience with dalbavancin use in various gram-positive infections within Aberdeen Royal Infirmary OPAT service. Infection 2024, 52, 567–576. [Google Scholar] [CrossRef]
- Davidson, H.; Dunstan, I.; Yau, T.; Houston, A.; Basarab, M.; Bicanic, T. O04 Use of the novel antifungal rezafungin in outpatient parenteral antibiotic therapy: Early experience from a single centre. JAC Antimicrob. Resist. 2025, 7, dlae217.004. [Google Scholar] [CrossRef]
- Rawson, T.M.; Eigo, T.; Wilson, R.; Husson, F.; Dhillon, R.; Seddon, O.; Holmes, A.; Gilchrist, M. Exploring patient acceptance of research within complex oral and IV outpatient parenteral antimicrobial therapy (COpAT) networks. JAC Antimicrob. Resist. 2022, 4, dlac087. [Google Scholar] [CrossRef]
- Seaton, R.A.; Ritchie, N.D.; Robb, F.; Stewart, L.; White, B.; Vallance, C. From ‘OPAT’ to ‘COpAT’: Implications of the OVIVA study for ambulatory management of bone and joint infection. J. Antimicrob. Chemother. 2019, 74, 2119–2121. [Google Scholar] [CrossRef]
- Seaton, R.A.; Gilchrist, M. Making a case for outpatient parenteral antimicrobial therapy (OPAT). J. Antimicrob. Chemother. 2024, 79, 1723–1724. [Google Scholar] [CrossRef] [PubMed]
- Reidy, P.; Breslin, T.; Muldoon, E. Outpatient parenteral antimicrobial therapy (OPAT) across the world: A comparative analysis—What lessons can we learn? JAC Antimicrob. Resist. 2024, 6, dlae111. [Google Scholar] [CrossRef] [PubMed]
- British Society for Antimicrobial Chemotherapy. United Kingdom Outpatient Parenteral Antimicrobial Therapy (OPAT) Initiative. Available online: www.e-opat.com (accessed on 20 December 2024).
- Nathwani, D.; Conlon, C. Outpatient and home parenteral antibiotic therapy (OHPAT) in the UK: A consensus statement by a working party. Clin. Microbiol. Infect. 1998, 4, 537–551. [Google Scholar] [CrossRef]
- Chapman, A.L.N.; Seaton, R.A.; Cooper, M.A.; Hedderwick, S.; Goodall, V.; Reed, C.; Sanderson, F.; Nathwani, D.; on behalf of the BSAC/BIA OPAT Project Good Practice Recommendations Working Group. Good practice recommendations for outpatient parenteral antimicrobial therapy (OPAT) in adults in the UK: A consensus statement. J. Antimicrob. Chemother. 2012, 67, 1053–1062. [Google Scholar] [CrossRef]
- Patel, S.; Abrahamson, E.; Goldring, S.; Green, H.; Wickens, H.; Laundy, M. Good practice recommendations for paediatric outpatient parenteral antibiotic therapy (p-OPAT) in the UK: A consensus statement. J. Antimicrob. Chemother. 2015, 70, 360–373. [Google Scholar] [CrossRef]
- Healthcare Financial Management Association. HFMA Introductory Guide to NHS Finance; HFMA: Bristol, UK, 2024; Available online: https://www.hfma.org.uk/publications/introductory-guide-nhs-finance (accessed on 2 February 2025).
- Dunn, P.; Ewbank, L.; Alderwick, H. Nine Major Challenges Facing Health and Care in England [Internet]; The Health Foundation: London, UK, 2023; Available online: https://www.health.org.uk/reports-and-analysis/briefings/nine-major-challenges-facing-health-and-care-in-england (accessed on 5 December 2024).
- Propper, C.; Stoye, G.; Zaranko, B. The wider impacts of the coronavirus pandemic on the NHS. Fisc. Stud. 2020, 41, 345–356. [Google Scholar] [CrossRef]
- Darzi, A. Independent Investigation of the National Health Service in England. 2024. Available online: https://assets.publishing.service.gov.uk/media/66f42ae630536cb92748271f/Lord-Darzi-Independent-Investigation-of-the-National-Health-Service-in-England-Updated-25-September.pdf (accessed on 3 February 2025).
- Savage, C. Streeting Says NHS ‘Broken But Not Beaten’ as He Seeks Private Sector Deal. The Independent [Internet], 8 September 2024. Available online: https://www.independent.co.uk/news/uk/nhs-wes-streeting-victoria-atkins-labour-bbc-b2609022.html (accessed on 4 January 2025).
- Boyce, S.H. The NHS is broken: We need to change how our healthcare system works and join the 21st century. Emerg. Med. J. 2023, 40, 155. [Google Scholar] [CrossRef]
- Devlin, K.; Mitchell, A.; Cooke, M. Keir Starmer Abolishes NHS England to Bring Health Service Back to ‘Heart of Government’. The Independent [Internet], 13 March 2025. Available online: https://www.independent.co.uk/news/uk/politics/nhs-england-health-starmer-government-reform-b2714378.html (accessed on 5 January 2025).
- Kwok, C.S.; Whittaker, J.J.; Malbon, C.; White, B.; Snape, J.; Lloyd, V.; Yazdani, F.; Kemp, T.; Duckett, S. Outpatient parenteral antimicrobial therapy (OPAT) service is associated with inpatient-bed cost savings. Br. J. Cardiol. 2021, 28, 38. [Google Scholar]
- Hitchcock, J.; Jepson, A.P.; Main, J.; Wickens, H.J. Establishment of an Outpatient and Home Parenteral Antimicrobial Therapy Service at a London Teaching Hospital: A Case Series. J. Antimicrob. Chemother. 2009, 64, 630–634. [Google Scholar] [CrossRef]
- Durojaiye, O.C.; Bell, H.; Andrews, D.; Ntziora, F.; Cartwright, K. Clinical efficacy, cost analysis and patient acceptability of outpatient parenteral antibiotic therapy (OPAT): A decade of Sheffield (UK) OPAT service. Int. J. Antimicrob. Agents 2018, 51, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Barr, D.A.; Semple, L.; Seaton, R.A. Outpatient parenteral antimicrobial therapy (OPAT) in a teaching hospital-based practice: A retrospective cohort study describing experience and evolution over 10 years. Int. J. Antimicrob. Agents 2012, 39, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Chapman, A.L.N.; Dixon, S.; Andrews, D.; Lillie, P.J.; Bazaz, R.; Patchett, J.D. Clinical efficacy and cost-effectiveness of outpatient parenteral antibiotic therapy (OPAT): A UK perspective. J. Antimicrob. Chemother. 2009, 64, 1316–1324. [Google Scholar] [CrossRef]
- Kesharwani, D.; Bista, A.; Singh, H.; Unnithan, A.; Das, G.; Bristoll, S.; Lewis, N.; Alnoori, N. Outpatient parenteral antimicrobial therapy practice in United Kingdom: A single-center experience. Oman Med. J. 2022, 37, e442. [Google Scholar] [CrossRef]
- Mohammed, S.A.; Roberts, J.A.; Cotta, M.O.; Rogers, B.; Pollard, J.; Assefa, G.M.; Erku, D.; Sime, F.B. Safety and efficacy of outpatient parenteral antimicrobial therapy: A systematic review and meta-analysis of randomized clinical trials. Int. J. Antimicrob. Agents 2024, 64, 107263. [Google Scholar] [CrossRef]
- Duncan, C.J.A.; Barr, D.A.; Ho, A.; Sharp, E.; Semple, L.; Seaton, R.A. Risk factors for failure of outpatient parenteral antibiotic therapy (OPAT) in infective endocarditis. J. Antimicrob. Chemother. 2013, 68, 1650–1654. [Google Scholar] [CrossRef]
- Durojaiye, O.C.; Morgan, R.; Chelaghma, N.; Kritsotakis, E.I. Clinical predictors of outcome in patients with infective endocarditis receiving outpatient parenteral antibiotic therapy (OPAT). J. Infect. 2021, 83, 644–649. [Google Scholar] [CrossRef]
- Partridge, D.G.; O’Brien, E.; Chapman, A.L. Outpatient parenteral antibiotic therapy for infective endocarditis: A review of 4 years’ experience at a UK centre. Postgrad. Med. J. 2012, 88, 377–381. [Google Scholar] [CrossRef]
- Seaton, R.; Sharp, E.; Bezlyak, V.; Weir, C. Factors associated with outcome and duration of therapy in outpatient parenteral antibiotic therapy (OPAT) patients with skin and soft-tissue infections. Int. J. Antimicrob. Agents 2011, 38, 243–248. [Google Scholar] [CrossRef]
- Zhang, J.; Moore, E.; Bousfield, R. OPAT for cellulitis: Its benefits and the factors that predispose to longer treatment. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 1013–1015. [Google Scholar] [CrossRef]
- Palit, J.; Cole, J.; Durojaiye, O.C. Clinical and operational factors associated with treatment duration for cellulitis in outpatient parenteral antimicrobial therapy (OPAT). Diagn. Microbiol. Infect. Dis. 2021, 100, 115305. [Google Scholar] [CrossRef] [PubMed]
- Durojaiye, O.C.; Slucka, A.; Kritsotakis, E.I. Retrospective analysis of outcomes of outpatient parenteral antimicrobial therapy (OPAT) for necrotising otitis externa. Eur. J. Clin. Microbiol. Infect. Dis. 2022, 41, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Dabrowski, H.; Wickham, H.; De, S.; Underwood, J.; Morris-Jones, S.; Logan, S.; Marks, M.; Pollara, G. Clinical outcomes of teicoplanin use in the OPAT setting. Int. J. Antimicrob. Agents 2020, 55, 105888. [Google Scholar] [CrossRef] [PubMed]
- Wareham, D.; Melzer, M. Clinical outcomes in OPAT patients treated with ceftriaxone 4 g and ceftazidime 6 g extended interval dosing regimens. JAC Antimicrob. Resist. 2024, 6, dlae079. [Google Scholar] [CrossRef]
- Mitchell, E.D.; Murray, C.C.; Meads, D.; Minton, J.; Wright, J.; Twiddy, M. Clinical and cost-effectiveness, safety and acceptability of community intravenous antibiotic service models: CIVAS systematic review. BMJ Open 2017, 7, e013560. [Google Scholar] [CrossRef]
- Pacheco, R.L.; Latorraca, C.d.O.C.; dos Santos, A.P.P.; Martimbianco, A.L.C.; Moreira, R.d.F.C.; Logullo, P.; Riera, R. Efficacy and safety of home-based intravenous antibiotic therapy among adults: A systematic review. Int. J. Antimicrob. Agents 2022, 59, 106555. [Google Scholar] [CrossRef]
- Bryant, P.A.; Katz, N.T. Inpatient versus outpatient parenteral antibiotic therapy at home for acute infections in children: A systematic review. Lancet Infect. Dis. 2018, 18, e45–e54. [Google Scholar] [CrossRef]
- Dimitrova, M.; Gilchrist, M.; Seaton, R.A. Outpatient parenteral antimicrobial therapy (OPAT) versus inpatient care in the UK: A health economic assessment for six key diagnoses. BMJ Open 2021, 11, e049733. [Google Scholar] [CrossRef]
- Vargas-Palacios, A.; Meads, D.M.; Twiddy, M.; Murray, C.C.; Hulme, C.; Mitchell, E.D.; Gregson, A.; Stanley, P.; Minton, J. Cost-effectiveness of outpatient parenteral antibiotic therapy: A simulation modelling approach. J. Antimicrob. Chemother. 2017, 72, 2392–2400. [Google Scholar] [CrossRef]
- Berrevoets, M.A.H.; Oerlemans, A.J.M.; Tromp, M.; Kullberg, B.J.; Oever, J.T.; Schouten, J.A.; Hulscher, M.E. Quality of outpatient parenteral antimicrobial therapy (OPAT) care from the patient’s perspective: A qualitative study. BMJ Open 2018, 8, e024564. [Google Scholar] [CrossRef]
- Corwin, P.; Toop, L.; McGeoch, G.; Than, M.; Wynn-Thomas, S.; Wells, J.E.; Dawson, R.; Abernethy, P.; Pithie, A.; Chambers, S.; et al. Randomised controlled trial of intravenous antibiotic treatment for cellulitis at home compared with hospital. BMJ 2005, 330, 129. [Google Scholar] [CrossRef] [PubMed]
- Twiddy, M.; Murray, C.J.C.; Mason, S.J.; Meads, D.; Wright, J.M.; Mitchell, E.D.; Minton, J.; on behalf of the CIVAS Study Team. A qualitative study of patients’ feedback about Outpatient Parenteral Antimicrobial Therapy (OPAT) services in Northern England: Implications for service improvement. BMJ Open 2018, 8, e019099. [Google Scholar] [CrossRef] [PubMed]
- Tonna, A.; Anthony, G.; Tonna, I.; Paudyal, V.; Forbes-McKay, K.; Laing, R.; Mackenzie, A.; Falconer, S.; McCartney, G.; Stewart, D. Home self-administration of intravenous antibiotics as part of an outpatient parenteral antibiotic therapy service: A qualitative study of the perspectives of patients who do not self-administer. BMJ Open 2019, 9, e027475. [Google Scholar] [CrossRef] [PubMed]
- Soni, S.; Harding, I.; Jones, C.; Wade, S.; Norton, J.; Pollock, J.S. A qualitative review of patient feedback for the OPAT (outpatient antimicrobial therapy) service in Bristol. Antibiotics 2024, 13, 420. [Google Scholar] [CrossRef]
- Carter, B.; Fisher-Smith, D.; Porter, D.; Lane, S.; Peak, M.; Taylor-Robinson, D.; Bracken, L.; Carrol, E. Being ‘at-home’ on outpatient parenteral antimicrobial therapy (OPAT): A qualitative study of parents’ experiences of paediatric OPAT. Arch. Dis. Child. 2020, 105, 276–281. [Google Scholar] [CrossRef]
- Durojaiye, O.C.; Kritsotakis, E.I. Evaluation of health-related quality of life in patients receiving outpatient parenteral antimicrobial therapy (OPAT) in a UK setting. Expert Rev. Anti Infect. Ther. 2024, 22, 987–995. [Google Scholar] [CrossRef]
- Wong, K.K.; Fraser, T.G.; Shrestha, N.K.; Fatica, C.; Deshpande, A. Low incidence of Clostridium difficile infection (CDI) in patients treated with outpatient parenteral antimicrobial therapy (OPAT). Infect. Control Hosp. Epidemiol. 2015, 36, 110–112. [Google Scholar] [CrossRef]
- Duncan, C.J.; Barr, D.A.; Seaton, R.A. Outpatient parenteral antimicrobial therapy with ceftriaxone, a review. Int. J. Clin. Pharm. 2012, 34, 410–417. [Google Scholar] [CrossRef]
- Aberdein, J.; Chapman, A.L. Clostridium difficile infection following outpatient parenteral antimicrobial therapy. J. Hosp. Infect. 2015, 90, 171–172. [Google Scholar] [CrossRef]
- Bellamy, R. Outpatient parenteral antimicrobial therapy. Br. J. Hosp. Med. 2018, 79, 12–17. [Google Scholar] [CrossRef]
- Afra, K.; Wong, M.; Chapman, M.G.; Mirzanejad, Y.; Deans, G.D. 750: Effectiveness, Safety, and Impact on Healthcare Decongestion by a Busy Canadian Infusion Centre for Outpatient Parenteral Antimicrobial Therapy. Open Forum Infect. Dis. 2014, 1, S212. [Google Scholar] [CrossRef]
- Jenkins, T.C.; Price, C.S.; Sabel, A.L.; Mehler, P.S.; Burman, W.J. Impact of routine infectious diseases service consultation on the evaluation, management, and outcomes of Staphylococcus aureus bacteremia. Clin. Infect. Dis. 2008, 46, 1000–1008. [Google Scholar] [CrossRef] [PubMed]
- Fowler, V.G., Jr.; Sanders, L.L.; Sexton, D.J.; Kong, L.; Marr, K.A.; Gopal, A.K.; Gottlieb, G.; McClelland, R.S.; Corey, G.R. Outcome of Staphylococcus aureus bacteremia according to compliance with recommendations of infectious diseases specialists: Experience with 244 patients. Clin. Infect. Dis. 1998, 27, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Durojaiye, O.C.; Kritsotakis, E.I.; Johnston, P.; Kenny, T.; Ntziora, F.; Cartwright, K. Developing a risk prediction model for 30-day unplanned hospitalization in patients receiving outpatient parenteral antimicrobial therapy. Clin. Microbiol. Infect. 2019, 25, 905.e1–905.e7. [Google Scholar] [CrossRef]
- Tice, A.D.; Rehm, S.J.; Dalovisio, J.R.; Bradley, J.S.; Martinelli, L.P.; Graham, D.R.; Gainer, R.B.; Kunkel, M.J.; Yancey, R.W.; Williams, D.N. IDSA. Practice guidelines for outpatient parenteral antimicrobial therapy. IDSA guidelines. Clin. Infect. Dis. 2004, 38, 1651–1672. [Google Scholar] [CrossRef]
- Laupland, K.B.; Valiquette, L. Outpatient parenteral antimicrobial therapy. Can. J. Infect. Dis. Med. Microbiol. 2013, 24, 9–11. [Google Scholar] [CrossRef]
- Brenon, J.R.; Shulder, S.E.; Munsiff, S.S.; Burgoyne, C.M.; Nagel, A.K.; Pillinger, K.E. Rate of broad-spectrum antibiotic overuse in patients receiving outpatient parenteral antibiotic therapy (OPAT). Antimicrob. Steward. Healthc. Epidemiol. 2021, 1, e36. [Google Scholar] [CrossRef]
- Krah, N.M.; Bardsley, T.; Nelson, R.; Esquibel, L.; Crosby, M.; Byington, C.L.; Pavia, A.T.; Hersh, A.L. Economic burden of home antimicrobial therapy: OPAT versus Oral therapy. Hosp. Pediatr. 2019, 9, 234–240. [Google Scholar] [CrossRef]
- Sharma, R.; Loomis, W.; Brown, R.B. Impact of mandatory inpatient infectious disease consultation on outpatient parenteral antibiotic therapy. Am. J. Med. Sci. 2005, 330, 60–64. [Google Scholar] [CrossRef]
- Heintz, B.H.; Halilovic, J.; Christensen, C.L. Impact of a multidisciplinary team review of potential outpatient parenteral antimicrobial therapy prior to discharge from an academic medical center. Ann. Pharmacother. 2011, 45, 1329–1337. [Google Scholar] [CrossRef]
- Shrestha, N.K.; Bhaskaran, A.; Scalera, N.M.; Schmitt, S.K.; Rehm, S.J.; Gordon, S.M. Contribution of infectious disease consultation toward the care of inpatients being considered for community-based parenteral anti-infective therapy. J. Hosp. Med. 2012, 7, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Sriskandarajah, S.; Hobbs, J.; Roughead, E.; Ryan, M.; Reynolds, K. Safety and effectiveness of ‘hospital in the home’ and ‘outpatient parenteral antimicrobial therapy’ in different age groups: A systematic review of observational studies. Int. J. Clin. Pract. 2018, 72, e13216. [Google Scholar] [CrossRef] [PubMed]
- Keller, S.C.; Williams, D.; Gavgani, M.; Hirsch, D.; Adamovich, J.; Hohl, D.; Gurses, A.P.; Cosgrove, S.E. Rates of and risk factors for adverse drug events in outpatient parenteral antimicrobial therapy. Clin. Infect. Dis. 2018, 66, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Briggs, S.; Smith, S.; Ritchie, S.; Fitzharris, P.; Ellis-Pegler, R. Late-onset bolus intravenous beta-lactam antibiotic adverse reaction: Short-lived symptoms during drug administration and associated laboratory abnormalities. Scand. J. Infect. Dis. 2014, 46, 14–20. [Google Scholar] [CrossRef]
- Ceroni, D.; Regusci, M.; Pazos, J.M.; Saunders, C.T.; Kaelin, A. Risks and complications of prolonged parenteral antibiotic treatment in children with acute osteoarticular infections. Acta Orthop. Belg. 2003, 69, 400–404. [Google Scholar]
- Lamb, H.M.; Ormrod, D.; Scott, L.J.; Figgitt, D.P. Ceftriaxone: An update of its use in the management of community-acquired and nosocomial infections. Drugs 2002, 62, 1041–1089. [Google Scholar] [CrossRef]
- Fallouh, N.; McGuirk, H.M.; Flanders, S.A.; Chopra, V. Peripherally inserted central catheter-associated deep vein thrombosis: A narrative review. Am. J. Med. 2015, 128, 722–738. [Google Scholar] [CrossRef]
- Shrestha, N.K.; Shrestha, J.; Everett, A.; Carroll, D.; Gordon, S.M.; Butler, R.S.; Rehm, S.J. Vascular access complications during outpatient parenteral antimicrobial therapy at home: A retrospective cohort study. J. Antimicrob. Chemother. 2016, 71, 506–512. [Google Scholar] [CrossRef]
- Lam, P.W.; Graham, C.; Leis, J.A.; Daneman, N. Predictors of Peripherally inserted central catheter occlusion in the outpatient parenteral antimicrobial therapy setting. Antimicrob. Agents Chemother. 2018, 62, e00900-18. [Google Scholar] [CrossRef]
- Barr, D.A.; Semple, L.; Seaton, R.A. Self-administration of outpatient parenteral antibiotic therapy and risk of catheter-related adverse events: A retrospective cohort study. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 2611–2619. [Google Scholar] [CrossRef]
- Underwood, J.; Marks, M.; Collins, S.; Logan, S.; Pollara, G. Intravenous catheter-related adverse events exceed drug-related adverse events in outpatient parenteral antimicrobial therapy. J. Antimicrob. Chemother. 2019, 74, 787–790. [Google Scholar] [CrossRef] [PubMed]
- Paje, D.; Walzl, E.; Heath, M.; McLaughlin, E.; Horowitz, J.K.; Tatarcuk, C.; Swaminathan, L.; Kaatz, S.; Malani, A.N.; Vaughn, V.M.; et al. Midline vs peripherally inserted central catheter for outpatient parenteral antimicrobial therapy. JAMA Intern. Med. 2025, 185, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Urtecho, M.; Roldan, V.D.T.; Nayfeh, T.; Suarez, N.R.E.; Ranganath, N.; Sampathkumar, P.; Chopra, V.; Safdar, N.; Prokop, L.J.; O’horo, J.C. Comparing complication rates of midline catheter vs peripherally inserted central catheter. A systematic review and meta-analysis. Open Forum Infect. Dis. 2023, 10, ofad024. [Google Scholar] [CrossRef] [PubMed]
- Cohoon, K.P.; Ashrani, A.A.; Crusan, D.J.; Petterson, T.M.; Bailey, K.R.; Heit, J.A. Is infection an independent risk factor for venous thromboembolism? A population-based, case-control study. Am. J. Med. 2018, 131, 307–316.e2. [Google Scholar] [CrossRef]
- Schmidt, M.; Horvath-Puho, E.; Thomsen, R.W.; Smeeth, L.; Sørensen, H.T. Acute infections and venous thromboembolism. J. Intern. Med. 2012, 271, 608–618. [Google Scholar] [CrossRef]
- Durojaiye, O.C.; Cole, J.; Kritsotakis, E.I. Risk of venous thromboembolism in outpatient parenteral antimicrobial therapy (OPAT): A systematic review and meta-analysis. Int. J. Antimicrob. Agents 2023, 62, 106911. [Google Scholar] [CrossRef]
- Ingram, P.R.; Kilgarriff, S.; Grzelak, M.; Jackson, G.; Carr, P.; Boan, P.; Italiano, C.; Dyer, J.; Raby, E. Risk factors for catheter related thrombosis during outpatient parenteral antimicrobial therapy. J. Vasc. Access 2022, 23, 738–742. [Google Scholar] [CrossRef]
- Saillen, L.; Arensdorff, L.; Moulin, E.; Voumard, R.; Cochet, C.; Boillat-Blanco, N.; Gardiol, C.; de Vallière, S. Patient satisfaction in an outpatient parenteral antimicrobial therapy (OPAT) unit practising predominantly self-administration of antibiotics with elastomeric pumps. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 1387–1392. [Google Scholar] [CrossRef]
- Voumard, R.; Gardiol, C.; André, P.; Arensdorff, L.; Cochet, C.; Boillat-Blanco, N.; Decosterd, L.; Buclin, T.; de Vallière, S. Efficacy and safety of continuous infusions with elastomeric pumps for outpatient parenteral antimicrobial therapy (OPAT): An observational study. J. Antimicrob. Chemother. 2018, 73, 2540–2545. [Google Scholar] [CrossRef]
- Ortonobes, S.; Mujal-Martínez, A.; de Castro Julve, M.; González-Sánchez, A.; Jiménez-Pérez, R.; Hernández-Ávila, M.; De Alfonso, N.; Maye-Pérez, I.; Valle-Delmás, T.; Rodríguez-Sánchez, A.; et al. Successful integration of clinical pharmacists in an OPAT program: A real-life multidisciplinary circuit. Antibiotics 2022, 11, 1124. [Google Scholar] [CrossRef]
- Lui, G.Y.; Dickson, H.G.P.; West, D.; Alexandrou, E.; Malone, M.P.; Breen, P.P.B. Elastomeric pump infusion failures caused by inadequate luerlock connector engagement to needleless connectors. J. Infus. Nurs. 2021, 44, 274–281. [Google Scholar] [CrossRef]
- Mizuuchi, M.; Yamakage, M.; Iwasaki, S.; Kimura, A.; Namiki, A. The infusion rate of most disposable, non-electric infusion pumps decreases under hypobaric conditions. Can. J. Anaesth. 2003, 50, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Munzert, E.; Jacobshagen, A. Malfunctions of non-electrical infusion pumps. Dtsch. Arztebl. Int. 2024, 121, 541–542. [Google Scholar] [CrossRef] [PubMed]
- Diamantis, S.; Dawudi, Y.; Cassard, B.; Longuet, P.; Lesprit, P.; Gauzit, R. Home intravenous antibiotherapy and the proper use of elastomeric pumps: Systematic review of the literature and proposals for improved use. Infect. Dis. Now 2021, 51, 39–49. [Google Scholar] [CrossRef]
- Hanely, E.F.; Hancock, R.E.W. Addressing antibiotic failure—Beyond genetically encoded antimicrobial resistance. Front. Drug Discov. 2022, 2, 892975. [Google Scholar]
- Mackintosh, C.L.; White, H.A.; Seaton, R.A. Outpatient parenteral antibiotic therapy (OPAT) for bone and joint infections: Experience from a UK teaching hospital-based service. J. Antimicrob. Chemother. 2011, 66, 408–415. [Google Scholar] [CrossRef]
- Shakoor, S.; Durojaiye, O.C.; Collini, P.J. Outcomes of outpatient parenteral antimicrobial therapy (OPAT) for urinary tract infections—A single center retrospective cohort study. Clin. Infect. Pract. 2023, 17, 100212. [Google Scholar] [CrossRef]
- Hatcher, J.; Costelloe, C.; Cele, R.; Viljanen, A.; Samarasinghe, D.; Satta, G.; Brannigan, E.; De Barra, E.; Sanderson, F.; Gilchrist, M. Factors associated with successful completion of outpatient parenteral antibiotic therapy (OPAT): A 10-year review from a large West London service. Int. J. Antimicrob. Agents 2019, 54, 207–214. [Google Scholar] [CrossRef]
- Stubbs, R.D.; Shorten, R.J.; Benedetto, V.; Muir, A. Does comorbidity index predict OPAT readmission? JAC Antimicrob. Resist. 2023, 5, dlad125. [Google Scholar] [CrossRef]
- WWolie, Z.T.; Roberts, J.A.; Gilchrist, M.; McCarthy, K.; Sime, F.B. Current practices and challenges of outpatient parenteral antimicrobial therapy: A narrative review. J. Antimicrob. Chemother. 2024, 79, 2083–2102. [Google Scholar] [CrossRef]
- Narayanan, S.; Ching, P.R.; Traver, E.C.; George, N.; Amoroso, A.; Kottilil, S. Predictors of nonadherence among patients with infectious complications of substance use who are discharged on parenteral antimicrobial therapy. Open Forum Infect. Dis. 2022, 10, ofac633. [Google Scholar] [CrossRef] [PubMed]
- Means, L.; Bleasdale, S.; Sikka, M.; Gross, A.E. Predictors of Hospital Readmission in Patients Receiving Outpatient Parenteral Antimicrobial Therapy. Pharmacotherapy 2016, 36, 934–939. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Hearn, B.; Gabriel, M.; Spencer, M.D.; McCurdy, L. Predictors of unplanned hospitalization in patients receiving outpatient parenteral antimicrobial therapy across a large integrated healthcare network. Open Forum Infect. Dis. 2017, 4, ofx086. [Google Scholar] [CrossRef] [PubMed]
- Madaline, T.; Nori, P.; Mowrey, W.; Zukowski, E.; Gohil, S.; Sarwar, U.; Weston, G.; Urrely, R.; Palombelli, M.; Pierino, V.F.; et al. Bundle in the Bronx: Impact of a transition-of-care outpatient parenteral antibiotic therapy bundle on all-cause 30-day hospital readmissions. Open Forum Infect. Dis. 2017, 4, ofx097. [Google Scholar] [CrossRef]
- Buehrle, D.J.; Shields, R.K.; Shah, N.; Shoff, C.; Sheridan, K. Risk factors associated with outpatient parenteral antibiotic therapy program failure among intravenous drug users. Open Forum Infect. Dis. 2017, 4, ofx102. [Google Scholar] [CrossRef]
- Bradley, A.C.; Wingler, M.J.B.; Artman, K.L.; Ward, L.M.; Lucar, J. An evaluation of risk factors for readmission in patients receiving outpatient parenteral antimicrobial therapy. Ther. Adv. Infect. Dis. 2023, 10, 20499361231195966. [Google Scholar] [CrossRef]
- Durojaiye, O.C.; Morgan, R.; Chelaghma, N.; Palit, J.; Keil, C.; Omer, R.; Cartwright, K.; Kritsotakis, E.I. External validity and clinical usefulness of a risk prediction model for 30 day unplanned hospitalization in patients receiving outpatient parenteral antimicrobial therapy. J. Antimicrob. Chemother. 2021, 76, 2204–2212. [Google Scholar] [CrossRef]
- Emilie, C.; de Nocker, P.; Saïdani, N.; Gilchrist, M.; Seaton, R.A.; Patel, S.; Beraud, G.; Kofteridis, D.; Schouten, J.; Thilly, N.; et al. Survey of delivery of parenteral antimicrobials in non-inpatient settings across Europe. Int. J. Antimicrob. Agents 2022, 59, 106559. [Google Scholar] [CrossRef]
- Mohammed, S.; Cotta, M.; Assefa, G.; Erku, D.; Sime, F. Barriers and facilitators for the implementation and expansion of outpatient parenteral antimicrobial therapy: A systematic review. J. Hosp. Infect. 2024, 147, 1–16. [Google Scholar] [CrossRef]
- Hamad, Y.; Lane, M.A.; Beekmann, S.E.; Polgreen, P.M.; Keller, S.C. Perspectives of United States-based infectious diseases physicians on outpatient parenteral antimicrobial therapy practice. Open Forum Infect. Dis. 2019, 6, ofz363. [Google Scholar] [CrossRef]
- Jones, G.R.; Cumming, D.V.E.; Honeywell, G.; Ball, R.; Seaton, R.A.; Healy, B.; Hedderwick, S.; Dryden, M.; Gilchrist, M.; on behalf of the BSAC OPAT Standing Committee; et al. How is income generated by outpatient parenteral antibiotic treatment (OPAT) in the UK? Analysis of payment tariffs for cellulitis. J. Antimicrob. Chemother. 2015, 70, 1236–1240. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.R.; Cumming, D.; Gilchrist, M.; Seaton, R.A. Rapid response to Chapman ALN. Outpatient parenteral antimicrobial therapy. BMJ 2013, 346, f1585. Available online: https://www.bmj.com/content/346/bmj.f1585/rapid-responses (accessed on 5 December 2024).
- Lane, M.A.; Marschall, J.; Beekmann, S.E.; Polgreen, P.M.; Banerjee, R.; Hersh, A.L.; Babcock, H.M. Outpatient parenteral antimicrobial therapy practices among adult infectious disease physicians. Infect. Control Hosp. Epidemiol. 2014, 35, 839–844. [Google Scholar] [CrossRef] [PubMed]
- Kaes, A.; Buyle, F.; Quintens, C.; Van Eijgen, A.; Zubryckyj, L.; Boussery, K.; Vanoverschelde, A. Organisational quality and hospital pharmacists’ roles of outpatient parenteral antimicrobial therapy (OPAT) in Belgian hospitals: A national survey. Acta Clin. Belg. 2025, 79, 341–349. [Google Scholar] [CrossRef]
- Stoorvogel, H.H.; Hulscher, M.E.J.L.; Wertheim, H.F.L.; Yzerman, E.P.F.; Scholing, M.; Schouten, J.A.; Oever, J.T. Current practices and opportunities for outpatient parenteral antimicrobial therapy in hospitals: A national cross-sectional survey. Antibiotics 2022, 11, 1343. [Google Scholar] [CrossRef]
- Esposito, S.; Noviello, S.; Leone, S.; Tice, A.; Seibold, G.; Nathwani, D.; Scaglione, F. Outpatient parenteral antibiotic therapy (OPAT) in different countries: A comparison. Int. J. Antimicrob. Agents 2004, 24, 473–478. [Google Scholar] [CrossRef]
- Lehmann, C.U.; Miller, M.R. Standardization and the practice of medicine. J. Perinatol. 2004, 24, 135–136. [Google Scholar] [CrossRef]
- Leotsakos, A.; Zheng, H.; Croteau, R.; Loeb, J.M.; Sherman, H.; Hoffman, C.; Morganstein, L.; O’Leary, D.; Bruneau, C.; Lee, P.; et al. Standardization in patient safety: The WHO High 5s project. Int. J. Qual. Health Care 2014, 26, 109–116. [Google Scholar] [CrossRef]
- Braithwaite, J.; Westbrook, J.; Pawsey, M.; Greenfield, D.; Naylor, J.; Iedema, R.; Runciman, B.; Redman, S.; Jorm, C.; Robinson, M. A prospective, multi-method, multi-disciplinary, multi-level, collaborative, social-organisational design for researching health sector accreditation [LP0560737]. BMC Health Serv. Res. 2006, 6, 113. [Google Scholar] [CrossRef]
- Sumpter, C.; Russell, C.D.; Mackintosh, C. Inequitable access to an outpatient parenteral antimicrobial therapy service: Linked cross-sectional study. Int. J. Equity Health 2020, 19, 150. [Google Scholar] [CrossRef]
- Farmer, E.C.W.; Seaton, R.A. Recent innovations and new applications of outpatient parenteral antimicrobial therapy. Expert Rev. Anti Infect. Ther. 2021, 19, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, C.; Owen-Smith, A.; Donovan, J.; Hollingworth, W. A systematic review of geographical variation in access to chemotherapy. BMC Cancer 2015, 16, 1. [Google Scholar] [CrossRef] [PubMed]
- Ashby, J.; Ahmed, N.; Goldmeier, D.; BASHH Sexual Dysfunction Specialist Interest Group. Sexual difficulties service provision within sexual health services in the UK: A casualty of postcode lottery and commissioning? Sex. Transm. Infect. 2019, 95, 397. [Google Scholar] [CrossRef]
- Durojaiye, O.C.; Jibril, I.; Kritsotakis, E.I. Effectiveness of telemedicine in outpatient parenteral antimicrobial therapy (Tele-OPAT): A systematic review. J. Telemed. Telecare 2024, 30, 1230–1237. [Google Scholar] [CrossRef]
- Mahoney, M.V.; Ryan, K.L.; Alexander, B.T. Evaluation of OPAT in the age of antimicrobial stewardship. Curr. Treat. Options Infect. Dis. 2020, 12, 158–177. [Google Scholar] [CrossRef]
- Gilchrist, M.; Seaton, R.A. Outpatient parenteral antimicrobial therapy and antimicrobial stewardship: Challenges and checklists. J. Antimicrob. Chemother. 2015, 70, 965–970. [Google Scholar] [CrossRef]
- Dryden, M.; Saeed, K.; Townsend, R.; Winnard, C.; Bourne, S.; Parker, N.; Coia, J.; Jones, B.; Lawson, W.; Wade, P.; et al. Antibiotic stewardship and early discharge from hospital: Impact of a structured approach to antimicrobial management. J. Antimicrob. Chemother. 2012, 67, 2289–2296. [Google Scholar] [CrossRef]
- Hersh, A.L.; Olson, J.; Stockmann, C.; Thorell, E.A.; Knackstedt, E.D.; Esquibel, L.; Sanderson, S.; Pavia, A.T. Impact of antimicrobial stewardship for pediatric outpatient parenteral antibiotic therapy. J. Pediatr. Infect. Dis. Soc. 2018, 7, e34–e36. [Google Scholar] [CrossRef]
- Conant, M.M.; Erdman, S.M.; Osterholzer, D. Mandatory infectious diseases approval of outpatient parenteral antimicrobial therapy (OPAT): Clinical and economic outcomes of averted cases. J. Antimicrob. Chemother. 2014, 69, 1695–1700. [Google Scholar] [CrossRef]
- Cassettari, V.; Novato, N.; Onuchic, M.H.F. Antimicrobial stewardship in the outpatient parenteral antimicrobial therapy (OPAT) setting: The impact of prescription assessment by an infectious diseases specialist. Braz. J. Infect. Dis. 2021, 25, 101560. [Google Scholar] [CrossRef]
- Jenkins, A.; Shanu, S.; Jamieson, C.; Santillo, M. Systematic review of the stability of antimicrobial agents in elastomeric devices for outpatient parenteral antimicrobial therapy services based on NHS Yellow Cover Document standards. Eur. J. Hosp. Pharm. 2022, 29, 304–307. [Google Scholar] [CrossRef] [PubMed]
- Steffens, E.; Quintens, C.; Derdelinckx, I.; Peetermans, W.E.; Van Eldere, J.; Spriet, I.; Schuermans, A. Outpatient parenteral antimicrobial therapy and antibiotic stewardship: Opponents or teammates? Infection 2019, 47, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Eii, M.N.; Walpole, S.; Aldridge, C. Sustainable practice: Prescribing oral over intravenous medications. BMJ 2023, 383, e075297. [Google Scholar] [CrossRef] [PubMed]
- NHS England and NHS Improvement. Delivering a ‘Net Zero’ National Health Service; NHSE: London, UK, 2020.
- Keller, R.L.; Muir, K.; Roth, F.; Jattke, M.; Stucki, M. From bandages to buildings: Identifying the environmental hotspots of hospitals. J. Clean. Prod. 2021, 319, 128479. [Google Scholar] [CrossRef]
- Cole, A.; Aspin, J.; Laird, S.; Acri, F.; Galley, S.; Collins, M. The environmental impact of intravenous antimicrobial therapies: A comparison of OPAT and inpatient administration care pathways. JAC Antimicrob. Resist. 2025, 7, dlaf030. [Google Scholar] [CrossRef]
- UK Health Security Agency (UKHSA). English Surveillance Programme for Antimicrobial Utilisation and Resistance (ESPAUR) Report 2022 to 2023; UKHSA: London, UK, 2024. Available online: https://assets.publishing.service.gov.uk/media/6734e208b613efc3f1823095/ESPAUR-report-2023-2024.pdf (accessed on 8 January 2025).
- Yan, Y.M.; Tonks, K.; Singh, M.; Kavi, J.; Langford, N.J.; Hospital, B.C. Delivering outpatient antibiotic therapy (OPAT) in an Acute Medical Unit. Acute Med. 2011, 10, 22–25. [Google Scholar] [CrossRef]
- Nazarko, L. Avoiding admission and facilitating early discharge through OPAT. Br. J. Nurs. 2014, 23, S30–S36. [Google Scholar] [CrossRef]
- O’Hanlon, S.; McGrail, P.; Hodgkins, P. Community intravenous therapy provision. Nurs. Stand. 2017, 31, 45–53. [Google Scholar] [CrossRef]
- Regan, K.; Morgan, J. Implementing a nurse-led community intravenous antibiotic service. Primary Health Care 2015, 25, 18–24. [Google Scholar] [CrossRef]
- Nazarko, L. Outpatient parenteral antimicrobial therapy: Its delivery in the community. Br. J. Community Nurs. 2013, 18, 163–167. [Google Scholar] [CrossRef]
- Kayley, J. IV therapy in the community. Nurs. Times 2011, 107, 15–18. [Google Scholar] [PubMed]
- Docherty, T.; Schneider, J.J.; Cooper, J. Clinic- and hospital-based home care, outpatient parenteral antimicrobial therapy (OPAT) and the evolving clinical responsibilities of the pharmacist. Pharmacy 2020, 8, 233. [Google Scholar] [CrossRef] [PubMed]
- Mirón-Rubio, M.; González-Ramallo, V.; Estrada-Cuxart, O.; Sanroma-Mendizábal, P.; Segado-Soriano, A.; Mujal-Martínez, A.; Del Río-Vizoso, M.; García-Lezcano, M.; Martín-Blanco, N.; Florit-Serra, L.; et al. Intravenous antimicrobial therapy in the hospital-at-home setting: Data from the Spanish outpatient parenteral antimicrobial therapy registry. Future Microbiol. 2016, 11, 375–390. [Google Scholar] [CrossRef]
- George, T.; Ali, B.; Clark, F.; Oakley, R. Outpatient Parenteral Antimicrobial Therapy (OPAT) Service Delivery of Antiretrovirals—Clinical Staff and Patient-Reported Outcomes Measures (PROMs); Paper Presented at British HIV Association (BHIVA) Spring Conference; BHIVA: Gateshead, UK, 2023. [Google Scholar]
- Rodriguez, G.D.; Wu, Y.; Karnik, K.; Ruddy, S.; Kula, A.; Warren, N.; Yashayev, R.; Sajid, F.; Prasad, N.; Yoon, J.; et al. Implementation of a collaborated antimicrobial stewardship program and outpatient parenteral antimicrobial therapy (OPAT) unit-driven monoclonal antibody therapy program for COVID-19 at an NYC Hospital. Int. J. Infect. Dis. 2022, 118, 214–219. [Google Scholar] [CrossRef]
- Savic, L.; Ardern-Jones, M.; Avery, A.; Cook, T.; Denman, S.; Farooque, S.; Garcez, T.; Gold, R.; Jay, N.; Krishna, M.T.; et al. BSACI guideline for the set-up of penicillin allergy de-labelling services by non-allergists working in a hospital setting. Clin. Exp. Allergy 2022, 52, 1135–1141. [Google Scholar] [CrossRef]
- Seaton, R.A.; Bell, E.; Gourlay, Y.; Semple, L. Nurse-led management of uncomplicated cellulitis in the community: Evaluation of a protocol incorporating intravenous ceftriaxone. J. Antimicrob. Chemother. 2005, 55, 764–767. [Google Scholar] [CrossRef]
- Deng, H.; Gross, A.E.; Trotter, A.B.; Touchette, D.R. Cost evaluation of a nurse coordinated outpatient parenteral antimicrobial therapy (OPAT) program. Antimicrob. Steward. Healthc. Epidemiol. 2024, 3, e252. [Google Scholar] [CrossRef]
- Epperson, T.M.; Bennett, K.K.; Kupiec, K.K.; Speigel, K.; Neely, S.B.; Resman-Targoff, B.H.; Kinney, K.K.; White, B.P. Impact of a pharmacist-managed outpatient parenteral antimicrobial therapy (OPAT) service on cost savings and clinical outcomes at an academic medical center. Antimicrob. Steward. Healthc. Epidemiol. 2023, 3, e15. [Google Scholar] [CrossRef] [PubMed]
- NHS England. Virtual Wards Operational Framework; NHSE: London, UK, 2024; Available online: https://www.england.nhs.uk/long-read/virtual-wards-operational-framework/#what-is-and-is-not-a-virtual-ward (accessed on 3 January 2025).
- Mahoney, M.V.; Childs-Kean, L.M.; Khan, P.; Rivera, C.G.; Stevens, R.W.; Ryan, K.L. Recent updates in antimicrobial stewardship in outpatient parenteral antimicrobial therapy. Curr. Infect. Dis. Rep. 2021, 23, 24. [Google Scholar] [CrossRef]
- Pertzborn, M.; Rivera, C.G.; Tai, D.B.G. Taking the route less traveled: On the way to COpAT. Ther. Adv. Infect. Dis. 2023, 10, 20499361231192771. [Google Scholar] [CrossRef]
- Iversen, K.; Ihlemann, N.; Gill, S.U.; Madsen, T.; Elming, H.; Jensen, K.T.; Bruun, N.E.; Høfsten, D.E.; Fursted, K.; Christensen, J.J.; et al. Partial oral versus intravenous antibiotic treatment of endocarditis. N. Engl. J. Med. 2019, 380, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-K.; Rombach, I.; Zambellas, R.; Walker, A.S.; McNally, M.A.; Atkins, B.L.; Lipsky, B.A.; Hughes, H.C.; Bose, D.; Kümin, M.; et al. Oral versus intravenous antibiotics for bone and joint infection. N. Engl. J. Med. 2019, 380, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Tamma, P.D.; Conley, A.T.; Cosgrove, S.E.; Harris, A.D.; Lautenbach, E.; Amoah, J.; Avdic, E.; Tolomeo, P.; Wise, J.; Subudhi, S.; et al. Association of 30-day mortality with oral step-down vs continued intravenous therapy in patients hospitalized with enterobacteriaceae bacteremia. JAMA Intern. Med. 2019, 179, 316–323. [Google Scholar] [CrossRef]
- Arnold, M.R.; Wormer, B.A.; Kao, A.M.; Klima, D.A.; Colavita, P.D.; Cosper, G.H.; Heniford, B.T.; Schulman, A.M. Home intravenous versus oral antibiotics following appendectomy for perforated appendicitis in children: A randomized controlled trial. Pediatr. Surg. Int. 2018, 34, 1257–1268. [Google Scholar] [CrossRef]
- Azamgarhi, T.; Shah, A.; Warren, S. Clinical experience of implementing oral versus intravenous antibiotics (OVIVA) in a specialist orthopedic hospital. Clin. Infect. Dis. 2021, 73, e2582–e2588. [Google Scholar] [CrossRef]
- Fang, J.; Zhang, X.S.; Zhang, C.H.; Zhou, Z.Y.; Han, L.; Wang, Y.X.; He, X.S.; Bian, X.L.; Lin, G.Y.; Jiao, Z.; et al. Model based identification of linezolid exposure-toxicity thresholds in hospitalized Patients. Front. Pharmacol. 2021, 12, 732503. [Google Scholar] [CrossRef]
- Medicines and Healthcare Products Regulatory Agency (MHRA). Fluoroquinolone Antibiotics: Must Now Only Be Prescribed When Other Commonly Recommended Antibiotics Are Inappropriate; MHRA: London, UK, 2024. Available online: https://www.gov.uk/drug-safety-update/fluoroquinolone-antibiotics-must-now-only-be-prescribed-when-other-commonly-recommended-antibiotics-are-inappropriate (accessed on 10 January 2025).
- Juskowich, J.J.; Ward, A.; Spigelmyer, A.E.; Howard, C.A.; Slain, D.; Guilfoose, J.A.; Edmond, M.B.; Sarwari, A.R. 1002. Complex outpatient antimicrobial therapy (COpAT) program at a rural academic medical center: Evaluation of first 100 patients. Open Forum Infect. Dis. 2022, 9, ofac492.843. [Google Scholar] [CrossRef]
- Lakota, E.A.; Ong, V.; Flanagan, S.; Rubino, C.M. Population pharmacokinetic analyses for Rezafungin (CD101) efficacy using phase 1 data. Antimicrob. Agents Chemother. 2018, 62, e02603-17. [Google Scholar] [CrossRef]
- Smith, J.R.; Roberts, K.D.; Rybak, M.J. Dalbavancin: A novel lipoglycopeptide antibiotic with extended activity against Gram-positive infections. Infect. Dis. Ther. 2015, 4, 245–258. [Google Scholar] [CrossRef]
- Van Hise, N.W.; Chundi, V.; Didwania, V.; Anderson, M.; McKinsey, D.; Roig, I.; Sharma, A.; Petrak, R.M. Treatment of acute osteomyelitis with once-weekly oritavancin: A two-year, multicenter, retrospective study. Drugs Real World Outcomes 2020, 7, 41–45. [Google Scholar] [CrossRef]
- Taylor, K.; Williamson, J.; Luther, V.; Stone, T.; Johnson, J.; Gruss, Z.; Russ-Friedman, C.; Ohl, C.; Beardsley, J. Evaluating the use of dalbavancin for off-label indications. Infect. Dis. Rep. 2022, 14, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Lueking, R.; Wei, W.; Mang, N.S.; Ortwine, J.K.; Meisner, J. Evaluation of dalbavancin use on clinical outcomes, cost-Savings, and adherence at a large safety net hospital. Microbiol. Spectr. 2023, 11, e0238522. [Google Scholar] [CrossRef] [PubMed]
- Bryson-Cahn, C.; Beieler, A.M.; Chan, J.D.; Harrington, R.D.; Dhanireddy, S. Dalbavancin as secondary therapy for serious Staphylococcus aureus infections in a vulnerable patient population. Open Forum Infect. Dis. 2019, 6, ofz028. [Google Scholar] [CrossRef] [PubMed]
- Ciccullo, A.; Giuliano, G.; Segala, F.V.; Taddei, E.; Farinacci, D.; Pallavicini, F. Dalbavancin as a second-line treatment in methicillin-resistant Staphylococcus aureus prosthetic vascular graft infection. Infection 2020, 48, 309–310. [Google Scholar] [CrossRef]
- Asumang, J.; Heard, K.L.; Troise, O.; Fahmy, S.; Mughal, N.; Moore, L.S.P.; Hughes, S. Evaluation of a thrice weekly administration of teicoplanin in the outpatient setting: A retrospective observational multicentre study. JAC Antimicrob. Resist. 2021, 3, dlab012. [Google Scholar]
- Tascini, C.; Tagliaferri, E.; Di Paolo, A.; Ciofi, L.; Del Tacca, M.; Lambelet, P.; Menichett, F. Three-times weekly teicoplanin as outpatient treatment of chronic osteoarticular infections. J. Chemother. 2009, 21, 421–425. [Google Scholar]
- Lamont, E.; Seaton, R.A.; Macpherson, M.; Semple, L.; Bell, E.; Thomson, A.H. Development of teicoplanin dosage guidelines for patients treated within an outpatient parenteral antibiotic therapy (OPAT) programme. J. Antimicrob. Chemother. 2009, 64, 181–187. [Google Scholar] [CrossRef]
- Suzuki, J.; Johnson, J.; Montgomery, M.; Hayden, M.; Price, C. Outpatient parenteral antimicrobial therapy among people who inject drugs: A review of the literature. Open Forum Infect. Dis. 2018, 5, ofy194. [Google Scholar]
- Ho, J.; Archuleta, S.; Sulaiman, Z.; Fisher, D. Safe and successful treatment of intravenous drug users with a peripherally inserted central catheter in an outpatient parenteral antibiotic treatment service. J. Antimicrob. Chemother. 2010, 65, 2641–2644. [Google Scholar] [CrossRef]
- Beieler, A.M.; Dellit, T.H.; Chan, J.D.; Dhanireddy, S.; Enzian, L.K.; Stone, T.J.; Dwyer-O’Connor, E.; Lynch, J.B. Successful implementation of outpatient parenteral antimicrobial therapy at a medical respite facility for homeless patients. J. Hosp. Med. 2016, 11, 531–535. [Google Scholar] [CrossRef]
- Morrisette, T.; Miller, M.A.; Montague, B.T.; Barber, G.R.; McQueen, R.B.; Krsak, M. Long-acting lipoglycopeptides: “lineless antibiotics” for serious infections in persons who use drugs. Open Forum Infect. Dis. 2019, 6, ofz274. [Google Scholar] [PubMed]
- Oliver, G. Optimising patient safety when using elastomeric pumps to administer outpatient parenteral antibiotic therapy. Br. J. Nurs. 2016, 25, S22–S27. [Google Scholar] [CrossRef] [PubMed]
- Karimaghaei, S.; Rao, A.; Chijioke, J.; Finch, N.; Nigo, M. Characteristics, safety and cost-effectiveness analysis of self-administered outpatient parenteral antibiotic therapy via a disposable elastomeric continuous infusion pump at two county hospitals in Houston, Texas, United States. J. Clin. Pharm. Ther. 2022, 47, 211–217. [Google Scholar]
- Jenkins, A.; Shanu, S.; Jamieson, C.; Santillo, M. Widening the net: A literature review of antimicrobial agents with potential suitability for outpatient parenteral antimicrobial therapy services-the importance of storage and stability. Eur. J. Hosp. Pharm. 2023, 30, 64–69. [Google Scholar]
- Allwood, M.C.; Stonkute, D.; Wallace, A.; Wilkinson, A.-S.; Hills, T.; Jamieson, C. Assessment of the stability of citrate-buffered flucloxacillin for injection when stored in two commercially available ambulatory elastomeric devices: INfusor LV (Baxter) and Accufuser (Woo Young Medical): A study compliant with the NHS Yellow Cover Document (YCD) requirements. Eur. J. Hosp. Pharm. 2020, 27, 90–94. [Google Scholar]
- Jamieson, C.; Ozolina, L.; Seaton, R.A.; Gilchrist, M.; Hills, T.; Drummond, F.; Wilkinson, A.S.; on behalf of BSAC Drug Stability Testing Working Group. Assessment of the stability of citrate-buffered piperacillin/tazobactam for continuous infusion when stored in two commercially available elastomeric devices for outpatient parenteral antimicrobial chemotherapy: A study compliant with the NHS Yellow Cover Document requirements. Eur. J. Hosp. Pharm. 2022, 29, 212–216. [Google Scholar]
- Sime, F.B.; Wallis, S.; Jamieson, C.; Hills, T.; Gilchrist, M.; Santillo, M.; Seaton, R.A.; Drummond, F.; Roberts, J.; on behalf of BSAC Drug Stability Testing Programme. Evaluation of the stability of temocillin in elastomeric infusion devices used for outpatient parenteral antimicrobial therapy in accordance with the requirements of the UK NHS Yellow Cover Document. Eur. J. Hosp. Pharm. 2023, 30, e76–e81. [Google Scholar] [CrossRef]
- Jamieson, C.; Allwood, M.C.; Stonkute, D.; Wallace, A.; Wilkinson, A.-S.; Hills, T. Investigation of meropenem stability after reconstitution: The influence of buffering and challenges to meet the NHS Yellow Cover Document compliance for continuous infusions in an outpatient setting. Eur. J. Hosp. Pharm. 2020, 27, e53–e57. [Google Scholar]
- Haleem, A.; Javaid, M.; Singh, R.P.; Suman, R. Telemedicine for healthcare: Capabilities, features, barriers, and applications. Sens. Int. 2021, 2, 100117. [Google Scholar] [CrossRef]
- Stoltzfus, M.; Kaur, A.; Chawla, A. The role of telemedicine in healthcare: An overview and update. Egypt. J. Intern. Med. 2023, 35, 49. [Google Scholar]
- Young, J.D.; Abdel-Massih, R.; Herchline, T.; McCurdy, L.; Moyer, K.J.; Scott, J.D.; Wood, B.R.; Siddiqui, J. Infectious Diseases Society of America Position Statement on Telehealth and Telemedicine as Applied to the Practice of Infectious Diseases. Clin. Infect. Dis. 2019, 68, 1437–1443. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.J.; Ingram, P.R.; Rothnie, A.J.; Whitmore, T.J.; Robinson, J.O.; Hatch, J.B.; Italiano, C.M.; Heath, C.H. Successful outpatient parenteral antibiotic therapy delivery via telemedicine. J. Antimicrob. Chemother. 2017, 72, 2898–2901. [Google Scholar] [CrossRef] [PubMed]
- Eron, L.; King, P.; Marineau, M.; Yonehara, C. Treating acute infections by telemedicine in the home. Clin. Infect. Dis. 2004, 39, 1175–1181. [Google Scholar] [CrossRef]
- Seaton, R.A.; Barr, D.A. Outpatient parenteral antibiotic therapy: Principles and practice. Eur. J. Intern. Med. 2013, 24, 617–623. [Google Scholar] [CrossRef]
- Durojaiye, O.C.; Jibril, I.; Kritsotakis, E.I. Palliative outpatient parenteral antimicrobial therapy (OPAT): A single center experience and systematic scoping review. Clin. Infect. Pract. 2022, 16, 100205. [Google Scholar] [CrossRef]
- Hart, E.; Snape, S.; Thomson, R. Palliative outpatient parenteral antibiotic therapy: A review of 5 years of patient data. JAC Antimicrob. Resist. 2020, 2, dlaa052. [Google Scholar] [CrossRef]
- Jumpertz, M.; Guilhaumou, R.; Million, M.; Parola, P.; Lagier, J.-C.; Brouqui, P.; Cassir, N. Subcutaneously administered antibiotics: A review. J. Antimicrob. Chemother. 2022, 78, 1–7. [Google Scholar] [CrossRef]
- Ferry, T.; Lodise, T.P.; Gallagher, J.C.; Forestier, E.; Goutelle, S.; Tam, V.H.; Mohr, J.F.; Roubaud-Baudron, C. Outpatient subcutaneous antimicrobial therapy (OSCAT) as a measure to improve the quality and efficiency of healthcare delivery for patients with serious bacterial infections. Front. Med. 2022, 7, 585658. [Google Scholar] [CrossRef]
- Forestier, E.; Paccalin, M.; Roubaud-Baudron, C.; Fraisse, T.; Gavazzi, G.; Gaillat, J. Subcutaneously administered antibiotics: A national survey of current practice from the French Infectious Diseases (SPILF) and Geriatric Medicine (SFGG) society networks. Clin. Microbiol. Infect. 2015, 21, 370.e1–370.e3. [Google Scholar] [CrossRef]
- Colin, E.; Baldolli, A.; Verdon, R.; Saint-Lorant, G. Subcutaneously administered antibiotics. Med. Mal. Infect. 2020, 50, 231–242. [Google Scholar] [CrossRef]
| Patient Benefits | Organisational Benefits |
|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Vascular Access Complications | Drug-Related Complications | Infusion Device Related | Others |
|---|---|---|---|
| Allergy to dressing | Antibiotic-associated diarrhoea | Battery failure (electronic pumps) | Communication errors |
| Breakage | Blood dyscrasia | Device leakage | Non-adherence |
| Dislodgement | Clostridioides difficile infection | Device malfunction | Readmission |
| Infection | Electrolyte disturbances | Disconnection or accidental removal | Treatment failure |
| Migration | Gastrointestinal effects | Flow rate irregularities | |
| Occlusion | Hepatotoxicity | Incomplete infusion | |
| Thrombophlebitis | Nephrotoxicity | ||
| Thrombosis | Neurotoxicity | ||
| Rash |
| Patient-Related | Infection-Related | Antimicrobial- Related | Vascular Access-Related | OPAT Structure |
|---|---|---|---|---|
| Existing comorbidities (e.g., chronic renal disease, malignancy) High CCI Score | Condition treated (e.g., endovascular, prosthetic infection) | Adverse drug events Antimicrobial agent (e.g., glycopeptides, aminoglycosides) | Infection Line patency issue Thrombosis | Inadequate OPAT follow-up Mode of OPAT delivery (e.g., skilled nursing facility, subacute rehabilitation centre) |
| Older age Prior hospital admission Substance misuse | Multidrug-resistant Organisms Prolonged therapy | Concurrent IV therapy | Non-availability of test results |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Durojaiye, O.C.; Fiori, C.; Cartwright, K. Delivery of Outpatient Parenteral Antimicrobial Therapy (OPAT) in an Ever-Changing National Health Service (UK): Benefits, Barriers, and Opportunities. Antibiotics 2025, 14, 451. https://doi.org/10.3390/antibiotics14050451
Durojaiye OC, Fiori C, Cartwright K. Delivery of Outpatient Parenteral Antimicrobial Therapy (OPAT) in an Ever-Changing National Health Service (UK): Benefits, Barriers, and Opportunities. Antibiotics. 2025; 14(5):451. https://doi.org/10.3390/antibiotics14050451
Chicago/Turabian StyleDurojaiye, Oyewole Christopher, Charlotte Fiori, and Katharine Cartwright. 2025. "Delivery of Outpatient Parenteral Antimicrobial Therapy (OPAT) in an Ever-Changing National Health Service (UK): Benefits, Barriers, and Opportunities" Antibiotics 14, no. 5: 451. https://doi.org/10.3390/antibiotics14050451
APA StyleDurojaiye, O. C., Fiori, C., & Cartwright, K. (2025). Delivery of Outpatient Parenteral Antimicrobial Therapy (OPAT) in an Ever-Changing National Health Service (UK): Benefits, Barriers, and Opportunities. Antibiotics, 14(5), 451. https://doi.org/10.3390/antibiotics14050451







